Combined treatment of 3-hydroxypyridine-4-one derivatives and green tea extract to induce hepcidin expression in iron-overloadedβ-thalassemic mice
Supranee Upanan,Kanjana Pangjit,Chairat Uthaipibull,Suthat Fucharoen,Andrew T.McKie,Somdet Srichairatanakool*Department of Biochemistry,Faculty of Medicine,Chiang Mai University,Chiang Mai 000,Thailand
2College of Medicine and Public Health,Ubon Ratchathani University,Ubon Ratchathani 34190,Thailand
3National Center for Genetic Engineering and Biotechnology,National Science and Technology Development Agency,Thailand Science Park,Pathum Thani 12120,Thailand
4Thalassemia Research Center,Institute of Molecular Biosciences,Mahidol University,Salaya Campus,Nakornpathom 73170,Thailand
5Division of Diabetes and Nutritional Sciences,School of Medicine,King's College London,London,SE1 9NH,United
Kingdom
Combined treatment of 3-hydroxypyridine-4-one derivatives and green tea extract to induce hepcidin expression in iron-overloadedβ-thalassemic mice
Supranee Upanan1,Kanjana Pangjit2,Chairat Uthaipibull3,Suthat Fucharoen4,Andrew T.McKie5,Somdet Srichairatanakool1*
1Department of Biochemistry,Faculty of Medicine,Chiang Mai University,Chiang Mai 50200,Thailand
2College of Medicine and Public Health,Ubon Ratchathani University,Ubon Ratchathani 34190,Thailand
3National Center for Genetic Engineering and Biotechnology,National Science and Technology Development Agency,Thailand Science Park,Pathum Thani 12120,Thailand
4Thalassemia Research Center,Institute of Molecular Biosciences,Mahidol University,Salaya Campus,Nakornpathom 73170,Thailand
5Division of Diabetes and Nutritional Sciences,School of Medicine,King's College London,London,SE1 9NH,United
Kingdom
ARTICLE INFO
Article history:
in revised form 17 Aug 2015
Accepted 6 Sep 2015
Available online 17 Oct 2015
Thalassemia
Iron overload
Hepcidin
Iron chelator
Green tea
Hydroxypyridinone
Objective:To evaluate the efficacy of deferiprone(DFP),1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one(CM1)or green tea extract(GTE)in enhancing expression of hepatic hepcidin1(Hamp1)mRNA and relieving iron overload inβ-globin knockout thalassemic mice.
Methods:Theβ-globin knockout thalassemic mice were fed with a ferrocenesupplemented diet for 2 months and oral administration of deionized water,DFP(50 mg/kg),CM1(50 mg/kg),GTE(50 mg epigallocatechin 3-gallate equivalent/kg),GTE along with DFP(50 mg/kg),and GTE along with CM1(50 mg/kg)every day for 3 months.Levels of hepatic Hamp1 mRNA,plasma non-transferrin bound iron,plasma alanine aminotransferase activity and tissue iron content were determined.
Results:All chelation treatments could reduce plasma non-transferrin bound iron concentrations.Additionally,hepatic Hamp1 mRNA expression was significantly upregulated in the mice in a GTE+DFP combined treatment,correlating with a decrease in the plasma alanine aminotransferase activity and tissue iron deposition.
Conclusions:The GTE+DFP treatment could ameliorate iron overload and liver oxidative damage in non-transfusion dependentβ-thalassemic mice,by chelating toxic iron in plasma and tissues,and increasing hepcidin expression to inhibit duodenal iron absorption and iron release from hepatocytes and macrophages in the spleen.There is probably an advantage in giving GTE with DFP when treating patients with iron overload.
Original articlehttp://dx.doi.org/10.1016/j.apjtb.2015.09.007
1.Introduction
Secondary iron overload inβ-thalassemia patients is caused by multiple blood transfusions and an increase of duodenal ironabsorption[1].Apparently,toxic forms of iron known as nontransferrin bound iron(NTBI),labile plasma iron and labile iron pools(LIP)are detectable in these patients[2,3].Effective iron chelators are required to remove this iron to prevent oxidative damage in the vital organs,particularly the heart and liver.Nowadays,deferoxamine(DFO),deferiprone(DFP)and deferasirox(DFX)are the iron chelators which are usually used for the treatment ofβ-thalassemia patients with iron overload;however,they do produce adverse effects[4].1-(NAcetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one(CM1)has been synthesized and proposed as a new bidentate iron chelator whose chelating property and toxicity have so far been investigated in vitro and in animals[5-7].Green tea extract(GTE)has been reported as a strong antioxidant,as well as a naturalironchelatorbothinvitroandinvivo[8-10]. Furthermore,iron chelators have been reported to increase hepatichepcidinexpression[11,12].Importantly,increased hepcidinexpressionreducesironoverloadandimproves anemia effectively[13,14].
Hepcidin,a hepatic 25-amino acid peptide hormone,is synthesized and released into the blood circulation to regulate systemic iron metabolism [15,16].It inhibits iron flux from enterocytes,hepatocytes and macrophages to the blood stream by binding to the iron-exporter ferroportin causing its internalization and degradation[17,18].The regulations result in a retention of iron within the cells and a reduction of iron in the plasma[19-21].Recently,NTBIand hepcidin have been proposed to be novel reliable markers for iron metabolism,especially,iron overload condition[2,22,23].Urinary and serum hepcidinlevelsaredecreasedinβ-thalassemia,which exacerbates the condition leading to further iron overload[24]. Expression of hepcidin in patients with iron overload such as β-thalassemiaandmyelodysplaticsyndromesmaybe suppressed by the growth differentiation factor 15,the twisted gastrulation factor 1,the bone morphogenetic protein-binding endothelial cell precursor-derived regulator and/or the erythroferrone[2,25-28].Nonetheless,the mechanism of hepcidin regulation under these conditions is still unclear.In the present study,we investigated the expression and benefits of hepcidin in iron-loadedβ-globin knockout(BKO)thalassemic mice treated with single and combined iron chelators.Hopefully,iron chelators would lead to a negative iron balance in the body by enhancing hepcidin expression,resulting in lowering duodenal iron absorption and release of the iron from the liver and macrophages in the spleen.
2.Materials and methods
2.1.Chemicals and reagents
3-(2-Pyridyl)-5,6-diphenyl-1,2,4-triazine-p,p′-disulfonic acid monosodium salt hydrate(ferrozine),bis-(η5-cyclopentadienyl)-iron(ferrocene),ferrous ammonium sulfate,3-[N-morpholino]propanesulfonic acid,nitrolotriacetic acid trisodium salt(NTA),sodium acetate trihydrate,sodium dodecylsulphate,thioglycolic acid and trichloroacetic acid were purchased from Sigma-Aldrich Chemicals Co.Ltd.,St.Louis,MO,USA.TRIzol reagent was purchased from Invitrogen Company,UK.RNA isolation kit(Illustra RNAspin mini RNA isolation kit)was purchased from GE Healthcare Company,UK.High capacity cDNA reverse transcription kit was purchased from Applied Biosystems Company,UK.The EXPRESS SYBR®GreenER™qPCR Supermix universal kit was obtained from Invitrogen Company,UK.Alanine aminotransferase(ALT)assay kit was purchased from Biotech Co.Ltd.,Thailand.Acetonitrile[highperformanceliquidchromatography(HPLC)grade,density=0.782 g/cm3]was purchased from BDH,UK.
2.2.Iron chelators
DFP was kindly donated by the Government Pharmaceutical Organization of Thailand.CM1 was synthesized by Dr.Kanjana Pangjit,Ubon Ratchathani University,Thailand[5].1-Methyl-2-propyl-3-hydroxypyridine-4-one(CP22)was kindly donated by Professor Robert C.Hider,Institute of Pharmaceutical Science,King's College London,United Kingdom.
2.3.GTE
Fresh tea(Camellia sinensis)leaves were harvested from a local tea plantation in Chiang Mai,Thailand and immediately dried in a microwave cabinet[8].Hot water crude extract of green tea was prepared and epigallocatechin 3-gallate(EGCG)content was determined by using HPLC method[9,10].The GTE product containing 24% (w/w)EGCG was kept in the dark at-20°C until studied.
2.4.Animals
Male and female C57/BL6 mice of wild type(WT,muβ+/+),aged 2-3 months old and weighed 20-25 g,and heterozygous BKO(muβth-3/+)mice,were bred and supplied by the Thalassemia Research Center,Institute of Molecular Bioscience,Mahidol University,Salaya Campus,Thailand[29,30].The experimental protocol was conducted with the approval of the Animal Ethical Committee of Medical Faculty,Chiang Mai University,Thailand(Reference No.42/2556).WT and BKO mice(n=10;5 in each gender)fed with a CP 082 normal chow diet(N diet)(Perfect Companion Group Co.Ltd.,Samuthprakarn,Thailand)were observed as the normal diet control.For iron loading,six groups of BKO mice(n=10;5 in each gender)were fed with a 0.2% (w/w)ferrocenesupplemented diet(Fe diet)for 2 months(Day 0-60)[31].On the 60th day,tail vein blood samples were collected before the chelationtreatmentsforanalysisofplasmaNTBI concentrations and ALT activity.Afterwards,deionized water(DI),DFP(50 mg/kg),CM1(50 mg/kg),GTE(50 mg EGCG equivalent/kg),GTE(50 mg EGCG equivalent/kg)together with DFP(50 mg/kg),and the GTE(50 mg EGCG equivalent/ kg)together with CM1(50 mg/kg)were orally administered by using a gavage needle to the mice every day for 3 months(Day 61-150)[10].On the 150th day,all mice were sacrificed and their blood was collected through cardiac puncture into Na-heparin tubes for analysis of plasma NTBI concentrations and ALT activity.The liver,spleen and duodenum were collected,weighed and used for evaluation of the tissue iron by using histochemical Perl's Prussian blue staining technique and ferrozine colorimetric method.Organ weight index(OWI)was calculated with the following formula:
OWI(%)=organ weight(g)×100/body weight(g)
2.5.Quantification of hepatic hepcidin1(Hamp1)mRNA
RNA was extracted from 100 mg of mouse liver by using TRIzol reagent,and the genomic DNA was removed from the RNA samples by using the RNA isolation kit according to the manufacturer's protocol.A total of 1μg of RNA was reversely transcribed into cDNA by using a high capacity cDNA reverse transcription kit.Levels of Hamp1 mRNA in the liver were quantified by using the quantitative real-time PCR(qPCR)with ΔΔCT method[32].The housekeeping RNAβ-actin(Actb)was used as an endogenous control to normalize the cDNA samples for relative quantitation.The qPCR reaction of cDNA was performed by using the EXPRESS SYBR®GreenER™qPCRSupermixuniversalkitontheABI7500real-timePCRinstrument(Applied Biosystems,UK).The primer sequences used in qPCR were presented as follows:mHamp1 forward:CCTGAGCAGCACCACCTATC,mHamp1 reverse:TGCAACAGATACCACACTGGG,mActb forward:GGTCCACACCCGCCAC,and mActbreverse:GTCCTTCTGACCCATTCCCA.Relative mRNA expression in WT and BKO mice was acquired by normalizing Hamp1 mRNA to Actb mRNA.
2.6.Plasma ALT activity assay
Plasma ALT activity of the mice on Day 60 and Day 150 was examined by using ALT assay kit[33].Difference in the ALT activity was calculated.
2.7.Quantification of plasma NTBI
Plasma NTBI concentrations of the mice on Day 60 and Day 150 were measured by using the HPLC method[34].Briefly,plasma was incubated with a weak chelator NTA solution(at a final concentration of 80 mmol/L,pH 7.0)for 30 min at roomtemperaturetoproduceFe3+-(NTA)2complex. Subsequently,the complex was filtered through a membrane(NanoSep®,10-kDa cut-off,polysulfone type;Pall Life Sciences,USA)and analyzed by using the non-metallic HPLC system.
The Fe3+-(NTA)2representing NTBI was fractionated on a glass analytical column(ChromSep-ODS1,100 mm×3 mm,5μm particle size)and eluted at a flow rate of 1 mL/min with a mobile phase solvent containing 3 mmol/L CP22 in 19% acetonitrile buffered with 5 mmol/L 3-[N-morpholino]propanesulfonic acid(pH 7.0)to generate a Fe3+-(CP22)3product.Eluents were monitored and detected at 450 nm with a flow cell detector(SpecMonitor2300;LDC Milton-Roy Inc.,USA).Data analysis was manipulated by BDS software(BarSpec Ltd.,Israel).NTBI concentrations were represented by the Fe3+-(CP22)3,while the peak height was determined from a standard curve which was constructed from 0 to 16μmol/L Fe3+-(NTA)2in 80 mmol/L NTA.The difference in the NTBI concentrations was calculated.
2.8.Histochemical examination of tissue iron
Liver,spleen and duodenum tissues were fixed in 10% neutralized formalin.Fixed tissue sections were dehydrated with a gradual series of ethanol,embedded in paraffin,sectioned,and stained with potassium ferrocyanide solution(known as Perl's supravital dye)by using the standard protocol.The stained slides were analyzed under a light microscope by an expert pathologist and photographed with a digital camera.
2.9.Determination of tissue iron content(TIC)
TIC was measured by the ferrozine colorimetric method[35]. The liver,spleen and duodenum were dried at 120°C overnight in a hot air oven.The dried organs were weighed and homogenized in 0.5%(w/v)sodium dodecylsulphate solution. The homogenate was added to the protein precipitating agent(1mol/LHCl/10%trichloroaceticacidsolution),mixed vigorously,and heated at 95°C for 1 h.After cooled down to room temperature,the protein-precipitated solution was centrifuged at 12000 r/min for 10 min.Iron in the supernatant was then allowed to react with the chromogenic solution containing 0.508 mmol/L ferrozine,1.5 mol/L sodium acetate and 1.5%(v/v)thioglycolic acid for 30 min to generate the colored product.The optical density of the product was measured photometrically at 562 nm.Iron concentrations were determined from a calibration curve of 0-200μmol/L ferrous ammonium sulfate.The TIC was presented as mg/g organ dry weight.
2.10.Statistical analysis
Data were analyzed by using the IBM SPSS Statistic 20 program and presented as mean±SD.Statistical significance was determined by using One-way ANOVA test,and the results with P<0.05 were considered significant.
3.Results
3.1.OWI
As shown in Table 1,the OWI value of the spleen from the BKO-N diet mice was significantly increased when compared with the WT-N diet mice,and those of the liver and spleen from the BKO-Fe diet mice were significantly elevated when compared with the BKO-N diet mice.However,the chelators did not significantly change any OWI values in the treated BKOFe diet mice when compared with the untreated mice.
3.2.Hepatic Hamp1 mRNA expression
Hepatic Hamp1 mRNA expression was not found to be significantly different between genders in both WT and BKO mice(Figure 1).Importantly,the hepatic Hamp1 mRNA levels in the BKO-N diet mice were significantly lower than those in the WT-N diet mice,while the hepatic Hamp1 mRNA expression was increased in the BKO-Fe diet mice when compared with the BKO-N diet mice(P<0.05)(Figure 2).The hepatic Hamp1 mRNA levels were increased significantly in the BKOFe diet/GTE+DFP group when compared with the BKO-Fe diet/DI group.Nevertheless,there was no significant change in Hamp1 mRNA levels in the BKO-Fe diet/DFP,BKO-Fe diet/ CM1,BKO-Fe diet/GTE and BKO-Fe diet/GTE+CM1 groups(Figure 2).

Table 1 OWI of WT and BKO mice(n=10,5 in each gender)fed with N diet or Fe diet along with chelation treatments for 90 days.%.
3.3.Plasma ALT activity
The levels of plasma ALT activity in the BKO-N diet mice were not significantly different from those in the WT-N diet mice(Table 2).However,the levels of plasma ALT activity were increased significantly in the BKO-Fe diet mice when compared with the BKO-N diet mice,implying iron loadedoxidative liver damage in the BKO-Fe diet mice.
All single chelation treatments tended to lower the increased plasma ALT activity levels with efficacy in the order of DFP>CM1,GTE when compared with the non-treatment group(BKO-Fe diet/DI).Most importantly,the GTE+DFP treatment(meandifference=1.16IU/L),butnotthe GTE+CM1 treatment(mean difference=20.85 IU/L),was significantly more effective than DFP treatment alone(mean difference=6.31 IU/L)as well as GTE treatment(mean difference=11.49 IU/L)and was the most effective in decreasing plasma ALT activity when compared with the nontreatment group.
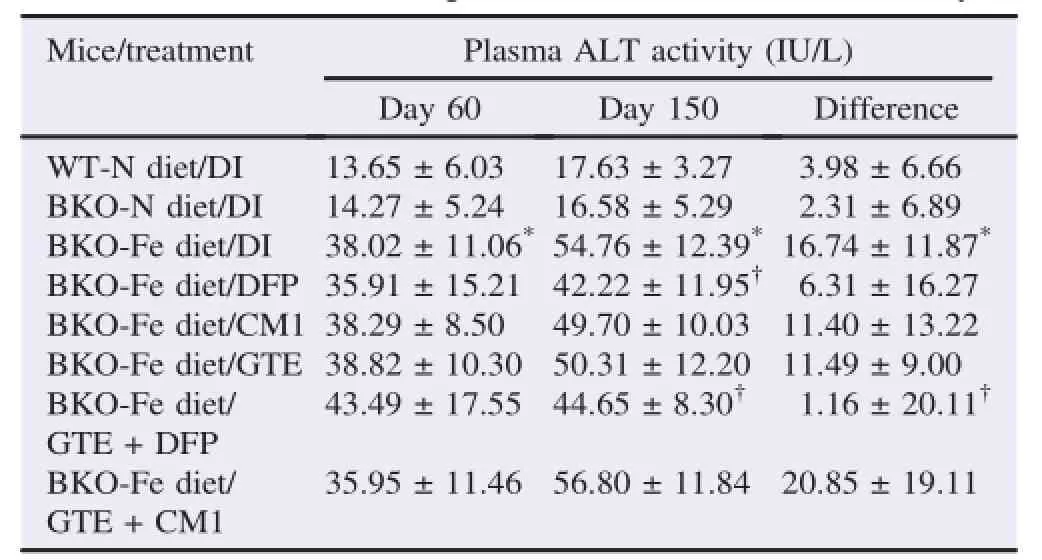
Table 2 Plasma ALT activity of WT and BKO mice(n=10,5 in each gender)fed with the N diet or Fe diet along with chelation treatments for 90 days.
Expectedly,plasma NTBI levels in the BKO-Fe diet mice were much higher than those in the BKO-N diet mice(P<0.05)(Table 3).All treatments reduced the plasma NTBI levels(P<0.05);however,the GTE+DFP treatment seemed to show the greatest effect.
Perl's Prussian blue staining results(Figure 3)revealed numerous hemosiderin in sinusoidal macrophages,hepatocytes and splenic macrophages in both red and white pulps of the BKO-Fe diet mice.The duodenum also showed iron accumulation with rare hemosiderin-laden macrophages in mucosa when compared with the BKO-N diet mice.The WT-N diet mice had no iron accumulation,and the BKO-N diet mice showed the iron accumulation in the spleen with scattered hemosiderinladen macrophages in both red and white pulps.Only the GTE+DFP combined treatment group showed less iron accumulation in the liver and spleen,as compared with the BKO-Fe diet group,but there was no iron accumulation in the duodenum. Consistently,results of TIC demonstrated that the BKO-Fe diet mice had significant iron accumulation in the liver,spleen and duodenum when compared with the BKO-N diet mice(Table 4). All chelation treatments reduced the iron deposition slightly in the liver and spleen,but not in the duodenum.
Obviously,the GTE+DFP combined treatment had the greatest effect in decreasing the iron deposition significantly in the liver and spleen.
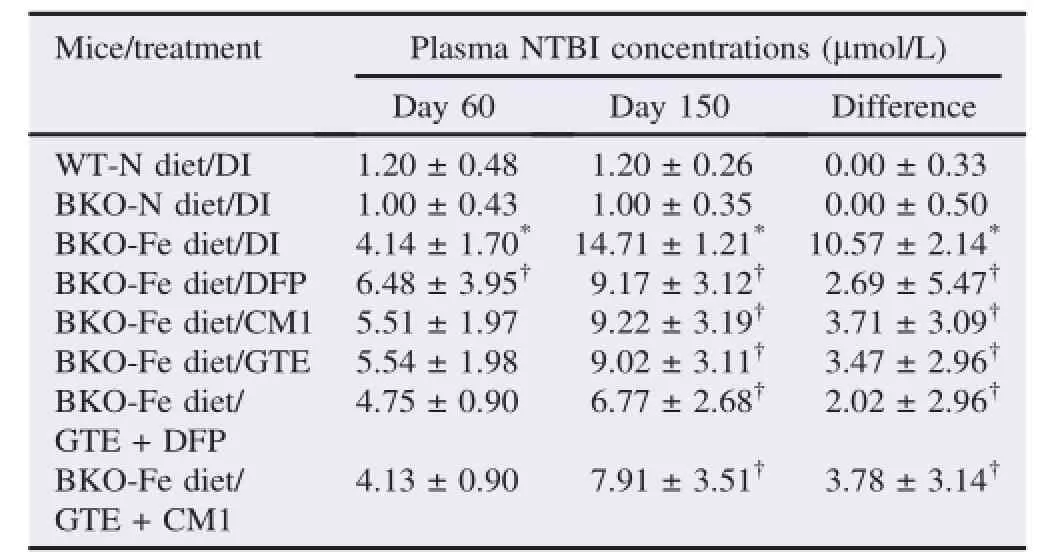
Table 3 Plasma NTBI concentrations of WT and BKO mice(n=10,5 in each gender)fed with the N diet or Fe diet along with chelation treatments for 90 days.
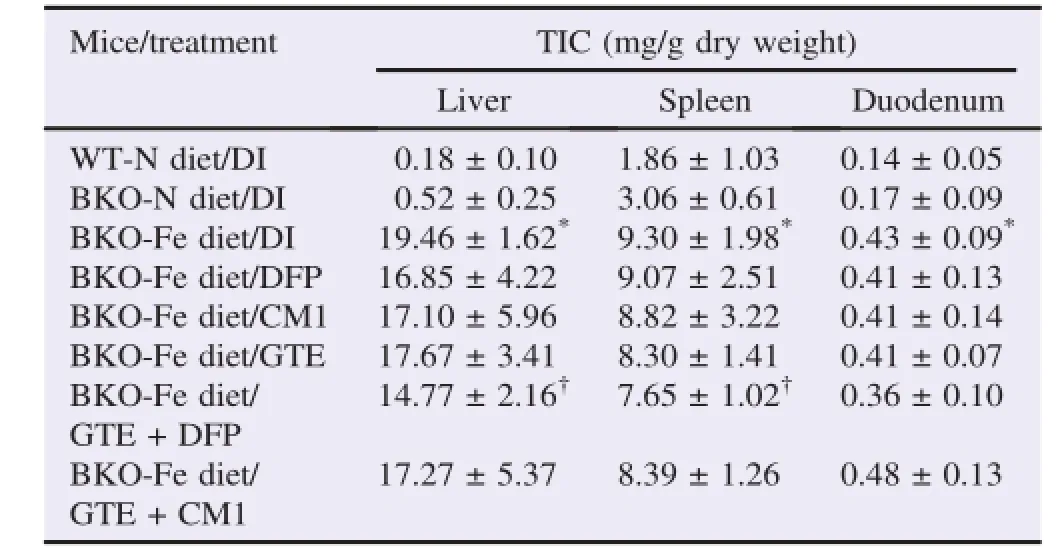
Table 4 TIC of WT and BKO mice(n=10,5 in each gender)fed with the N diet or Fe diet along with chelation treatments for 90 days.
4.Discussion
We used the BKO thalassemic mice loaded with ferrocene to imitate iron overload inβ-thalassemia intermediate patients.The BKO mice displayed ineffective erythropoiesis,mild anemia and splenic enlargement[29,36].Plasma ALT activity,plasma NTBI concentrations and liver iron accumulation in the BKO-N diet mice were not much different from those in the WT-N diet mice,whereas levels of these parameters were considerably increased in the BKO-Fe diet mice.Some other groups have demonstrated that mRNA expressions of iron-regulatory proteins such as hepcidin,ferroportin,and transferrin receptors vary between the genders of the mice[37,38].However,we did not find any difference in hepatic Hamp1 mRNA expression between male and female of WT and BKO mice in this study.That is in accordance with the hepcidin expression in Huh7 cells grown in the condition containing male and femaleβ-thalassemicpatient sera(Upanan S,unpublished data).Therefore,the gender of the mice does not appear to affect basal hepcidin expression in their livers.
We found that BKO mice had significantly lower Hamp1 mRNA levels than WT mice,which was consistent with previous reports inβ-thalassemic mice and humans[39,40]. Interestingly,the up-regulation of Hamp1 mRNA expression in the BKO-Fe diet mice found in this study would reflect the increase of their tissue iron stores or serum iron concentrations in order to suppress duodenal iron absorption and iron release from the liver and macrophages in the spleen.Gardenghi et al. also reported that levels of hepcidin were increased while iron concentrations in the organs were increased and hepcidin still partially responded to iron overload inβ-thalassemic mice[41]. The levels of iron-loading in this study may overcome the effects of hepcidin suppressors,resulting in hepcidin upregulation.Controversially,iron levels might not increase hepcidin expression inβ-thalassemia with ineffective erythropoiesis according to high levels of hepcidin suppressors(e.g.,growth differentiation factor 15,twisted gastrulation factor 1,bone morphogenetic protein-binding endothelial cell precursor-derived regulator or erythroferrone),which do have a greater influence on decreasing hepcidin levels[27,42,43]. However,the increased hepcidin levels in the GTE+DFP treatment could not result from the iron-loading effect with regard to the reduction of the plasma NTBI levels and iron accumulation in the tissues when compared with the nontreatment group.
Accordingly,GTE+DFP combined treatment was more effective than the DFP treatment alone in diminishing iron overloaded liver damage and consequently decreasing plasma levels of ALT activity in the BKO-Fe diet mice.We expect that green tea catechins may potentiate iron depletion in cooperation with DFP chelator by shuttling the iron from the Fe3+-(DFP)3complex.When DFP and DFO co-exist in plasma,DFP would firstly shuttle intracellular iron and plasma NTBI and transfer them to DFO to form a ferrioxamine,implying that DFP commits to removing labile iron in the liver cells[44,45].Similarly,the GTE catechins(particularly EGCG)could enhance DFP chelation by shuttling the iron from the iron-DFP complex to formtheiron-EGCGcomplex[9,10].Significantly,the GTE+DFP combined treatment lowers levels of plasma ALT activity and liver iron accumulation efficiently,suggesting that green tea polyphenols would improve iron-induced dysfunction and injury of the livers in BKO-Fe diet mice.A current study has supported that the combined chelation treatment synergized mobilization of labile iron pools in Huh7 cells,in which the DFX+DFP treatment was the most effective[45]. Though effect of green tea consumption on iron absorption is not conclusive definitely,we believe that it will not affect our study[46,47].
Consistently,green tea significantly increased glutathione peroxidase,catalase,quinine reductase and glutathione S-transferase activities in the liver and improved the liver function by its anti-oxidative effect against hepatotoxicity[48-50].Taken together,this may explain the increased hepatic hepcidin expression following the improvement of the liver functions. In addition,DFX chelation treatment could decrease plasma ALT levels in the patients with iron overload-associated liver dysfunction,and elevate serum hepcidin level in iron-overloaded patients with myelodysplastic syndrome[51].Unexpectedly,GTE+CM1 combined treatment neither induced hepcidin expression nor reduced the plasma ALT activity.Since CM1 moleculeismorelipophilic,butbiggerthanDFP,the GTE+CM1 combined treatment may be less efficient than the GTE+DFP combined treatment in removing the iron via the proposed iron-shuttling mechanism.In all likelihood as mentioned above,we imply that the GTE+DFP combined treatment is more effective than the GTE+CM1 combined treatment and the single chelation treatments in the reduction of plasma NTBI concentrations and iron accumulation in the liver and spleen.Consequently,the GTE+DFP treatment could relieve oxidative damage in the liver and enhance hepcidin expression and secretion,and the latter will limit iron absorption in the duodenum and iron release from hepatocytes and macrophages in the spleen.
In conclusion,GTE+DFP combined treatment is the most effective in increasing Hamp1 mRNA expression and reveals beneficial health effects by lowering plasma NTBI levels,tissue iron deposit and liver oxidative damage inβ-thalassemic mice with iron overload.Efficacy of GTE+DFP combined treatment on hepcidin expression/secretion and changes of iron parameters should be further investigated clinically inβ-thalassemia patients with iron overload.
Conflict of interest statement
We declare that we have no conflict of interest.
Acknowledgments
We thank Thalassemia Research Center,Mahidol University for supplying thalassemic mice;Medical Science Research Equipment Center,Faculty of Medicine,Chiang Mai University for supplying research instruments;and Professor Robert C. Hider,PhD.,Institute of Pharmaceutical Science,King's College London,United Kingdom for his comments and English proofreading.
[1]Nienhuis AW,Nathan DG.Pathophysiology and clinical manifestations of theβ-thalassemias.Cold Spring Harb Perspect Med 2012;2(12):a011726.
[2]Porter JB,Walter PB,Neumayr LD,Evans P,Bansal S,Garbowski M,et al.Mechanisms of plasma non-transferrin bound iron generation:insights from comparing transfused diamond blackfan anaemia with sickle cell and thalassaemia patients.Br J Haematol 2014;167(5):692-6.
[3]Breuer W,Ghoti H,Shattat A,Goldfarb A,Koren A,Levin C,et al. Non-transferrin bound iron in thalassemia:differential detection of redox active forms in children and older patients.Am J Hematol 2011;87(1):55-61.
[4]Neufeld EJ.Update on iron chelators in thalassemia.Hematol Am Soc Hematol Educ Program 2010;2010:451-5.
[5]Pangjit K,Banjerdpongchai R,Phisalaphong C,Fucharoen S,Xie YY,Lu ZD,et al.Characterisation of a novel oral iron chelator:1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one.J Pharm Pharmacol 2015;67(5):703-13.
[6]Kulprachakarn K,Chansiw N,Pangjit K,Phisalaphong C,Fucharoen S,Hider RC,et al.Iron-chelating and anti-lipid peroxidationpropertiesof1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one(CM1)in long-term iron loadingβ-thalassemic mice.Asian Pac J Trop Biomed 2014;4(8):663-8.
[7]Chansiw N,Pangjit K,Phisalaphong C,Porter JB,Evans P,Fucharoen S,et al.Effect of a novel oral active iron chelator:1-(N-acetyl-6-aminohexyl)-3-hydroxy-2-methylpyridin-4-one(CM1)iniron-overloaded and non-overloaded mice.Asian Pac J Trop Med 2014;7(Suppl 1):S155-61.
[8]Srichairatanakool S,Ounjaijean S,Thephinlap C,Khansuwan U,Phisalpong C,Fucharoen S.Iron-chelating and free-radical scavenging activities of microwave-processed green tea in iron overload.Hemoglobin 2006;30(2):311-27.
[9]Thephinlap C,Ounjaijean S,Khansuwan U,Fucharoen S,Porter JB,Srichairatanakool S.Epigallocatechin-3-gallate and epicatechin-3-gallatefromgreenteadecreaseplasmanontransferrin bound iron and erythrocyte oxidative stress.Med Chem 2007;3(3):289-96.
[10]SaewongT,Ounjaijean S,MundeeY,PattanapanyasatK,Fucharoen S,Porter JB,et al.Effects of green tea on iron accumulation and oxidative stress in livers of iron-challenged thalassemic mice.Med Chem 2010;6(2):57-64.
[11]Cermanova J,Kadova Z,Dolezelova E,Zagorova M,Safka V,Hroch M,et al.Deferoxamine but not dexrazoxane alleviates liver injury induced by endotoxemia in rats.Shock 2014;42(4): 372-9.
[12]Zhao N,Nizzi CP,Anderson SA,Wang J,Ueno A,Tsukamoto H,et al.Low intracellular iron increases the stability of matriptase-2. J Biol Chem 2015;290(7):4432-46.
[13]Zhao N,Zhang AS,Enns CA.Iron regulation by hepcidin.J Clin Invest 2013;123(6):2337-43.
[14]Camaschella C.Iron and hepcidin:a story of recycling and balance. Hematol Am Soc Hematol Educ Program 2013;2013:1-8.
[15]Fung E,Nemeth E.Manipulation of the hepcidin pathway for therapeutic purposes.Haematologica 2013;98(11):1667-76.
[16]K¨uhn LC.Iron regulatory proteins and their role in controlling iron metabolism.Metallomics 2015;7(2):232-43.
[17]Rishi G,Wallace DF,Subramaniam VN.Hepcidin:regulation of the master iron regulator.Biosci Rep 2015;35(3):e00192.
[18]CasanovasG,BanerjiA,d'AlessioF,MuckenthalerMU,Legewie S.A multi-scale model of hepcidin promoter regulation reveals factors controlling systemic iron homeostasis.PLoS Comput Biol 2014;10(1):e1003421.
[19]Galy B,Ferring-Appel D,Becker C,Gretz N,Gr¨one HJ,Sch¨umann K,et al.Iron regulatory proteins control a mucosal block to intestinal iron absorption.Cell Rep 2013;3(3):844-57.
[20]De Domenico I,Lo E,Yang B,Korolnek T,Hamza I,Ward DM,et al.The role of ubiquitination in hepcidin-independent and hepcidin-dependent degradation of ferroportin.Cell Metab 2011;14(5):635-46.
[21]Rochette L,Gudjoncik A,Guenancia C,Zeller M,Cottin Y,Vergely C.The iron-regulatory hormone hepcidin:a possible therapeutic target?Pharmacol Ther 2015;146:35-52.
[22]Chauhan R,Sharma S,Chandra J.What regulates hepcidin in polytransfusedβ-thalassemia major:erythroid drive or store drive?Indian J Pathol Microbiol 2014;57(1):39-42.
[23]Ikuta K.Two novel potential markers for iron metabolism:hepcidin and non-transferrin-bound iron(NTBI).Rinsho Ketsueki 2015;56(2):194-203.
[24]Aboul-Enein A,El-Beshlawy A,Hamdy M,Shaheen I,El-Saadany Z,Samir A,et al.Peripheral expression of hepcidin gene in Egyptianβ-thalassemia major.Gene 2015;564(2):206-9.
[25]Gu S,Song X,Zhao Y,Guo J,Fei C,Xu F,et al.The evaluation of iron overload through hepcidin level and its related factors in myelodysplastic syndromes.Hematology 2013;18(5):286-94.
[26]PatelN,MasaratanaP,Diaz-CastroJ,Latunde-DadaGO,Qureshi A,Lockyer P,et al.BMPER protein is a negative regulator of hepcidin and is up-regulated in hypotransferrinemic mice.J Biol Chem 2012;287(6):4099-106.
[27]Kautz L,Jung G,Valore EV,Rivella S,Nemeth E,Ganz T. Identification of erythroferrone as an erythroid regulator of iron metabolism.Nat Genet 2014;46(7):678-84.
[28]Yun S,Vincelette ND.Update on iron metabolism and molecular perspective of common genetic and acquired disorder,hemochromatosis.Crit Rev Oncol Hematol 2015;95(1):12-25.
[29]Jamsai D,Zaibak F,Khongnium W,Vadolas J,Voullaire L,Fowler KJ,et al.A humanized mouse model for a commonβ0-thalassemia mutation.Genomics 2005;85(4):453-61.
[30]Jamsai D,Zaibak F,Vadolas J,Voullaire L,Fowler KJ,Gazeas S,et al.A humanized BAC transgenic/knockout mouse model for HbE/β-thalassemia.Genomics 2006;88(3):309-15.
[31]Valerio LG Jr,Petersen DR.Characterization of hepatic iron overload following dietary administration of dicyclopentadienyl iron(Ferrocene)to mice:cellular,biochemical,and molecular aspects.Exp Mol Pathol 2000;68(1):1-12.
[32]Livak KJ,Schmittgen TD.Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T))method.Methods 2001;25(4):402-8.
[33]Yang Y,Yang J,Jiang Q.The protective effect of huperzine A against hepatic ischemia reperfusion injury in mice.Transplant Proc 2014;46(5):1573-7.
[34]Singh S,Hider RC,Porter JB.A direct method for quantification of non-transferrin-bound iron.Anal Biochem 1990;186(2):320-3.
[35]Pourkhalili A,Mirlohi M,Rahimi E.Heme iron content in lamb meat is differentially altered upon boiling,grilling,or frying as assessed by four distinct analytical methods.ScientificWorldJournal 2013;http://dx.doi.org/10.1155/2013/374030.
[36]Nai A,Pagani A,Mandelli G,Lidonnici MR,Silvestri L,Ferrari G,et al.Deletion of TMPRSS6 attenuates the phenotype in a mouse model ofβ-thalassemia.Blood 2012;119(21):5021-9.
[37]Ramos E,Kautz L,Rodriguez R,Hansen M,Gabayan V,Ginzburg Y,et al.Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice.Hepatology 2011;53(4):1333-41.
[38]Brewer CJ,Wood RI,Wood JC.mRNA regulation of cardiac iron transporters and ferritin subunits in a mouse model of iron overload.Exp Hematol 2014;42(12):1059-67.
[39]Parrow NL,Gardenghi S,Ramos P,Casu C,Grady RW,Anderson ER,et al.Decreased hepcidin expression in murineβthalassemia is associated with suppression of Bmp/Smad signaling. Blood 2012;119(13):3187-9.
[40]Frazer DM,Wilkins SJ,Darshan D,Badrick AC,McLaren GD,Anderson GJ.Stimulated erythropoiesis with secondary iron loading leads to a decrease in hepcidin despite an increase in bone morphogenetic protein 6 expression.Br J Haematol 2012;157(5): 615-26.
[41]Gardenghi S,Marongiu MF,Ramos P,Guy E,Breda L,Chadburn A,et al.Ineffective erythropoiesis inβ-thalassemia is characterized by increased iron absorption mediated by downregulation of hepcidin and up-regulation of ferroportin.Blood 2007;109(11):5027-35.
[42]Pasricha SR,Frazer DM,Bowden DK,Anderson GJ.Transfusion suppresses erythropoiesis and increases hepcidin in adult patients withβ-thalassemia major:a longitudinal study.Blood 2013;122(1):124-33.
[43]Athiyarath R,George B,Mathews V,Srivastava A,Edison ES. Association of growth differentiation factor 15(GDF15)polymorphisms with serum GDF15 and ferritin levels inβ-thalassemia. Ann Hematol 2014;93(12):2093-5.
[44]Evans P,Kayyali R,Hider RC,Eccleston J,Porter JB.Mechanisms for the shuttling of plasma non-transferrin-bound iron(NTBI)onto deferoxamine by deferiprone.Transl Res 2010;156(2):55-67.
[45]Vlachodimitropoulou Koumoutsea E,Garbowski M,Porter J. Synergistic intracellular iron chelation combinations:mechanisms and conditions for optimizing iron mobilization.Br J Haematol 2015;170(6):874-83.
[46]Beverly AB,Zhu L,Fish TL,Thannhauser T,Rutzke MA,Miller DD.Green tea ingestion by rats does not affect iron absorption but does alter the composition of the saliva proteome. J Food Sci 2012;77(5):H96-104.
[47]Koutelidakis AE,Kizis D,Argyri K,Kyriakou A,Komaitis M,Kapsokefalou M.The effect of iron and fat in a diet containing green tea extract(Camellia sinensis)on the antioxidant capacity of some organs and the mRNA expression of specific genes in mice. J Med Food 2014;17(11):1232-8.
[48]Thangapandiyan S,Miltonprabu S.Epigallocatechin gallate effectively ameliorates fluoride-induced oxidative stress and DNA damage in the liver of rats.Can J Physiol Pharmacol 2013;91(7): 528-37.
[49]Liu Y,Flynn TJ,Ferguson MS,Hoagland EM,Yu LL.Effects of dietary phenolics and botanical extracts on hepatotoxicity-related endpoints in human and rat hepatoma cells and statistical models for prediction of hepatotoxicity.Food Chem Toxicol 2011;49(8): 1820-7.
[50]B´artikov´aH,Sk´alov´aL,Valentov´aK,Matouˇskov´aP,Szot´akov´a B,Martin J,et al.Effect of oral administration of green tea extract in various dosage schemes on oxidative stress status of mice in vivo.Acta Pharm 2015;65(1):65-73.
[51]Ghoti H,Fibach E,Westerman M,Gordana O,Ganz T,Rachmilewitz EA.Increased serum hepcidin levels during treatment with deferasirox in iron-overloaded patients with myelodysplastic syndrome.Br J Haematol 2011;153(1):118-20.
3 Aug 2015
Somdet Srichairatanakool,Department of Biochemistry,Faculty of Medicine,Chiang Mai University,Chiang Mai 50200,Thailand.
Tel:+66 53 945322
Fax:+66 53 894031
E-mail:ssrichai@med.cmu.ac.th
Peer review under responsibility of Hainan Medical University.
Foundation Project:Supported by Royal Golden Jubilee PhD Program of Thailand Research Fund(Grant No.PHD/0345/2552),Faculty of Medicine Research Fund,Chiang Mai University,Thailand,and Chair Professor Grant of National Science and Technology Development Agency through Professor Suthat Fucharoen,MD.
 Asian Pacific Journal of Tropical Biomedicine2015年12期
Asian Pacific Journal of Tropical Biomedicine2015年12期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- Cocktail of Theileria equi antigens for detecting infection in equines
- Antiplasmodialactivity oftraditionalpolyherbalremedyfrom Odisha,India: Their potential for prophylactic use
- Deletion of Salmonella enterica serovar typhimurium sipC gene
- Anticancer activity of Cyanothece sp.strain extracts from Egypt:First record
- Influence of CD133+expression on patients'survival and resistance of CD133+cells to anti-tumor reagents in gastric cancer
- Vascular endothelial growth factor before and after locoregional treatment and its relation to treatment response in hepatocelluar carcinoma patients
