Hydrothermal Synthesis and High Activity of Bi2S3/BiOCl Com posite Photocatalysts
LIHui-quan,ZHU Liang-jun,SUN Qian,WANG Gao,MIAO Hui,CUIYu-min*,JIA Qing-feng
(1.College of Chemistry and Materials Engineering,Fuyang Normal College,Fuyang 236037,China;
2.Anhui Provincial Key Laboratory for Degradation and Monitoring of Pollution of The Environment,Fuyang 236037,China)*Corresponding Author,E-mail:cuiyumin0908@163.com
Hydrothermal Synthesis and High Activity of Bi2S3/BiOCl Com posite Photocatalysts
LIHui-quan1,2,ZHU Liang-jun1,2,SUN Qian1,WANG Gao1,MIAO Hui1,2,CUIYu-min1,2*,JIA Qing-feng1
(1.College of Chemistry and Materials Engineering,Fuyang Normal College,Fuyang 236037,China;
2.Anhui Provincial Key Laboratory for Degradation and Monitoring of Pollution of The Environment,Fuyang 236037,China)*Corresponding Author,E-mail:cuiyumin0908@163.com
Bi2S3/BiOCl composite photocatalysts with variousmass fractions of Bi2S3were successfully synthesized by a facile hydrothermal process at433 K,characterized by various techniques,and evaluated by the photo-degradation ofmethyl orange(MO)in an aqueous solution under the irradiation of ultraviolet(UV)light.The results showed that the photocatalytic activity of Bi2S3/BiOCl catalysts was greatly enhanced,compared with that of pure Bi2S3and BiOCl.Especially,the photocatalytic activity of the 26.5%Bi2S3/BiOCl sample is very close to that of commercial Degussa P-25(TiO2),which has been generally recognized as an efficient photocatalyst under UV light irradiation.The remarkably enhanced photocatalytic activity could bemainly attributed to the effective transfer of the photo-generated electrons and holes at the heterojunction interface of Bi2S3and BiOCl,which reduced the recombination of electron-hole pairs.
hydrothermalmethod;Bi2S3/BiOCl;composite photocatalysts;methyl orange
1 Introduction
The photocatalyticmaterial has been an exciting and rapidly growing research area owing to its great potential applications in solar energy utilization,photocatalytic hydrogen evolution,organic wastewater treatment and air purification[1-8].Bismuth oxychloride(BiOCl),as one of the bestnanomaterials,has recently attracted intensive attention in the degradation of environmental pollutants due to its interesting chemical and physical properties[9-11],such as electrical and optical performances,band gaps,and special micro/nanostructures.However,the low quantum efficiency of photocatalytic reactions resulting from the high rate of electron-hole recombination,impair its applications to great extent.As a result,in order to overcome this disadvantage,many attempts have been done,including doping or composition tuning,controlling the morphologies,and forming heterojunctions or composite structures[12-14].
Bi2S3has a narrow band-gap energy of~1.3 eV[15],therefore,itmay be a potential sensitizer to sensitize BiOClwith a wide band-gap energy(3.19-3.60 eV)[10].In 2012,Kang et al.[16]reported that the production of hydrogen of Bi2S3-loaded TiO2(Bi2S3/TiO2)was higher than that of pure TiO2and Bi2S3under identical experiment conditions.The improved performance could be attributed to the fact that Bi2S3-loaded TiO2facilitated the separation of photo-generated carriers.Hence,Bi2S3/BiOCl compositemay be an ideal system to enhance the separation efficiency of photo-generated charge carriers,and then achieve a high photocatalytic activity.Recent studies have also showed that Bi2S3-sensitized BiOCl photocatalyst[17],Bi2S3/BiOCl composites[18]and Bi2S3nanocrystals/BiOCl hybrid architectures[19]synthesized by an ion exchange method had higher visible light photocatalytic activities than single Bi2S3and BiOCl.However,to the best of our knowledge,the researches on the UV light photocatalytic activity and mechanism in the Bi2S3/BiOCl system prepared by a hydrothermalmethod have not been reported.
In thiswork,Bi2S3/BiOCl composite photocatalystswith differentmass fraction of Bi2S3were successfully synthesized by a facile hydrothermal process at 433 K,and the obtained samples were characterized by various techniques.As a representative of organic dyes released in the textile effluents,methyl orange(MO)was used to evaluate the photocatalytic activity of Bi2S3/BiOCl catalysts under UV light irradiation,and the photocatalytic mechanism of Bi2S3/BiOCl catalysts was discussed.The facile preparation of Bi2S3/BiOCl with high activity could be hopefully applied in the near future.
2 Experiments
2.1 M aterials
All of the reagentswere provided by Sinopharm Chemical Reagent Co.,Ltd.(China)except that TiO2powder(Degussa P-25)was purchased from Degussa Co.(Frankfurt,Germany),and P25 was used as the reference.Bismuth nitrate pentahydrate[Bi(NO3)3·5H2O],thiourea(CH4N2S),sodium hydroxide(NaOH),absolute ethanol(CH3CH2OH),nitric acid(HNO3),potassium chloride(KCl)and ethylene glycol(OH-CH2CH2-OH)were of analytical grade.
2.2 Catalyst Preparation
The Bi2S3/BiOCl catalysts were synthesized by a hydrothermal method.In a typical preparation,different stoichiometric amounts of Bi(NO3)3·5H2O and 0.27 g thioureawere dissolved in 20mL of0.10 mol/L HNO3solution.The mixture was stirred and sonicated until the Bi(NO3)3·5H2O was dissolved,and the pH value ofmixed solution was adjusted to 7.0 with a certain concentration of NaOH solution,followed by the addition of0.15 g KCl and 30 mL ethylene glycol solution.Then the mixture was transferred to a Teflon-lined stainless steel autoclave to perform hydrothermal process at 433 K for 24 h.After cooling down to room temperature,the obtained products were separated centrifugally,washed with absolute ethanol and de-ionized water and dried at 373 K in air.The final Bi2S3/BiOCl samples with various mass fractions of Bi2S3of 0,13.3%,26.5%and 39.8%,respectively,were denoted as 0,13.3%,26.5%and 39.8%Bi2S3/ BiOCl,respectively.For comparison,pure Bi2S3sample was also synthesized by adopting the same method at the absence of KCl and ethylene glycol.
2.3 Catalyst Characterization
X-ray diffraction(XRD)was performed on a Philips X’pert diffractometer equipped with Ni-filtered Cu Kαradiation source(λ=0.154 18 nm). X-ray photoelectron spectroscopy(XPS)measurementswere carried out by using Multilab 2000 XPS system with a monochromatic Mg Kαsource and a charge neutralizer.All binding energies were calibrated using C 1s peak at284.6 eV.The Brunauer-Emmett-Teller(BET)surface areas and pore volume of samples were determined from N2adsorption isotherms at77 K using a Micromeritics ASAP 2020 instrument with a computer-controlled measurement system.High-resolution transmission electron microscopy(HR-TEM)images were taken using a JEM-2100 electron microscope.The photoluminescence(PL)spectra,obtained at room temperature with an excitation wavelength of 280 nm,were recorded on a CARY Eclipse(America)fluorescence spectrophotometer.UV-Vis diffuse reflection spectroscopy of the catalysts was determined with a Shimadzu UV-3600 spectrophotometer(Japan).
2.4 Photocatalytic Reaction
The photocatalytic activities of Bi2S3/BiOCl catalystswere evaluated by the degradation of MO in an aqueous solution.UV lightwas obtained by a 300 W high-pressure mercury lamp(λmax=365 nm). For each UV light test,40 mL MO(6.11×10-5mol/L)aqueous solution and 0.05 g catalyst sample were used.A general procedure was carried out as follows.First,MO aqueous solution was placed into a water-jacketed reactor maintained at 298 K,and then the catalyst sampleswere suspended in the solution.The suspension was stirred vigorously for60min in the dark to establish the adsorption-desorption equilibrium of MO,then irradiated under UV light. About2.5 mL solution was withdrawn from the reactor periodically and centrifuged and analyzed for the degradation ofMO using a TU-1901 spectrophotometer.
For the detection of·OH,catalyst samples(0.05 g)were dispersed in a 10 mL of terephthalic acid(TA)solution whose concentration was set at 5×10-4mol/L in 2×10-3mol/L NaOH solution. At a fixed time intervals,the suspension solution was sampled,centrifuged and measured on a Varian Cary Eclipse type fluorescence spectrophotometer. The excitation wavelength was 315 nm.
In order to study the effect of·OH,isopropanol(IPA)quencher(5.0μL),as an·OH scavenger,was introduced into the photocatalytic degradation process of MO in amanner similar to the photo-degradation experiment.
3 Results and Discussion
3.1 Catalyst Structure
Fig.1 shows the XRD patterns of Bi2S3/BiOCl samples with different Bi2S3mass fraction.Five main diffraction peaks of Bi2S3were found and could be respectively indexed to the(130),(211),(221),(431)and(351)planes of orthorhombic Bi2S3(JCPDS card No.17-0320).The characteristic diffraction peaks of BiOCl including(101),(110),(112),(200),(211)and(212)are in good accordance with the standard card of tetragonal BiOCl(JCPDS card No.06-0249).For the Bi2S3/ BiOCl samples,the intensities of corresponding diffraction peaks of Bi2S3strengthened gradually along with the increase of the Bi2S3contents in the Bi2S3/ BiOCl composites,while those of BiOCl weakened simultaneously for its depletion.No other characteristic peaks of impurity were detected,indicating that Bi2S3/BiOCl composites are only composed of Bi2S3and BiOCl phases at the same time.

Fig.1 XRD patterns of Bi2 S3/BiOCl samples with different Bi2 S3 mass fraction:(a)0.00,(b)13.3,(c)26.5,(d)39.8,(e)100.
The average crystallite sizes of Bi2S3and BiOCl in the Bi2S3/BiOCl samples were calculated by the Scherrer formula(Eq.(1))[20],respectively,as shown in Table 1.
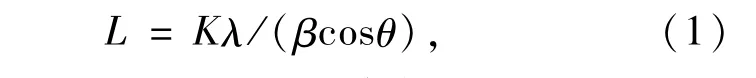
Where L is taken as average crystallite size,K is a constant equalling to 0.89,λis0.154 nm,βis the full-width of half-maximum measured in radians on the 2θscale,θis the Bragg angle for the diffraction peaks.
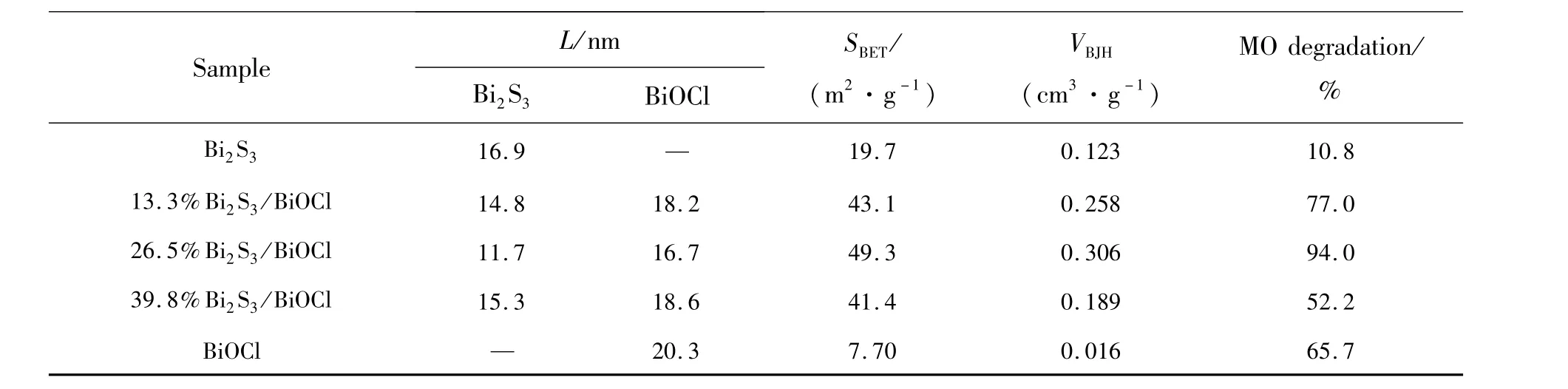
Table 1 Average crystallite size(L),BET surface areas(S BET),pore volumes(V BJH)and photocatalytic activity of different catalyst(reaction for the first one hour)
Fig.2 presents the HR-TEM image of 26.5% Bi2S3/BiOCl sample,in which the interface of Bi2S3and BiOCl can be observed.Moreover,clear fringe with an interval of 0.360 nm can be indexed to(130)lattice plane of orthorhombic Bi2S3and that of 0.275 nm agreed with the(110)lattice plane of tetragonal BiOCl,which further demonstrates the existence of the heterojunction formed between Bi2S3and BiOCl.

Fig.2 HR-TEM image of 26.5%Bi2 S3/BiOCl sample
The chemical state of as-prepared 26.5%Bi2S3/ BiOCl samplewas investigated by using X-ray photoelectron spectroscopy(XPS),and the results are shown in Fig.3.The typical XPSsurvey spectrum in Fig.3(a)shows that 26.5%Bi2S3/BiOCl was composed of Bi,Cl,S and O elements(C 1s peak can be ascribed to the adventitious hydrocarbon from XPS instrument itself).The Bi4f peaks of the sample appeared at164.5 and 159.2 eV in Fig.3(b),and the Cl 2p peaks of the sample appeared at 198.0 and 199.4 eV in Fig.3(c).The binding energies of both Bi4f and Cl 2p were in agreementwith the reported values in the literature[21].In Fig.3(d),the O 1s binding energy of530.1 eV could be attributed to the lattice oxygen in crystalline BiOCl[22].
Fig.4(a),(b)and(c)display the UV-Vis diffuse reflectances spectroscopy of Bi2S3,BiOCl and 26.5%Bi2S3/BiOCl samples.It can be seen that the absorption edge of BiOCl is located at about 378 nm,while Bi2S3has strong absorption over the whole visible light region.As for 26.5%Bi2S3/ BiOCl sample,its absorption edge(~370 nm)shifts to shorter wavelengths compared to that of BiOCl,which may be attributed to small particle sizes of 26.5%Bi2S3/BiOCl.
As a crystalline semiconductor,the optical absorption near the band edge follows the formulaαhv= A(hv-Eg)n/2,whereα,v,Eg,and A are the absorption coefficient,light frequency,band-gap energy,and a constant,respectively[23].As reported previously,the n values of Bi2S3[17]and BiOCl[24]are 1 and 4,respectively.The band-gap energies(Eg)of BiOCl and Bi2S3can be thus estimated from a plot of(αhv)1/2and(αhv)2vs.photon energy(hv),respectively.As shown in Fig.4(d),the intercept of the tangent to the x-axis will give a good approximation of the band-gap energies for the BiOCl and Bi2S3powders,respectively.The estimated Egvalues are about 3.28 and 1.28 eV for pure BiOCl and Bi2S3,respectively,which are very close to the values reported in the literatures[10,25].

Fig.3 XPS survey spectra of26.5%Bi2 S3/BiOCl(a)and the corresponding high-resolution XPS spectra of Bi4f(b),Cl 2p(c)and O 1s(d).
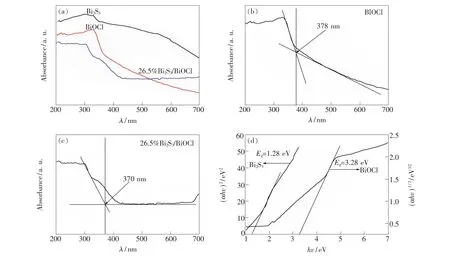
Fig.4 UV-Vis diffuse reflectance spectra of Bi2 S3,BiOCl and 26.5%Bi2 S3/BiOCl samples(a-c),and plot of(αhv)2/n vs. energy(hv)for the band-gap energy of Bi2 S3 and BiOCl(d).
The data related to BET surface areas and pore volumes obtained from N2adsorption-desorption analysis are also summarized in Table 1.It can be seen that with Bi2S3content increasing,the BET surface areas and pore volumes of Bi2S3/BiOCl catalysts first increase,reaching themaximums at Bi2S3mass fraction of 26.5%,respectively,and then decrease with further increasing Bi2S3contents.The BET surface areas and pore volumes of Bi2S3/BiOCl catalysts are obviously higher than those of pure Bi2S3and BiOCl,but the corresponding BET surface areas of 13.3%,26.5%and 39.8%Bi2S3/BiOCl catalysts did not have obvious difference,which demonstrates that the BET surface area is not themain influencing factor for the photocatalytic activity of different Bi2S3/BiOCl catalysts.
3.2 Photocatalytic Activity
The effect of Bi2S3mass fraction on the photocatalytic activity of Bi2S3/BiOCl catalysts has been investigated by MO degradation in an aqueous solution under UV light irradiation.Fig.5(a)shows the UV light photocatalytic activity of Bi2S3/BiOCl catalystswith different Bi2S3content.It can be seen that the self-degradation of MO is very low under UV light irradiation,indicating that the photolysis can be ignored.While Bi2S3,BiOCl and Bi2S3/BiOCl catalysts exhibit obviously the photo-degradation of MO,and Bi2S3content in BiOCl exerts great influences on the photocatalytic activity of Bi2S3/BiOCl catalysts.With the increasing of Bi2S3mass fraction,the photocatalytic activity of Bi2S3/BiOCl catalysts first increases,reaching a maximum at Bi2S3mass fraction of 26.5%,then decreases,and under 60 min UV light irradiation,themaximum MO degradation ratio reaches to 94.0%,being very close to that of commercial Degussa P-25,which has been generally recognized as an efficient photocatalyst under UV light irradiation.
In generally,the photocatalytic activity of composite catalyst is related to its BET surface areas,component contents,band structure matching and heterojunction interface,etc.But the corresponding BET surface areas(Table 1)of 13.3%,26.5%,39.8% Bi2S3/BiOCl catalysts do not have obvious difference,which demonstrates that the main influencing factor for the enhanced photocatalytic performance of Bi2S3/BiOCl catalysts is more likely to be the heterojunction structure rather than both BET surface areas.With the increasing of Bi2S3mass fraction from 13.3%to 26.5%,the more Bi2S3-BiOCl heterojunction interface will be formed,which facilitates the efficient separation of photo-induced electrons and holes,leading to the enhanced photocatalytic performance.However,if themass fraction of Bi2S3increases continuously to excess,more Bi2S3and Bi2S3contacts will be established,which dominates the Bi2S3and BiOCl contacts.The Bi2S3and Bi2S3contacts are not as effective as Bi2S3and BiOCl contacts for an effective charge separation.Moreover,the excessive Bi2S3with narrow band gap can act as the recombination center of electrons and holes,impeding the photocatalytic activity on the contrary[15-16,21,25].Thus,26.5%Bi2S3/BiOCl exhibits the highest photocatalytic activity.
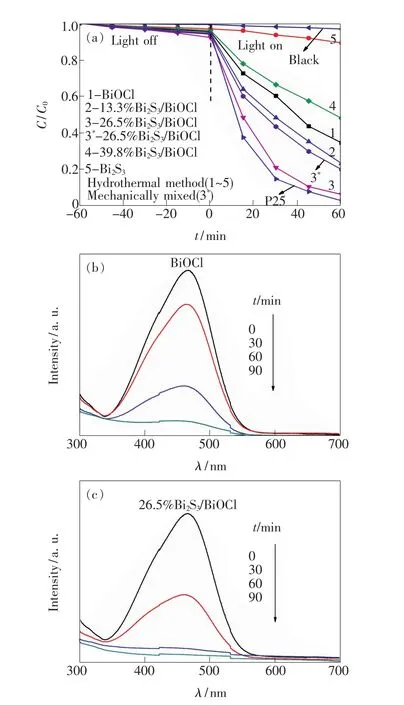
Fig.5 Effects of Bi2 S3 mass fraction in the Bi2 S3/BiOCl catalysts on the degradation of MO under UV light irradiation(a),and UV-Vis spectra of the MO aqueous solution under UV light irradiation in the presence of BiOCl(b)and 26.5%Bi2 S3/BiOCl(c)catalysts.
In order to confirm the advantage of heterojunction between Bi2S3and BiOCl,26.5%Bi2S3/BiOCl is compared with the mechanical mixture of Bi2S3and BiOClwith the similar composition under identical photocatalytic degradation conditions,as shown in Fig.5(a).Obviously,the UV light photocatalytic activity ofmechanically mixed 26.5%Bi2S3/BiOCl are lower than that of 26.5%Bi2S3/BiOCl prepared by hydrothermal method.This is mainly because Bi2S3and BiOCl behave as independent photocatalysts rather than a coupled system in themechanical mixture of Bi2S3and BiOCl,which is unfavorable to the transfer of charge carriers from one phase to another.However,the photocatalytic activity of mechanical mixture of Bi2S3and BiOCl is enhanced compared with that of pure Bi2S3and BiOCl,even themathematical sum of them.Thismight be owing to the inevitable partial contact between Bi2S3and BiOCl in the mechanical mixture of Bi2S3and BiOCl,which is in favor of the photocatalytic activity to some extent but is still far inferior to the effect of Bi2S3/BiOCl heterostructure[25].The results show that the Bi2S3and BiOCl heterojunction fabricated in the Bi2S3/BiOCl catalysts can improve the photocatalytic activity greatly.
UV-Vis spectra of MO aqueous solution as a function of UV light irradiation time in the presence of pure BiOCl and 26.5%Bi2S3/BiOCl catalysts are illustrated in Fig.5(b)and Fig.5(c),respectively.It can be seen that the visible region peak intensities in the photo-degradation of MO by the 26.5% Bi2S3/BiOCl catalyst decrease more obviously than those of pure BiOCl catalystafter60min UV light irradiation,which is in agreement with the results of Fig.5(a).Since no new peak appears,the loss of absorbance can bemainly attributed to the degradation reaction.
3.3 Discussion of The Photocatalytic Mechanism
3.3.1 Hydroxyl Radical(·OH)Involved in The Photocatalytic Process
Hydroxyl radical(·OH)has been considered to be an important species in the photocatalytic degradation ofmany hazardous chemical compounds owing to its high reaction ability to attack organicmolecule.The formation of·OH on the surface of Bi2S3,BiOCl and 26.5%Bi2S3/BiOCl catalysts was detected by a photoluminescence(PL)techniquewith terephthalic acid as a probe molecule.Terephthalic acid reacted with·OH readily to produce a highly fluorescent product,2-hydroxy terephthalic acid,whose PL peak intensity was in proportion to the amount of·OH radicals produced in water,which was reported in the literature[26].After UV light irradiation for a fixed time,the reaction solution was filtrated tomeasure the PL intensity at425 nm of 2-hydroxyterephthalic acid.
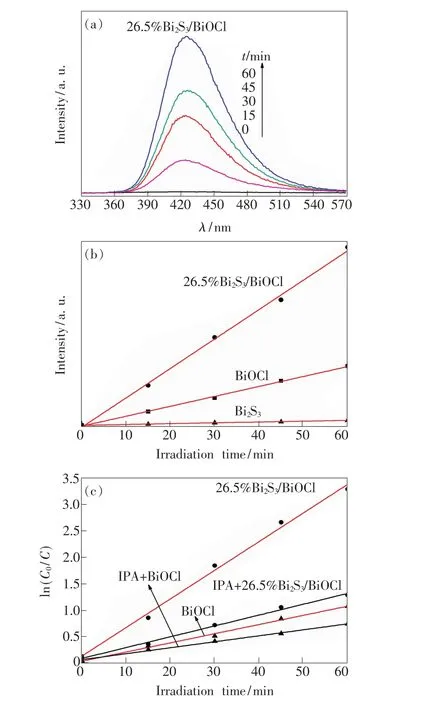
Fig.6 Changes of PL spectra over 26.5%Bi2 S3/BiOCl(a),linear transform of PL spectra over different catalyst recorded during UV light illumination in terephthalic acid solution(5×10-4 mol/L,excitation at315 nm)(b),and the kinetics of photocatalytic degradation of MO using different catalyst under UV light illumination(c).
Fig.6(a)indicates the changes of PL spectra of 26.5%Bi2S3/BiOCl catalyst in a 5×10-4mol/L terephthalic acid solution with UV irradiation time. It can be seen that no PL signal is observed in the absence of irradiation.The fluorescence intensity at 425 nm gradually increase with the irradiation time,which elucidates that·OH on 26.5%Bi2S3/BiOCl have been really produced under UV light irradiation.
As shown in Fig.6(b),the PL peak of26.5% Bi2S3/BiOCl is higher than that of pure BiOCl and Bi2S3under UV light irradiation,and it suggests that the formation rate of OH radicals on its surface is much higher than that of pure BiOCl and Bi2S3,which implies the photocatalytic activity of the 26.5% Bi2S3/BiOCl catalyst is much higher than that of pure BiOCl and Bi2S3.This result is also verified in the Fig.5(a).
In order to identify the role of·OH in the MO degradation,IPA,an efficient·OH quencher[27]was added into BiOCl and 26.5%Bi2S3/BiOCl reaction systems.The results are shown in Fig.6(c).It can be seen that the degradation efficiency(kapp)of MO decreases significantly after the addition of IPA under UV light irradiation.That is to say,the·OH species plays an important role in the degradation of MO under UV light irradiation.
3.3.2 Origin of·OH
Taking the kinds of·OH species involved in the photocatalysis into account,the photocatalytic process of Bi2S3/BiOCl catalysts can be described as the following Eqs.(2)-(6):
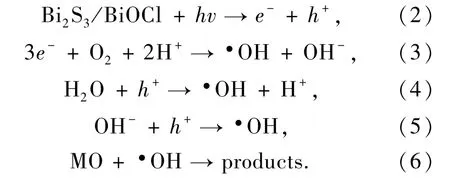
In the above process,electron-hole pairs are directly produced by catalyst after UV light illumination.Then,the photo-generated electrons transfer to CB bottom of the catalyst and react with the adsorbed O2and H+on the surface of catalyst to form ·OH.Meanwhile,the holes are left on the VB top,reacting with the adsorbed H2O or OH-on the surface of catalyst to form·OH.After that,the·OH species oxidized MO.
3.3.3 Mechanism of Photocatalytic Activity Enhancement of Bi2S3/BiOCl
It is well known that the energy level and the band gap of semiconductors play important roles in determining their photocatalytic activities.Using two semiconductors in contactwith different redox energy levels of conduction band(CB)and valence band(VB)can be used to improve photo-generated carriers separation and enhance the efficiency of the interfacial charge transfer[28].In the light of the experiment results and by referring to the literatures[13-17,25],the transfer behaviors of the photogenerated electrons and holes in Bi2S3/BiOCl catalysts are illustrated in Fig.7.As shown,the CB and VB of Bi2S3lie above those of BiOCl.Clearly,the difference between energy bands of BiOCl and Bi2S3(Fig.7)allows the efficient transfer of electron and hole between them.When the Bi2S3/BiOCl catalyst is irradiated by UV light,BiOCl and Bi2S3are both excited;then,a large number of electrons and holes are produced in the system.Electrons will transfer from Bi2S3to CB of BiOCl and holes shift from BiOCl to VB of Bi2S3at the same time.In thisway,the photo-generated electron-hole pairs are effectively separated,and the better separation of electrons and holes in the Bi2S3/BiOCl catalysts can be confirmed by photoluminescence(PL)emission spectra in Fig.8.
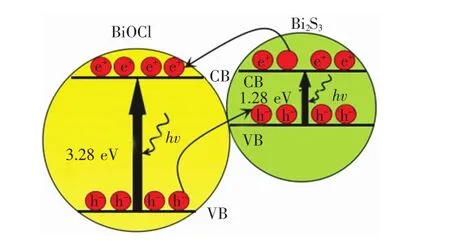
Fig.7 Diagrams of photo-generated electron-hole pairs transfer of Bi2 S3 and BiOCl
PL emission spectra have been widely used to investigate the separation efficiency of the photo-generated charge carriers in a semiconductor[29].The comparison of PL spectra(excited by 280 nm)of pure BiOCl and 26.5%Bi2S3/BiOCl at room temperature is shown in Fig.8.It can be seen that the two catalysts have a broad emission peak,and the strongest emitting peaks are similar,while PL emission intensity of the 26.5%Bi2S3/BiOCl sample isdramatically weakened compared with thatof pure BiOCl,indicating that the recombination of photo-generated charge carriers is greatly inhibited by the Bi2S3introduction.In other words,a proper content of Bi2S3in the BiOCl is helpful to separate the photo-generated charge carriers and increase the lifetime of charge carriers,and then enhance the photocatalytic activity of Bi2S3/BiOCl catalysts.
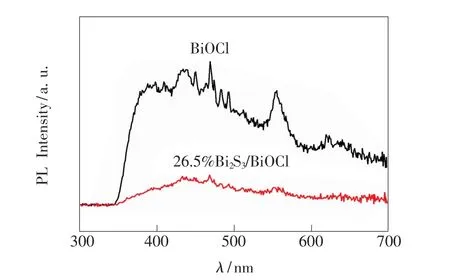
Fig.8 Photoluminescence(PL)spectra of pure Bi2 S3 and 26.5%Bi2 S3/BiOCl samples recorded at room temperature with the excitation wavelength of 280 nm
4 Conclusion
In summary,we have successfully synthesized the Bi2S3/BiOCl composite photocatalysts with different Bi2S3mas fraction by a facile hydrothermal process at 433 K.The photocatalytic activity of Bi2S3/BiOCl catalyst is greatly enhanced,compared with that of pure Bi2S3and BiOCl.Especially,the UV light photocatalytic activity of 26.5%Bi2S3/BiOCl catalyst is very close to that of commercial Degussa P-25.The remarkably enhanced photocatalytic performance can be mainly attributed to the effective transfer of the photo-generated electrons and holes at the heterojunction interface of Bi2S3and BiOCl,which can reduce the recombination of electron-hole pairs.In this study,the simple and low-cost preparation route can not only reduce the energy consumption for the fabrication of Bi2S3/BiOCl catalysts but also might extend the utilization of Bi2S3/BiOCl at low temperature in the near future.
[1]Chen X B,Liu L,Yu P Y,et al.Increasing solar absorption for photocatalysis with blank hydrogenated titanium dioxide nanocrystals[J].Science,2011,331:746-750.
[2]Schwinghammer K,Mesch M B,Duppel V,et al.Crystalline carbon nitride nanosheets for improved visible-lighthydrogen evolution[J].J.Am.Chem.Soc.,2014,136(5):1730-1733.
[3]Li H Q,HongW S,Cui Y M,etal.Enhancementof the visible light photocatalytic activity of Cu2O/BiVO4catalysts synthesized by ultrasonic dispersion method at room temperature[J].Mater.Sci.Eng.B,2014,181:1-8.
[4]Oncescu T,Stefan M I,Oancea P.Photocatalytic degradation of dichlorvos in aqueous TiO2suspensions[J].Environment.Sci.Technol.,2010,17(3):1158-1166.
[5]Li H Q,Cui Y M,HongW S,et al.Photodegradation ofmethyl orange by BiOI-sensitized TiO2[J].RareMetals(稀有金属),2012,31(6):604-610(in English).
[6]Chen SF,Hu Y F,Meng SG,et al.Study on the separationmechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3[J].Appl.Catal.B:Environ.,2014,150-151:564-573.
[7]Chen SF,Zhang S J,Liu W,et al.Preparation and activity evaluation of p-n junction photocatalyst NiO/TiO2[J].J. Hazard.Mater.,2008,155(1-2):320-326.
[8]Li H Q,Hong W S,Cui Y M,et al.High photocatalytic activity of C-ZnSn(OH)6catalysts prepared by hydrothermal method[J].J.Mol.Catal.A:Chem.,2013,378:164-173.
[9]Shenawi-Khalil S,Uvarov V,Menes E,et al.New efficient visible light photocatalyst based on heterojunction of BiOClbismuth oxyhydrate[J].Appl.Catal.A:General,2012,413-414:1-9.
[10]Mu Q H,Zhang Q H,Wang H Z,et al.Facile growth of vertically aligned BiOCl nanosheet arrays on conductive glass substrate with high photocatalytic properties[J].J.Mater.Chem.,2012,22(33):16851-16857.
[11]Ye L Q,Deng K J,Xu F,etal.Increasing visible-lightabsorption for photocatalysiswith black BiOCl[J].Phys.Chem. Chem.Phys.,2012,14(1):82-85.
[12]Li T B,Chen G,Zhou C,et al.New photocatalyst BiOCl/BiOIcompositeswith highly enhanced visible light photocatalytic performances[J].Dalton Trans.,2011,40(25):6751-6758.
[13]XiongW,Zhao Q D,Li X Y,et al.One-step synthesis of flower-like Ag/AgCl/BiOCl composite with enhanced visiblelight photocatalytic activity[J].Catal.Commun.,2011,16(1):229-233.
[14]Shamaila S,Sajjad A K L,Chen F,et al.WO3/BiOCl,a novel heterojunction as visible light photocatalyst[J].J. Colloid Interf.Sci.,2011,356(2):465-472.
[15]Bessekhouad Y,Robert D,Weber JV.Bi2S3/TiO2and CdS/TiO2heterojunctions as an available configuration for photocatalytic degradation of organic pollutant[J].J.Photochem.Photobio.A:Chem.,2004,163(3):569-580.
[16]Kim J,Kang M.Photocatalytic hydrogen production over the band gap-tuned urchin-like Bi2S3-loaded TiO2composites system[J].Int.J.Hydrogen Energy,2012,37:8249-8256.
[17]Cao J,Xu B Y,Lin H L,etal.Novel Bi2S3-sensitized BiOClwith highly visible lightphotocatalytic activity for the removal of rhodamine B[J].Catal.Commun.,2012,26:204-208.
[18]Jiang SH,Zhou K Q,Shi Y Q,et al.Laser-assisted implantation of gold nanoparticles,formed under surface plasmonpolariton resonant conditions in polymer layer[J].Appl.Surf.Sci.,2014,290:313-319.
[19]Cheng H F,Huang B B,Qin X Y,et al.A controlled anion exchange strategy to synthesize Bi2S3nanocrystals/BiOCl hybrid architectureswith efficient visible light photoactivity[J].Chem.Commun.,2012,48(1):97-99.
[20]Sarwan B,Pare B,Acharya A D,et al.Mineralization and toxicity reduction of textile dye neutral red in aqueous phase using BiOCl photocatalysis[J].J.Photochem.Photobio.B:Bio.,2012,116:48-55.
[21]Li H Q,Hong W S,Cui Y M,et al.Effect of Mo2C content on the structure and photocatalytic property of Mo2C/TiO2catalysts[J].J.Alloys Compd.,2013,569:45-51.
[22]Zhang X,Ai ZH,Jia F L,et al.Generalized one-pot synthesis,characterization,and photocatalytic activity of hierarchical BiO X(X=Cl,Br,I)nanoplatemicrospheres[J].J.Phys.Chem.C,2008,112(3):747-753.
[23]Cao J,Xu B Y,Lin H L,etal.Novel heterostructured Bi2S3/BiOIphotocatalyst:Acile preparation,characterization and visible light photocatalytic performance[J].Dalton Trans.,2012,41(37):11482-11490.
[24]Pare B,Sarwan B,Jonnalagadda SB.Photocatalytic mineralization study ofmalachite green on the surface of Mn-doped BiOCl activated by visible light under ambient condition[J].Appl.Surf.Sci.,2011,258(1):247-253.
[25]Yang LX,SunW S,Luo SL,etal.White fungus-likemesoporous Bi2S3ball/TiO2heterojunction with high photocatalytic efficiency in purifying 2,4-dichlorophenoxyacetic acid/Cr(Ⅵ)contaminated water[J].Appl.Catal.B:Environ.,2014,156-157:25-34.
[26]Chen SF,Yang Y G,Liu W.Preparation,characterization and activity evaluation of TiN/F-TiO2photocatalyst[J].J. Hazard.Mater.,2011,186(2-3):1560-1567.
[27]Cao J,Xu BY,Lin H L,etal.Chemical etching preparation of BiOI/BiOBr heterostructureswith enhanced photocatalytic properties for organic dye removal[J].Chem.Eng.J.,2012,185-186:91-99.
[28]Zhao D,Chen CC,Yu C L,etal.Photoinduced electron storage in WO3/TiO2nanohybridmaterial in the presence of oxygen and postirradiated reduction of heavymetal ions[J].J.Phys.Chem.C,2009,113(30):13160-13165.
[29]Li H Q,Cui Y M,Hong W S.High photocatalytic performance of BiOI/Bi2WO6toward toluene and reactive brilliant red[J].Appl.Surf.Sci.,2013,264:581-588.

李慧泉(1973-),男,江西萍乡人,博士,教授,2012年于南京大学获得博士学位,主要从事光催化纳米材料的设计与制备、结构与性能调控、光解水和水溶液中有机污染物的光催化降解等方面的研究。
E-mail:huiquanli0908@163.com

崔玉民(1963-),男,安徽毫州人,教授,1990年于延边大学获得硕士学位,主要从事光催化方面的研究。
E-mail:cuiyumin0908@163.com
Bi2S3/BiOCl复合光催化剂的水热合成及其高活性
李慧泉1,2,朱良俊1,2,孙 倩1,王 高1,苗 慧1,2,崔玉民1,2*,郏青峰1
(1.阜阳师范学院化学与材料工程学院,安徽阜阳 236037;2.安徽环境污染物降解与监测省级实验室,安徽阜阳 236037)
采用一种简便的水热法在433 K的温度下成功合成了具有不同Bi2S3质量分数的Bi2S3/BiOCl复合光催化剂,利用各种技术对其进行了表征。在紫外光照射下,以甲基橙水溶液的光催化降解为模型反应,评价了Bi2S3/BiOCl复合光催化剂的活性。研究结果表明:与纯Bi2S3和纯BiOCl相比,Bi2S3/BiOCl样品明显具有更高的光催化性能,尤其当Bi2S3在Bi2S3/BiOCl中的质量分数为26.5%时,Bi2S3/BiOCl复合催化剂的光催化活性与商业P25(TiO2)的活性非常接近,而这种商业P25在紫外光照射下是公认的高效光催化剂。这种明显提高的光催化活性主要归功于光生电子和空穴在Bi2S3和BiOCl形成异质结界面上的有效转移,降低了电子-空穴对的复合。
水热法;Bi2S3/BiOCl;复合光催化剂;甲基橙
2014-11-03;
2014-12-02
国家自然科学基金(21201037);安徽省高校自然科学基金(kj2014a191);安徽省自然科学基金(1408085mb35);安徽省高校青年人才基金(2013sqrl058zd);功能性分子固体省部共建教育部重点实验室项目(14010);阜阳师范学院和安徽环境污染物降解与监测省级实验室项目(fsb201401003,2014kjfh01)资助
1000-7032(2015)02-0176-10
O634 Document code:A
10.3788/fgxb20153602.0176

