Comparative anatomical studies of Artocarpus altilis (Parkinson) Fosberg and Artocarpus communis (J. R. & G. Forster) in Nigeria
Akinwumi J. Akinloye, Temitope I. Borokini, Kehinde A. Adeniji, Funmilola M. Akinnubi
1. Department of Botany, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria
2. Plant Genetic Resource Unit, National Centre for Genetic Resources and Biotechnology, Ibadan, Oyo State, Nigeria
3. Forestry Research Institute of Nigeria, P.M.B. 5054, Jericho, Ibadan, Oyo state, Nigeria
Comparative anatomical studies of Artocarpus altilis (Parkinson) Fosberg and Artocarpus communis (J. R. & G. Forster) in Nigeria
Akinwumi J. Akinloye1*, Temitope I. Borokini2, Kehinde A. Adeniji3, Funmilola M. Akinnubi1
1. Department of Botany, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria
2. Plant Genetic Resource Unit, National Centre for Genetic Resources and Biotechnology, Ibadan, Oyo State, Nigeria
3. Forestry Research Institute of Nigeria, P.M.B. 5054, Jericho, Ibadan, Oyo state, Nigeria
Comparative anatomy of two Artocarpus species was carried out to identify and describe anatomical characters in search of distinctive characters that could possibly be used to delimit the two taxa. Transverse, tangential and radial longitudinal sections and macerated samples of the stem and root wood were prepared onto microscopic slides. Epidermal peels and cleared leaves of the two species were made. Characteristic similarity disparity in the tissues arrangement as well as cell inclusions were noted for description and delimitation. The two Artocarpus species studied had essentially the same anatomical features; however, there were characters that seem to be taxon specific. The study revealed that at the transverse plane of the root, A. communis (J. R. & G. Forster) have predominantly solitary vessel, whereas pore multiple was predominant in A. altilis (Parkinson) Fosberg. Tyloses in vessels of the root were more frequent in A. communis than in A. altilis. In the cleared leaves, venation pattern also revealed some differences in the two species. The veins of A. communis were more or less straight and that of A. altilis were undulating especially in the secondary veins. Prismatic crystals were found in the cortex of the petiole in A. communis but not in A. altilis. Similarly, tannins were found in the root bark of A. communis but not in A. altilis. Trichomes and scales were more abundant in A. communis than in A. altilis. In the transverse section of the leaves, abaxial and adaxial epidermis were uniseriate in A. communis but only the abaxial epidermis was uniseriate in A. altilis, the adaxial epidermis was made up of 2 to 3 layers of cells. The epidermal cells in A. communis were predominantly short cylindrical shaped cells but were not so in A. altilis.
anatomy; macromorphology; characters; breadfruit; Artocarpus
1 Introduction
Breadfruit belongs to the genus Artocarpus (Moraceae), which consists of approximately 60 species native to the Indian subcontinent, Southeast Asia, and Australasia (Jarrett, 1959a,b; Kochummen, 2000). The genus name Artocarpus is derived from the Greek Word 'Artos', bread and 'karpus' which refer to the bread like quality of breadfruit when baked. The tree is found throughout the tropics and cultivated on most Pacific Islands. The trees are monoecious, with male and female flowers growing on the same tree, mostly pollinated by wind (Jarrett, 1959a,b; Brantjes, 1981). They grow to heights of 15 to 21 m or more and the trunksmay be as large as 2 m in diameter at the base. The trees begin fruit bearing in 3–5 years and are productive for many decades. Single-trunked trees grow with a spreading, evergreen canopy. Leaves are alternate, broadly obovate to broadly ovate, almost entire, with only slight lobing to deeply pinnately lobed, with sinuses up to 2/3 or more of the distance from margin to midrib, with up to six pairs of lobes and a large apical tip. Leaf blade is generally smooth, glossy, dark green with green or yellow-green veins, and few to many white to reddish-white trichomes on the midrib and veins. Leaves on new shoots and root suckers are generally larger and more hirsute than leaves on mature branches. Size is variable depending on the variety, ranging in 15–60 cm long. Fruits are variable in shape, size, and surface texture (Figure 1). They are usually round, oval, or oblong ranging from 9 to 20 cm wide and more than 30 cm long, weighing 0.25–6.00 kg.

Figure 1 Fruits of A. communis (a) and A. altilis (b)
Seeded breadfruit (Figure 2a) produces abundant nutritious fruits that are typically consumed as a starchy staple when firm and mature. Seeds are high in protein and low in fat and a good source of vitamins and minerals. All parts are used medicinally in the Pacific and Caribbean, especially the latex, leaf tips, and inner bark. The latex is massaged into the skin to treat broken bones and is bandaged on the spine to relieve sciatica. It is commonly used to treat skin ailments and fungus diseases such as "thrush", which is also treated with crushed leaves. Diluted latex is taken internally to treat diarrhea, stomach aches, and dysentery. The sap from crushed stems and leaves is used to treat ear infections or sore eyes. The root is astringent and used as a purgative; when macerated it is used as a poultice for skin ailments. The bark is also used to treat headaches in several islands. In the West Indies, the yellow leaf is brewed into tea and taken to reduce high blood pressure and relieve asthma. The tea is also thought to control diabetes.
The seeds are edible and are of high nutritional value (Keay et al., 1989). When the seeds are cooked, they are a fair source of thiamine and vitamin C (Amusa et al., 2002). The seeds could be cooked for the main dish, roasted for snacks or even converted to flour, which can be used for snacks or as soup thickener (Anazonwu-Bello, 1986).
In Nigeria, seedless breadfruit (Figure 2b) is regarded as the poor man's substitute for yam (Dioscorea esculenta (Lour.) Burkill and D. cayenensis Lam.) due to the fact that it is used in several traditional food preparations in replacement for yam and also costs <1/3 the price of yams at the market (Mayaki et al., 2003).

Figure 2 Seeded fruit of A. communis (a) and seedless fruit of A. atilis (b)
Among the Yoruba tribe of Nigeria, it is pounded into a paste, like pounded yam and eaten with various types of soups. Sometimes, it is boiled and eaten like yams. It is prepared into flour and reconstituted with hot water into paste and eaten with soup. The flour is reconstituted with water and fried into puff-puff. When the ripe fruit is used it is highly sugary. The fruit is also peeled and fried into chips.
Breadfruit exhibits great morphological variability, ranging from true seedless varieties to those with several small aborted seeds, or one to a few viable seeds, to varieties with numerous viable seeds.
Two varieties, seeded and seedless fruits, were observed for breadfruit. Since many cultivars of breadfruit are seedless, it has been inferred that fruit development is due to parthenocarpy (Barrau, 1976). Hasan and Razak (1992) showed that the fruits of breadfruit develop normally without pollination. Data available for seeded breadfruit basically indicate diploidy with 2n = 2x = 56; seedless cultivars are commonly triploid with 2n = 3x = 84 (Jarrett, 1959b; Barrau, 1976). Ragone (2001) attributed the loss of fertility in sterile diploid and triploid breadfruit to hybridization. Zerega et al. (2005) noted much confusion in the systematics of breadfruit with regards to the binomial, demonstrated by inconsistency regarding the correct name and specific epithet. Furthermore, the species delimitations within the breadfruit is complex that includes up to three species, A. altilis (domesticated breadfruit), A. mariannensis Tre'cul, and A. camanis Lanco, which pose a huge task in plant systematics. Morphological diversity is partitioned differently among these species according to various authors. Jarrett (1959b) published the most recent treatment for the breadfruit complex and took a conservative approach, recognizing one highly variable species, A. communis, which encompasses the diversity represented by both domesticated breadfruit and its closest relatives. However, Jarrett (1959b) acknowledged that the material examined was inadequate and mostly sterile, and suggested that further detailed studies were necessary.
This study was conducted to investigate the anatomical features in the seeded and seedless breadfruit with the view of assessing the species delimitation and providing useful taxonomic information about the taxa.
2 Materials and methods
2.1Collection and preparation of plant samples
Samples of mature root, wood, leaves and fruits of both A. communis (seeded) and A. altilis (seedless) were collected on the campus of Obafemi Awolowo University, Osun State, Nigeria (7°31'9.86''N; 4°31'34.23"E). The collected samples were preserved in fixative of 50% ethanol.
2.2Sectioning
Transverse (TS), tangential longitudinal (TLS) and radial longitudinal sections (RLS) of the wood and root as well as the TS of leaf and petiole of each plant were cut at 10 micron thickness. All the sections were done using a sledge microtome (Reichert, Austria).
2.3Leaf peeling
Mature leaves were cut into sizeable portions and kept in concentrated nitric acid until the surfaces becomes swollen under the hot sun. The pieces of leaves with swollen surfaces were transferred into water in a glass petri dish. The swollen surfaces were peeled off using fine tip forceps. The peels were preserved in 50% ethanol.
2.4Leaf clearing
Matured leaves were cut into sizeable portions and boiled in absolute ethanol for forty minutes, rinsed in water twice and soaked in 5% sodium hydroxide for 16 hours. After rinsed in water twice, they were transferred into 5% domestic bleach (JIK). They remained in the bleach until the whole leaves became completely white. They were then rinsed in water thrice and preserved in 50% ethanol.
2.5Staining of sections
The wood, root, leaf and petiole sections were stained for three minutes in Safranin O, rinsed in water twice and counter stained for three minutes in Alcian blue and again rinsed in water twice. The washed and counter stained sections were treated in a series of ethanol solution (50%, 70%, 80%, 90%, and 100%) to remove water molecules (dehydration process) and to remove excess stain (differentiation process). The dehydrated and differentiated sections were transferred into absolute xylene in two series to remove the last trace of water, to clear the sections (making it more transparent) and to remove the last trace of ethanol. Each section was then mounted on a glass slide in DPX(R)mountant.
2.6Maceration and staining of wood and root macerates
Stem and root wood from each species were sliced into small pieces using a pen knife and macerated using Schulze's fluid obtained by mixing equal volume of 10% chromic acid [dissolved 1 g potassium nitrate (KNO3) in 50 mL concentrated nitric acid (HNO3)] and10% nitric acid. The maceration was carried out in a beaker and kept in the oven at 90 °C for three hours. The macerated wood samples were washed in five changes of water and stained for three minutes in Safranin O then mounted in 25% glycerol on a glass slide and covered with a glass cover slip. The edges of the cover slip were sealed to the slide using nail polish.
2.7Staining of peels and clear leaves
The epidermal peels and cleared leaves of the Artocarpus species were stained for three minutes in Safranin O. They were rinsed thrice in water and mounted in 25% glycerol on a glass slides and covered with a glass cover slip, whose edges were sealed with nail polish.
2.8Microscopy
Microscopic observation of each slide was made and recorded. Photo micrographs of the slides were made using an Accu-scope trinocular microcope (ACCU-scope 3001 LED Trinocular microscope with 3.2 MP CMOS digital camera). Tissues and cells identification and description of wood samples of the Artocarpus species was done according to IAWA hardwood features list, definition and illustration (IAWA, 1989).
Tissues and cell identification and description of leaf and petiole was done according to Esau (1977), Fahn (1977), Cutter (1978), Bilgrami et al. (1983), and Metcalfe and chalk (1989).
3 Results
1) There are striking similarities in the macromorphology (Figure 3) and the sections of the root wood, stem bark, root bark, leaf, petiole and leaf macromorphological characters of A. altilis and A. communis. However, in spite of the similarities, several differences were also observed among the two species. The xylem vessels in the stem wood of both species are described as diffuse porous system, with presence of few tyloses; xylem parenchyma is made up of apotracheal parenchyma; rays are homogenous and procumbent and uniseriate ray scanty (Table 1, Figure 4). Furthermore, stem wood fibres were non-septate, non-storeyed and no pitting in both species with large lumen; starch granules were present in the wood and crystal druses were also observed in the parenchyma cells of the pith and phloem (Table 1, Figure 4). However, vessel length differs in both species, being 379±1 µm in A. altilis and 184±1 µm in A. communis (Table 1). In addition, the stem wood fibre maceration show differences in lumen size, wall thickness, fibre diameter and fibre length between samples of the two species (Table 1, Figures 5, 6). Also, root wood of both species show a diffuse porous xylem vessel system, simple pitting on the vessel element, apotracheal type of xylem parenchyma, non-storeyed, homogenous and procumbent rays and non-septate root wood fibre with large lumen (Table 2). Prismatic crystals were observed in the root of A. altilis while crystal druses were seen in A. communis (Table 3, Figures 7, 8).

Figure 3 Abaxial leaf surfaces of A. altilis (a) and A. communis (d), adaxial leaf surfaces of A. altilis (b) and A. communis (e), and leaf margins of A. altilis (c) and A. communis (f)
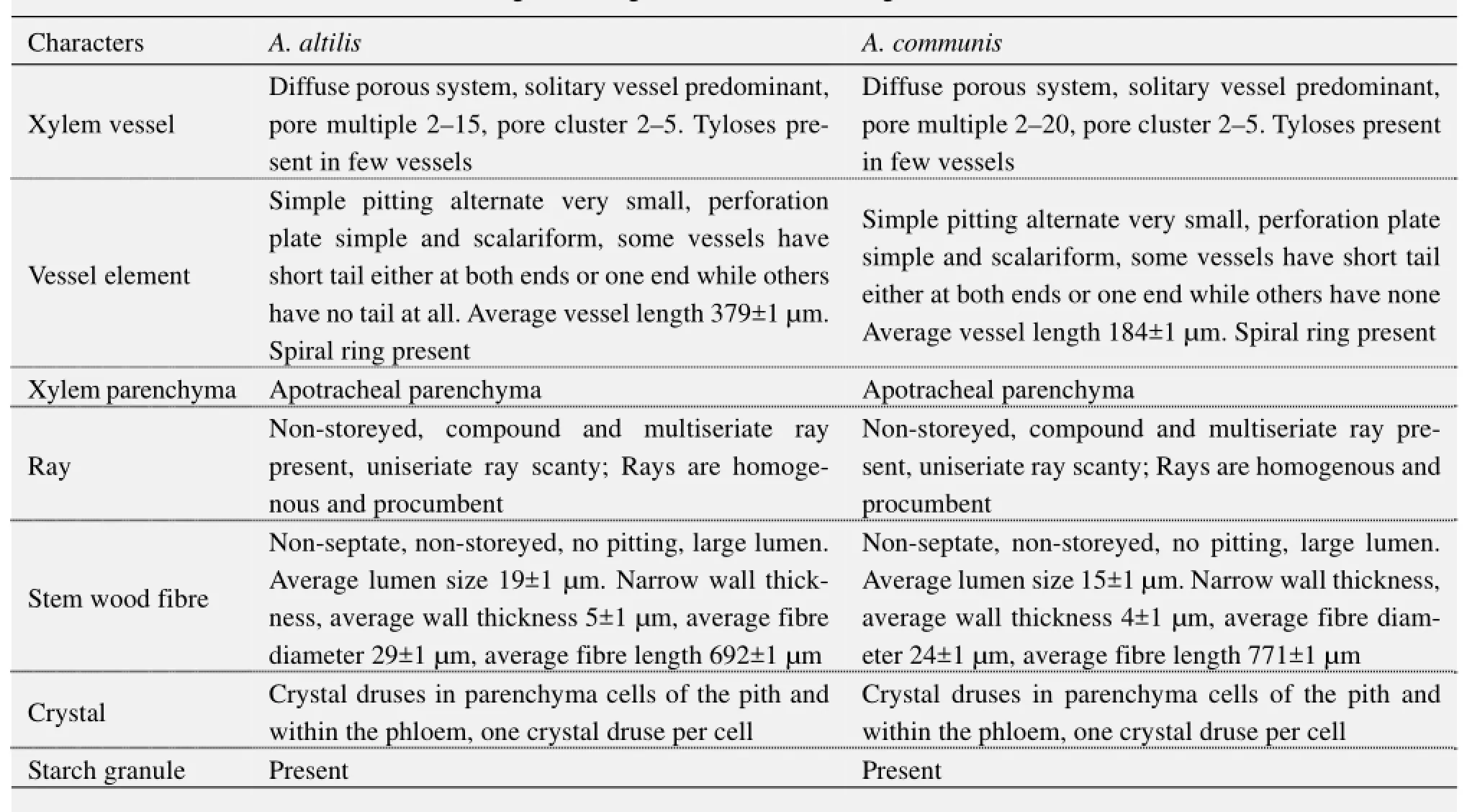
Table 1 Transverse, tangential longitudinal and radial longitudinal sections of stem wood
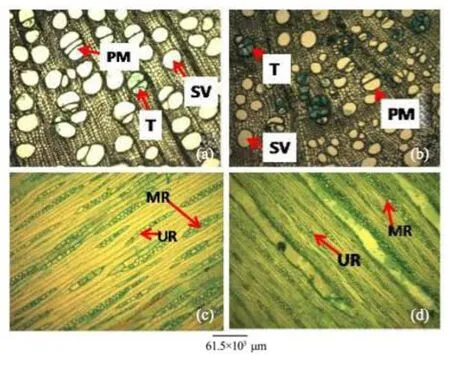
Figure 4 Stem transverse sections of A. atilis (a) and A. communis (b). Stem tangential longitudinal sections of A. altilis (c) and A. communis (d). PM: Pore multiple; T: Tylose; SV: Solitary vessel; MR: Multiseriate ray; UR: Uniseriate ray
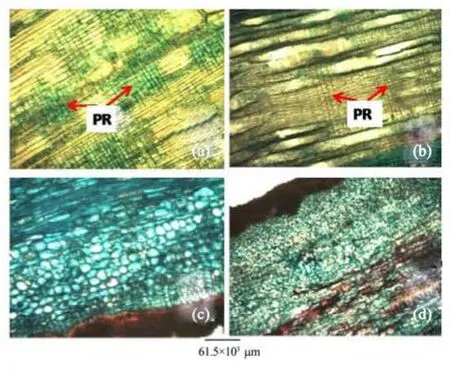
Figure 5 Stem radial longitudinal sections of A. altilis (a) and A. communis (b). Stem bark longitudinal sections of A. altilis (c) and A. communis (d). PR: Procumbent ray
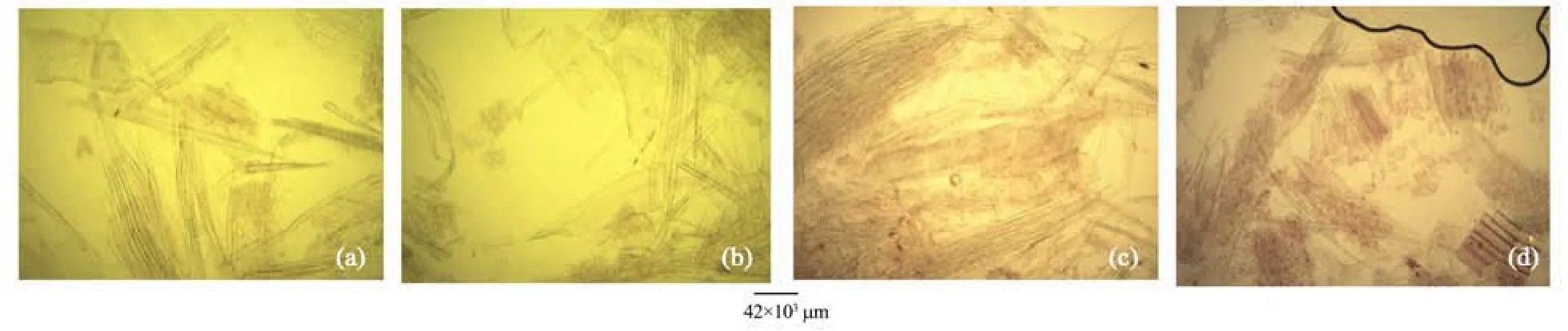
Figure 6 Stem macerate of A. altilis (a, b) and A. communis (c, d)
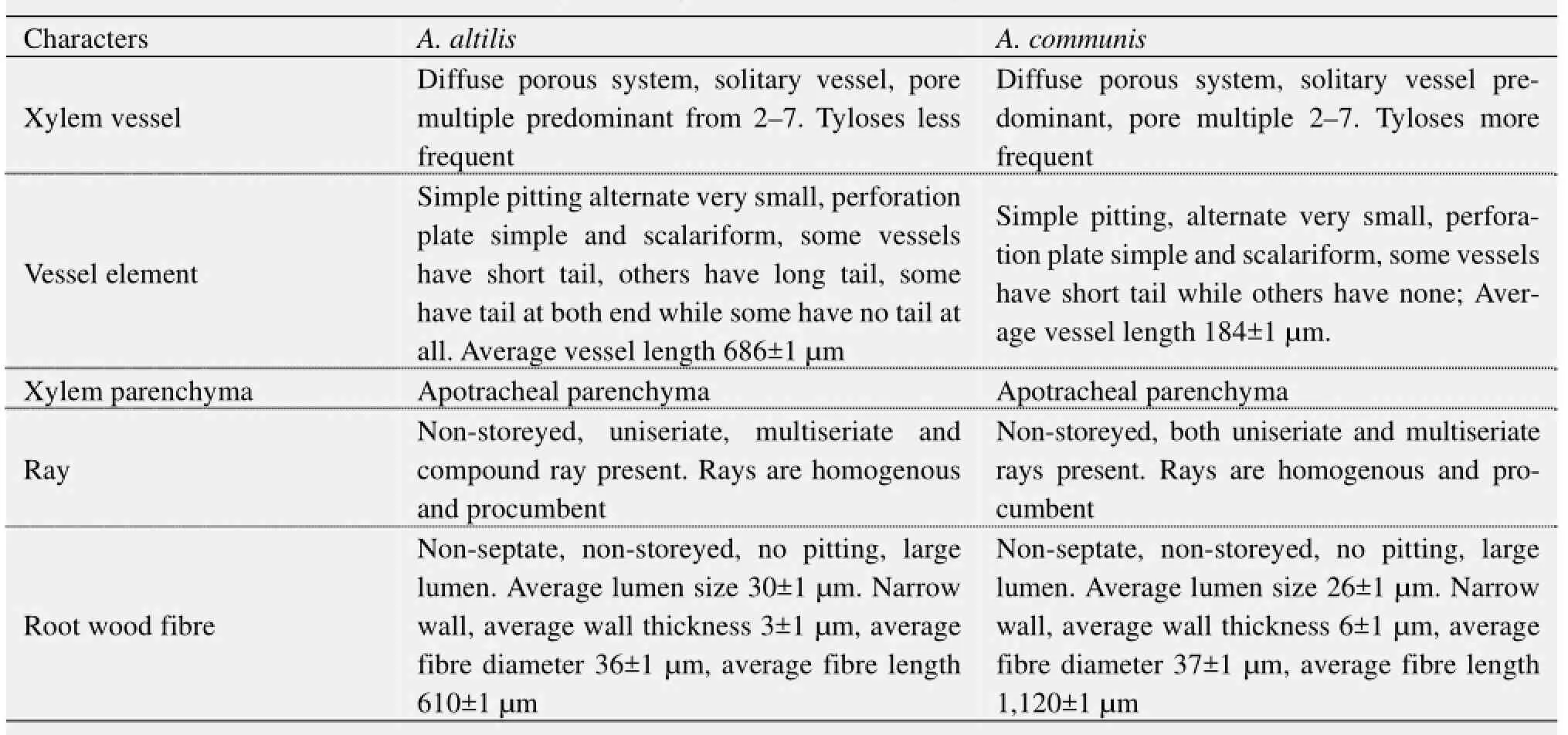
Table 2 Transverse, tangential longitudinal and radial longitudinal sections of root wood
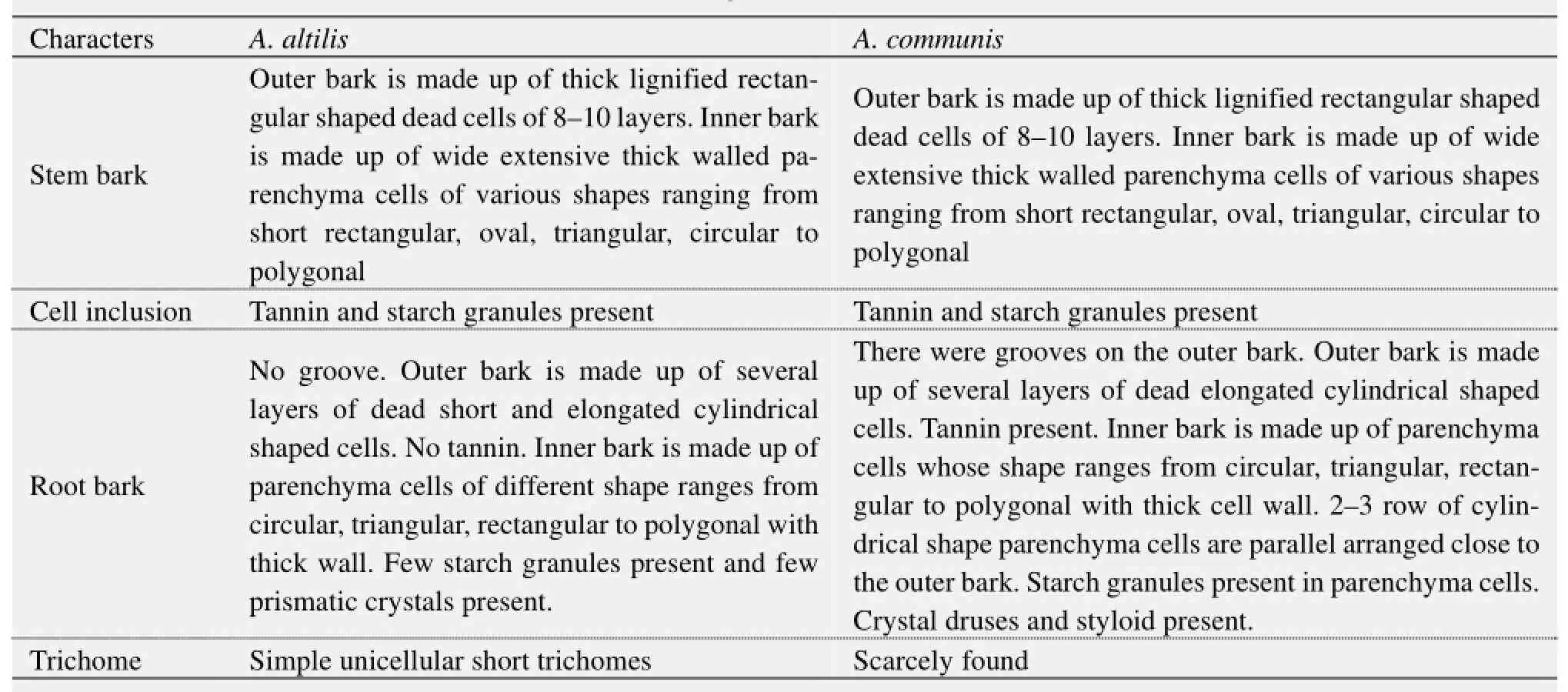
Table 3 Transverse and longitudinal sections of stem and root bark

Figure 7 Root transverse sections of A. altilis (a, b) and A. communis (c). CD: Crystal druses; PC: Prismatic crystal
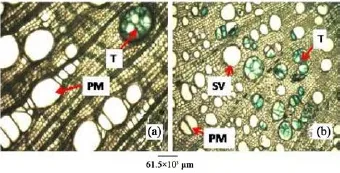
Figure 8 Root transverse sections of A. altilis (a) and A. communis (b). T: Tylose; PM: Pore multiple; SV: Solitary vessel
2) Tyloses are more frequent in A. communis than in A. altilis, while pore multiple is predominant in A. altilis in contrast to the predominance of solitary vessel in A. communis (Table 2). Just like in the stem wood fibre macerates, the root wood fibre macerate for the two species show significant differences in lumen size, wall thickness, fibre diameter and fibre length for the 2 species (Table 2, Figure 9). In cleared and peel leaves of both species the micromorphological features were the same except that the secondary veins were undulating in A. altilis but straight in A. communis (Table 4).
3) The stem bark of both species were observed to have many similarities, including the presence of tannins and starch granules, while the outer bark is made up of lignified rectangular shaped dead cells, and an inner layer of thick walled parenchyma cells (Table 3). Likewise, the outer rootbark section of both species is made up of dead elongated cylindrical shaped cells, while the inner root bark layer is also made up of different shaped parenchyma cells (Table 3, Figure 10). In contrast, more trichomes were found in A. altilis stem bark than in A. communis (Table 3), while groves were found in the bark of A. communis, which were absent in A. altilis (Table 3). Furthermore, the presence of tannins and styloids only in A. communis also distinguished the two species (Table 3, Figure 10). Leaf samples in both species have thin and non-striated cuticle (Figure 11). Abaxial epidermis was thin and single layered, while the adaxial epidermis was 2–3 layered (Figure 12).
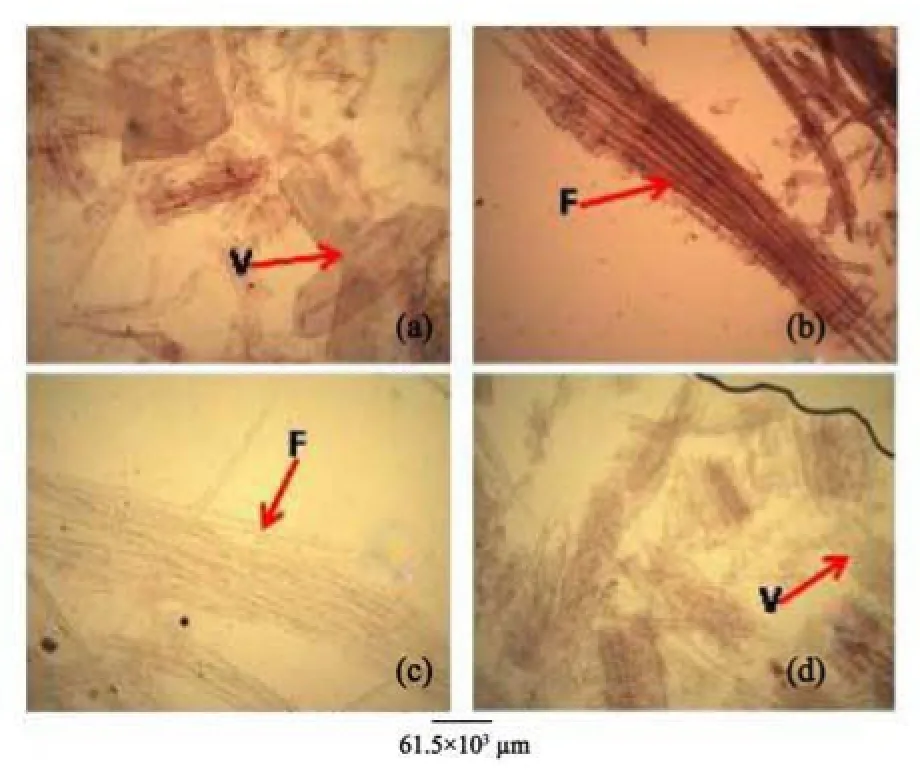
Figure 9 Root macerates of A. altilis (a, b) and A. communis (c, d). F: Fibre; V: Vessel element
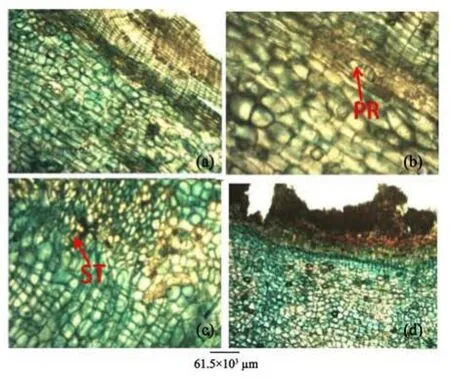
Figure 10 Root bark transverse sections of A. altilis (a, b) and A. communis (c, d). PR: Prismatic crystal; ST: Styloid crystal
Table 4 Cleared leaf and leaf epidermal peels
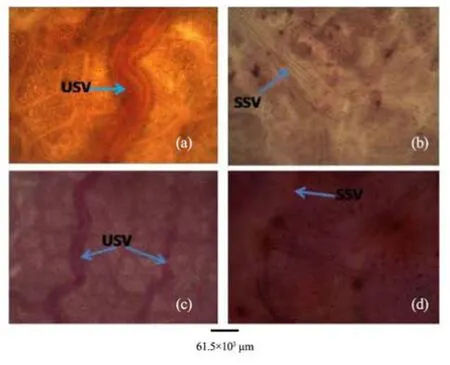
Figure 11 Cleared leaves of A. altilis (a, c) and A. communis (b, d). USV: undulating secondary vein; SSV: straight secondary vein
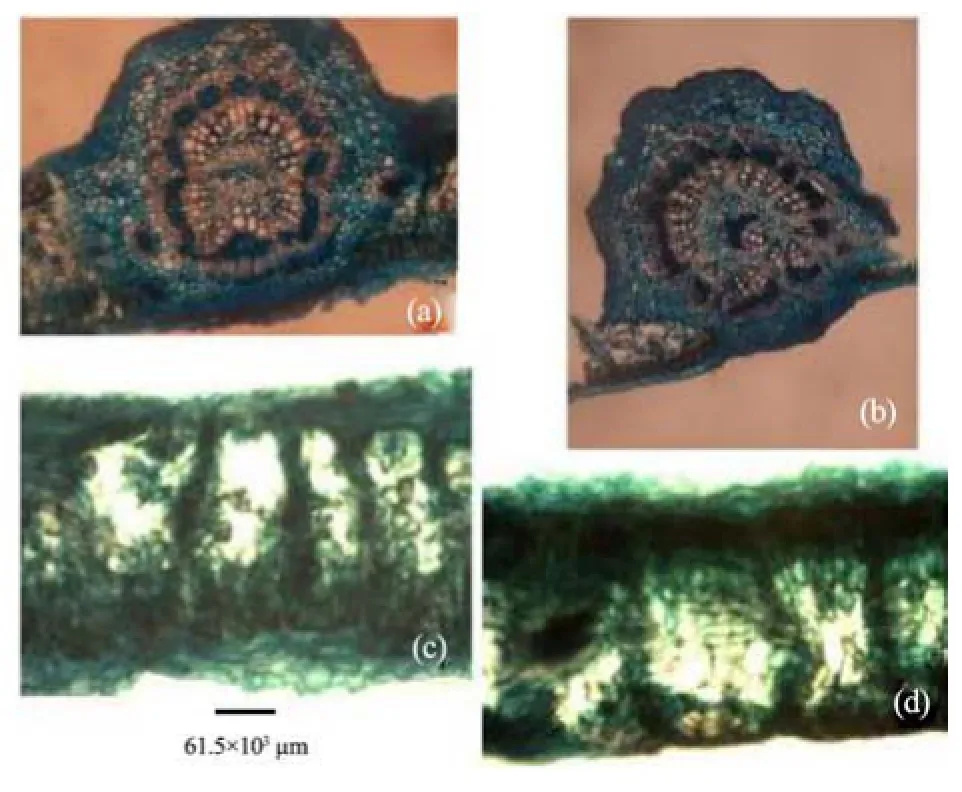
Figure 12 Leaf transverse sections of A. altilis (a, c) and A. communis (b, d)
4) Vascular bundles were closed conjoint collateral in both species, while the distribution of sclerenchyma in the vascular bundles was similar in both species (Table 5). The palisade and spongy mesophyll layers as well as crystal druses in the parenchyma cells were similar in both species. Furthermore, medullary bundles were between 1–4 in A. altilis, but up to 8 in A. communis. In addition, short cylindrical cells were predominant only in A. communis (Tables 5, 6; Figure 13). However, while both epidermal surfaces were uniseriate and thin in A. communis, similar to abaxial epidermis of A. altilis, the adaxial surface was made up of 2–3 layers of polygonal cells in A. altilis. In the transverse section of the root wood of A. altilis it was pore multiple that was predominant while solitary vessel predominate that of A. communis (Table 7). The root bark also revealed some differences. Tannin was not presented in A. altilis but was found in A. communis, few prismatic crystalis presented in A. altilis while crystal druses and styloid were found in A. communis. Few starch granules were seen in A. altilis and but numerous in A. communis (Table 8). In the cleared leaf, the secondary vein is undulating in A. altilis but straight in A. communis (Table 9).
5) Observations on the micromorphological characters of cuticle, epidermis, cortex and outer bundles of the vascular bundles show similarities between petioles of the two species (Table 10, Figure 14). However, in addition to the presence of prismatic crystals only in A. communis, the petiole samples from A. altilis are less hairy with short trichomes, but those of A. communis are more hairy with longer trichomes (Table 10).
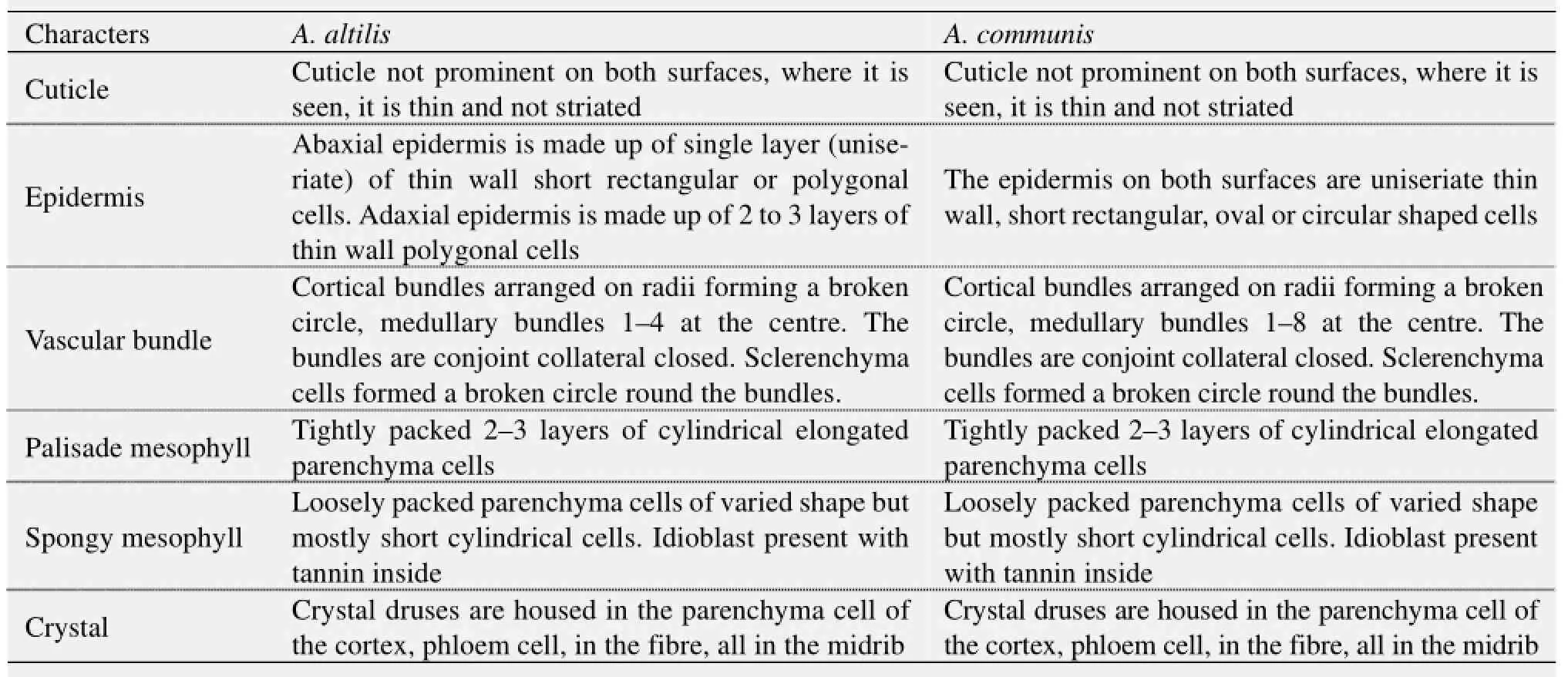
Table 5 Transverse section of leaf
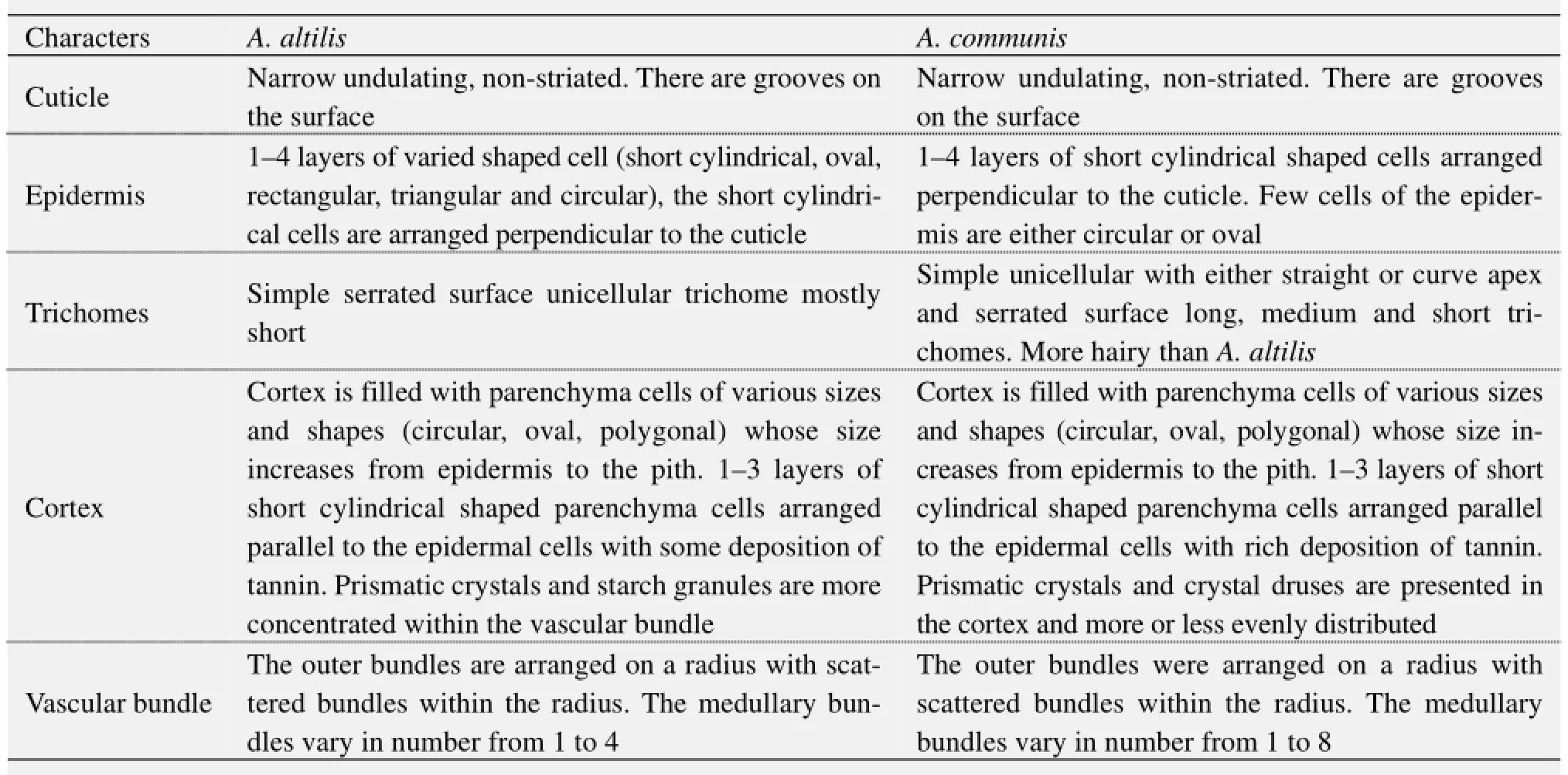
Table 6 Transverse section of petiole
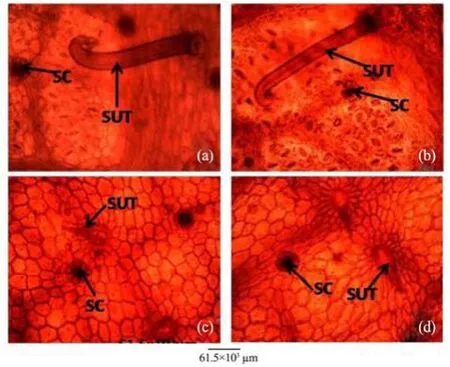
Figure 13 Leaf abaxial epidermis of A. altilis (a) and A. communis (b), and leaf adaxial epidermis of A. altilis (c) and A. communis (d). SC: Scale; SUT: Simple unicellular trichome
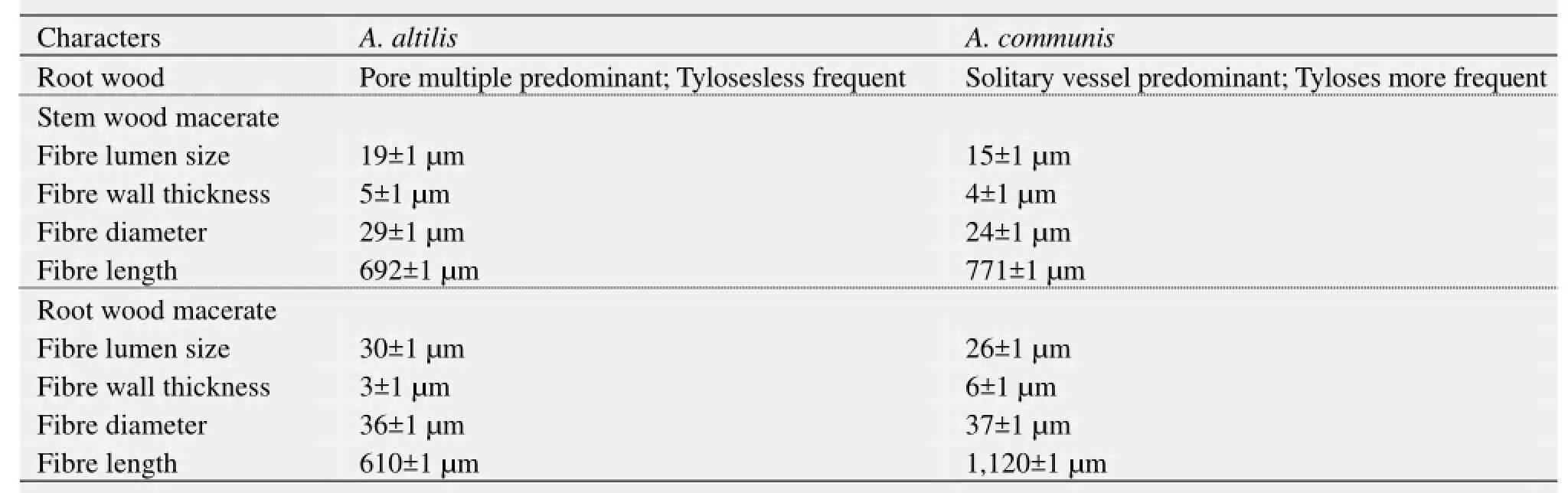
Table 7 Transverse section of root wood, stem wood macerate, and root wood macerate

Table 8 Transverse section of root bark
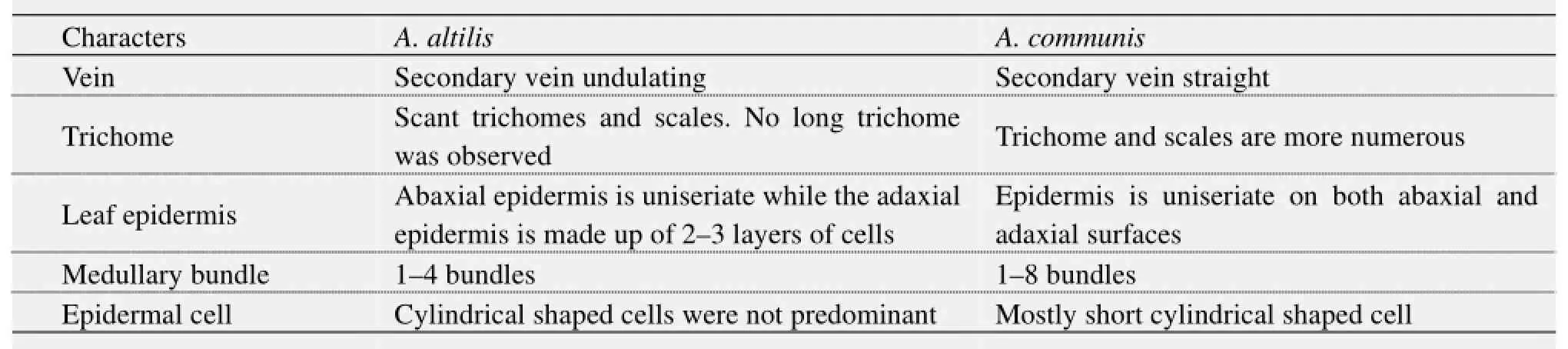
Table 9 Cleared and transverse section of leaf

Table 10 Transverse section of leaf petiole
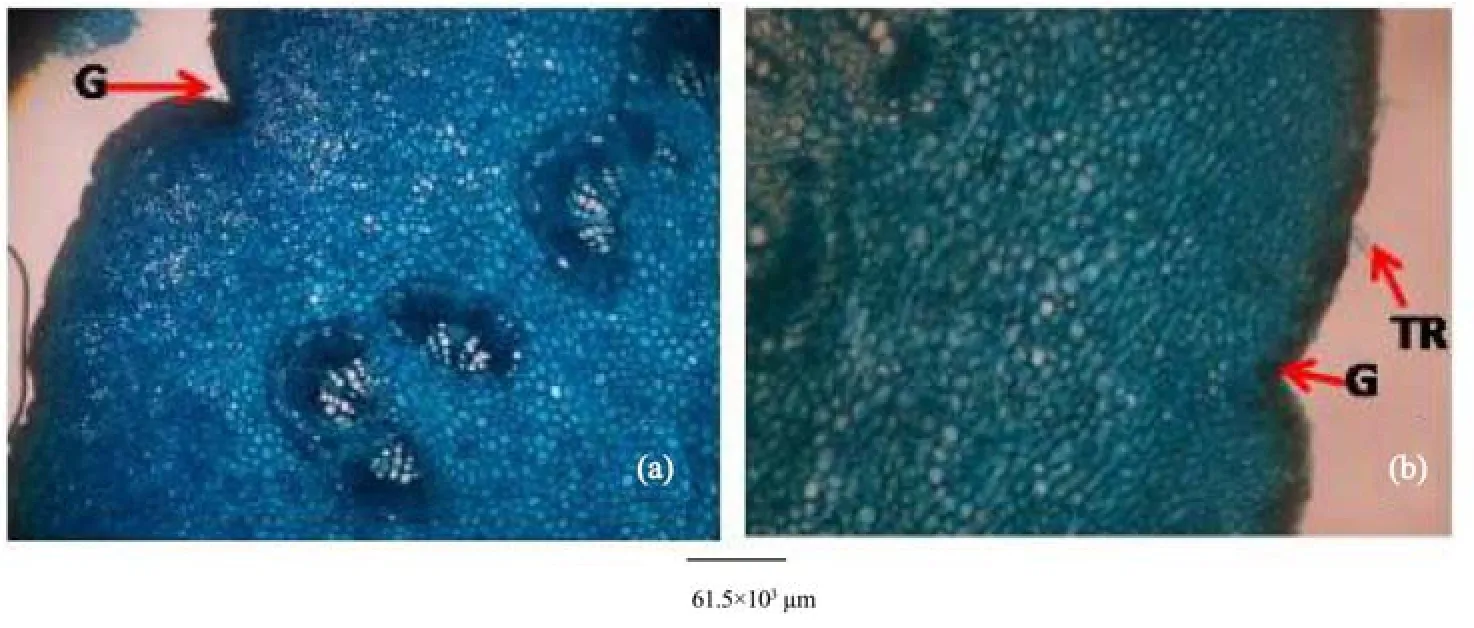
Figure 14 Petiole transverse sections of A. altilis (a) and A. communis (b). G: Groove; TR: Trichome
4 Discussion
Prominent similarities were observed in the sections of the root, stem, leaf petiole and leaf morphological character of the two species. In spite of these similarities, several differences were noted among the two species. The pores were diffuse porous system in the stem of the two species with presence of few tyloses, xylem parenchyma are apotracheal, rays are homogenous and procumbent, uniseriate ray scanty (Table 1, Figure 4). In both species, stem wood fibres are non septate, non-storeyed and no pitting with large lumen, starch granules are presented in the wood and crystal druses are also presented in the parenchyma cells of the pith and phloem (Table 1, Figure 4). However, vessel length differs in both species, being 379±1 µm in A. altilis and 184±1 µm in A. communis (Table 1). In addition, the stem wood fibre macerate revealed difference in lumen size, wall thickness, fibre diameter and fibre length between the two species (Table 1, Figure 6).
Similarly, root wood of both species revealed diffuse porous system. Simple pitting on vessel element. Xylem parenchyma is apotracheal, rays are non-storeyed, homogenous and procumbent, fibres are non-septate with large lumen (Table 2, Figure 5). However, tyloses are more frequent in A. commnis than in A. altilis, while pore multiple is predominant inA. altilis in contrast to the predominance of solitary vessels in A. communis (Table 2). In the two species, the root wood fibre macerate show significant differences in lumen size, wall thickness, fibre diameter and fibre length just as it was in the stem wood (Table 2, Figure 9). In both species, stem bark revealed some similarities, this includes presence of tannins and starch granules, outer bark is made up of lignified rectangular shaped dead cells and inner layer of thick walled parenchyma cells (Table 3). Likewise, the outer root bark sections of both species are made up of dead elongated cylindrical shaped cells, while the root bark layer are also occupied by different shaped parenchyma cells (Table 3, Figure 10). In contrast, more trichomes were found in A. altilis stem bark than A. communis (Table 3) while grooves are found on the bark of A. communis which are absent in A. altilis (Table 3), Furthermore, the presence of crystal druses and styloids only in A. communis also distinguished the two species (Table 3, Figure 10). Striking similarities were noted in the areole shapes, position of the scale, the presence and distribution of trichomes and serrated trichomes in the cleared leaves of both species (Table 3, Figure 11). Again, micromorphological studies on the leaf epidermal characters of both species indicated that the leaves were hypostomatic, with stomata present only on the abaxial surfaces, while the epidermal cells were polygonal-shaped on both leaf surfaces in the two species studied (Table 4, Figure 13). However, secondary veins were undulating in A. altilis but straight in A. communis. Furthermore, trichomes and scales were more numerous in A. communis than in A. altilis (Table 4). In the two species studied, the leaf cuticle is thin and non-striated, abaxial epidermis is a thin single layered, and whole adaxial epidermis is 2–3 layers. Vascular bundles are closed conjoint collateral in both species, while the distribution of sclerenchyma in the vascular bundles were similar in both species (Table 4). However, while both epidermal surfaces were uniseriate and thin in A. communis, similar to abaxial epidermis of A. altilis, the adaxial surface is made up of 2–3 layers of polygonal cells in A. altilis. Medullary bundles are between 1–4 in A. altilis but up to 8 in A. communis, while short cylindrical cells are predominant only A. communis (Table 5, Figures 12 and 13). The palisade and spongy mesophyll layers as well as crystal druses in the parenchyma cells are similar in both species (Table 5, Figures 12 and 13). The micromorphological characters of the cuticle, epidermis, cortex and the outer bundles of the vascular bundles revealed similarities between the petiole of the two species (Table 4, Figure 14). It was only in A. communis that prismatic crystals were sited. The petiole sample of A. altilis is less hairy with short trichomes, but those of A. communis are more hairy with longer trichomes (Tables 4 and 6). Macromorphological features of the fruits and seeds of these two Artocarpus species are taxon specific. The fruits of A. altilis have a smooth body surface and seedless while that of A. communis have spines and seeded. In fact, these are the only visible characters that distinguished these species in the field (Figures 1 and 2) for the leaves of the two species revealed more or less the same macromorphological features (Figure 3).
In addition, the stem wood of the two species revealed no visible anatomical differences (Figures 4 and 5). Also, the wood macerate of both stem and root of the two species revealed little or no anatomical differences except in the root wood fibre length (Figures 6 and 9).
The use of crystals as a diagnostic tool have been discussed by several authors (Amos, 1951; Terwelle, 1976; Illoh and Inyang, 1998). The presence of crystal druses is a common occurrence in the two species but then styloids was sited in A. communis root bark while prismatic crystals was sited in that of A. altilis (Figures 7, 10).
The anatomical study of A. altilis and A. communis revealed that both had more classificatory characters than delimiting specific character. However, there are characters that seem to be taxon specific. The study revealed that A. communis have solitary vessel predominant whereas it was pore multiple that was predominant in A. altilis at the transverse section of the root. Tyloses in vessels of the root were more frequent in A. communis than A. altilis (Figure 8).
In the midrib of the leaf at transverse plane, the medullary bundles in A. communis varied from 1–8 while 1–4 in A. altilis (Figure 12).
Furthermore in the cleared leaf, venation patterns also revealed some differences in the two species studied. While the veins of A. communis are more or less straight, that of A. altilis are undulating especially in the secondary veins (Figure 11).
In addition, prismatic crystals were found in the petiole cortex of A. communis but not seen in A. altilis. A similar observation by Akinloye et al. (2012) was used in distinguishing taxa in genus Cola. Also, starch granules were seen in the root bark of the two species but were more numerous in A. communis. A. communis has tannin in the root bark but this substance was not sited in the root bark of A. altilis.
In the cleared leaf, trichomes and scales were more abundant in A. communis than in A. altilis. This was in addition to the observation that petioles were more hairy on A. communis than on A. altilis (Figure 14). In fact, trichomes were graded as short (average length 124±1 µm), medium (average length 286±1 µm), and long (average length 770±1 µm) in A. communis. In A. altilis, only short (average length 77±1 µm) and medium (average length 156±1 µm) trichomes are presented. At transverse plane of the leaf, the adaxial andabaxial epidermis were uniseriate in A. communis but only the abaxial epidermis was uniseriate in A. altilis, and the adaxial epidermis was made up of 2–3 layers of cell.
Similarly, differences were observed in the epidermis of the two species, in terms of epidermal cell shape as well as uniseriate appearances of the epidermal surfaces. The epidermal cells in A. communis were predominantly short cylindrical shaped cell but in A. altilis cylindrical shaped cells were not predominant.
The non-prominence of the cuticle may probably be due to the fact that the plant always grows around stream/river or water logged area, therefore it does not need much of a water retention mechanism.
The taxonomic importance of morphological and anatomical characters of different plant parts for species delimitation have been reported by several authors (Metcalfe, 1968; Waly, 1999; Turki et al., 2006; Oladipo and Illoh, 2012). Waly (1999) proved that wood anatomical characters were found useful for the identification of Tamarix species. Furthermore, Oladipo and Illoh (2012) noted variations in the quantitative wood anatomical features among different Jatropha species. They concluded that wood anatomic features are taxon specific and species could be delimited by their quantitative wood characters. Metcalfe (1968) observed that anatomy of the vegetative organs could help in establishing the inter-relations of the taxa at infra- and supra-specific levels. Zaki et al. (1991) proved that the characters of the epidermal cells, the cortical zone and vascular cylinder of the young stem provided reliable characters for the distinction of closely allied species of section Tamarix. Denford (1980) reported that differences in epidermal characters of comparable organs seem always to reflect genetic differences in the plants.
Similarly, Akcin et al. (2011) reported wide variations in the anatomical structures of petioles of seven species in Lamiaceae. While leaf anatomy was reported to have no taxonomic value at generic and infrageneric levels, stem anatomy had biosystematic significance among species of the tribe Genisteae of Fabaceae in Argentina (Norverto et al., 1994). Tantawy and Naseri (2003) reported the taxonomic importance of achene surface pattern of species in Rosoideae tribe (Rosaceae) for species delimitation. Several variations and homogeneity in the micro and macro-morphological features of Hordeum species was reported by Amer et al. (2013). In the same vein, Shaheen (2007) reported diverse anatomical differences among species of Caesalpiniodeae, including vascular trace shape, pericyclic fibre forms, number of abaxial and adaxial vascular bundles, number and relative position of secondary vascular bundles, accessory vascular bundle status, the tendency of abaxial vascular bundles to divide, distribution of sclerenchyma, distribution of cluster crystals, and type of petiole trichomes, among other areas, which he concluded gave valuable data for taxonomic delimitation of the investigated species. In contrast, Sczepanik-Janyszek and Klimko (1999) reported that the observed morphological differences were not sufficient for taxonomic divisions among the studied taxa of Carex.
Likewise in this study, the variations noted in the anatomical features may not be sufficient to delimit these taxa, leaving only fruits and seed morphology as the most reliable distinguishing features between the two taxa. However, the authors suggest a comprehensive DNA analysis, as it may help provide further taxonomic data about the two species.
Abdel-Samai MS, 2007. Characteristics of the stem-leaf transitional zone in some species of Caesalpinioideae (Leguminosae). Turkish Journal of Botany, 31: 297–310.
Akcin OE, Ozyurt MS, Senel G, 2011. Petiole anatomy of some Lamiaceae Taxa. Pakistan Journal of Botany, 43: 1437–1443.
Akinloye AJ, Illoh HC, Olagoke OA, 2012. Significant of wood anatomical features to the taxonomy of five Cola Species. Sustainable Agriculture Research, 1(2): 21–26.
Amer NM, Hegazy AK, Azer SA, 2013. Taxonomic revision of genus Hordeum L. (Gramineae) in Egypt. International Journal of Biodiversity and Conservation, 5(4): 198–208.
Amos G, 1951. Some silicious timber of British Guyana. Carribians Forest, 12: 133–137.
Amusa NA, Kehinde IA, Ashaye OA, 2002. Biodeterioration of breadfruit (Artocarpuscommunis) in storage and its effect on the nutrient composition. African Journal of Biotechnology, 1: 57–60.
Anazonwu-Bello JN, 1986. Indigenous foods and nutritional adequacy. In: Proceedings on Development of Indigenous Technology. Ministry of Science Technology, pp. 29–30.
Barrau J, 1976. Breadfruit and relatives. In: Simmonds NW (ed.). Evolution of Crop Plants. London, UK: Longman, pp. 201–202.
Bilgrami KS, Srivastava LM, Shreemali JL, 1983. Fundamentals of Botany. Shahdara, Delhi (India): Vikas Publishing House PVT Ltd..
Brantjes NBM, 1981. Nectar and pollination of breadfruit: Artocarpusaltilis (Moraceae). Acta Botanica Neerlandia, 30: 345–352.
Cutter EG, 1978. Plant Anatomy Part 1: Cells and Tissues (2nded.). London: William Clowe and Sons Ltd..
Denford DKE, 1980. Flavenol glycosides and seed coat structure in certain species of Epilobiuma correlation. Experientia, 36: 299–300.
Esau K, 1977. Anatomy of Seed Plants (2nded.). New York: John Wiley and Sons.
Fahn A, 1977. Plant Anatomy (2nded.). New York: Pergamon Press.
Hasan SMZ, Razak AR, 1992. Parthenocarpy in seedless breadfruit (Arthocarpus incircus (Thunb.) L.). Acta Horticulturae, 321: 648–652.
IAWA, 1989. Hardwood features list: definitions and illustration. IAWA Bulletin, 10(3): 219–332.
Illoh HC, Inyang UE, 1998. Foliar Epidermis and petiole anatomy in some Nigeria Solanum Linn. Species in the Sub-Genus Leptostemanum (BITT) DUN Glinpses in Plant Research, 12: 73–86.
Jarrett FM, 1959a. Studies in Artocarpus and allied genera, I. General considerations. Journal of the Arnold Arboretum, 40: 1–29.
Jarrett FM, 1959b. Studies in Artocarpus and allied genera, III. A revision of Artocarpus subgenus Artocarpus. Journal of the Arnold Arboretum, 40: 113–155, 298–368.
Keay RWJ, Onochie CFA, Stanfield DFP, 1989. Trees of Nigeria. Oxford: Clarendia Press, pp. 204–205, 300.
Kochummen KM, 2000. Artocarpus J. R. & G. Forster, nominative conservation. In: Soepadmo E, Saw LG (eds.). Tree Flora of Sabah and Sarawak. Kuala Lumpur: Sabah Forestry Department, Forest Research Institute Malaysia, and Sarawak Forestry Department, pp. 187–212.
Mayaki OM, Akingbala JO, Baccus-Taylor GSH, et al., 2003. Evaluation of Breadfruit (Artocarpus communis) in traditional stiff porridge foods. Journal of Food, Agriculture and Environment, 1: 54–59.
Metcalfe CR, 1968. Current developments in systematic plantanatomy. In: Heywoods VH (ed.). Modern Methods in Plant Taxonomy. London, NewYork: Academic Press, pp. 45–57.
Metcalfe CR, Chalk L, 1989. Anatomy of the Dicotyledons (2nded.). Oxford: Charendon Press.
Norverto CA, Gonzalez-Andres F, Ortiz JM, 1994. Leaf and stem anatomy of species of Cytisophyllum, Cytisus, Chamaecytisus, Genista and Genista Sect. Teline (Fabaceae: Genisteae) as an aid for taxonomy. Israel Journal of Plant Sciences, 42: 213–225.
Oladipo OT, Illoh HC, 2012. Comparative wood anatomy of some members of the genus Jatropha (Euphorbiaceae) found in Nigeria. Phytologia Balcanica, 18(2): 141–147.
Onana J, 1995. Revue-d' Elevage-et de-medicine velerincuria-des-pays-tropicaus, 48: 213–219.
Ragone D, 2001. Chromosome numbers and pollen stainability of three species of Pacific Island Breadfruit (Artocarpus, Moraceae). American Journal of Botany, 88(4): 693–696.
Sczepanik-Janyszek M, Klimko M, 1999. Application of anatomical methods in the taxonomy of Sedges (Carex L.) from section Muehlenbergianae (L. H. Bailey) Kuk. Occuring in Poland. Rocc. Akad. Rol. W. Pozaniu-cccxvi Seria Botanika, (2): 97–107.
Shaheen ASM, 2007. Characteristics of the Stem –Leaf Transitional zone in some Species of Caesalpinioideae (Leguminosae). Turkish Journal of Botany, 31: 297–310.
Tantawy ME, Naseri MM, 2003. A contribution to the achene Knowledge of Rosoideae (Rosaceae): Light Microscope and Scanning Electron Microscope. International Journal of Agriculture and Biology, 5: 105–112.
Terwelle BJH, 1976. Silica grains in woody plants in neotropic especially Surinam Leiden Botanical Series. 3. Leiden University Press, pp. 107–142.
Turki Z, El-Shayeb F, Shehata F, 2006. Taxonomic studies in the Camphorosmeae (Chenopodiaceae) in Egypt. 1. Subtribe Kochiinae (Bassia, Kochia and Chenolea). Flora Mediterranea, 16: 275–294.
Wafaa MA, Ahmad KH, Safwat AA, 2013. Taxonomic revision of genus Hordeum L. (Gramineae) in Egypt. International Journal of Biodiversity and Conservation, 5(4): 198–208.
Waly NM, 1999. Wood anatomical characters of the Egyptian Tamarix L. species and its taxonomic significance. Taeckholmia, 19(2): 115–125.
Zaki MA, Hosni HA, Araffa S, 1991. Morphological and anatomical features of species of Tamarix in Egypt. Journal of Applied Science, 6(10): 502–511.
Zerega NJC, Ragone D, Motley TJ, 2005. Systematics and species limits of breadfruit (Artocarpus, Moraceae). Systematic Botany, 30(3): 603–615.
Akinloye AJ, Borokini TI, Adeniji KA, et al., 2015. Comparative anatomical studies of Artocarpus altilis (Parkinson) Fosberg and Artocarpus communis (J. R. & G. Forster) in Nigeria. Sciences in Cold and Arid Regions, 7(6): 0709-0721. DOI: 10.3724/SP.J.1226.2015.00709.
*Correspondence to: Akinwumi J. Akinloye, Department of Botany, Obafemi Awolowo University, Ile-Ife, Osun State, Nigeria. Tel: +234-708-6506868; E-mail: akinloye_johnson@yahoo.com
October 10, 2014 Accepted: May 15, 2015
 Sciences in Cold and Arid Regions2015年6期
Sciences in Cold and Arid Regions2015年6期
- Sciences in Cold and Arid Regions的其它文章
- Water accounting for conjunctive groundwater and surface water irrigation sources:A case study in the middle Heihe River Basin of arid northwestern China
- Analysis of permanent deformations of railway embankments under repeated vehicle loadings in permafrost regions
- Evaluation of eco-economic effects in relation to resettlement policy in Shulehe River Basin
- Land use and land cover change processes in China's eastern Loess Plateau
- Comparative anatomical studies of Artocarpus altilis(Parkinson)Fosberg and Artocarpus communis(J.R.&G.Forster)in Nigeria
- Vertical distribution of soil moisture and surface sandy soil wind erosion for different types of sand dune on the southeastern margin of the Mu Us Sandy Land,China
