硫酸钠水合物在网络微区域中的相变机制
吴晓琳史 剑符显珠孙 蓉,2
1(中国科学院深圳先进技术研究院 深圳 518055)
2(香港中文大学 香港 999077)
硫酸钠水合物在网络微区域中的相变机制
吴晓琳1史 剑1符显珠1孙 蓉1,2
1(中国科学院深圳先进技术研究院 深圳 518055)
2(香港中文大学 香港 999077)
水合盐作为一种固-液相变材料因具有高的能量储存密度而备受关注。然而,其过冷和相分离的缺陷限制了它的应用。为了解决过冷及相分离问题,文章提出了采用在硫酸钠饱和溶液中原位聚合的方法制备硅胶。根据实验结果,硅胶中饱和硫酸钠水溶液的潜热为 238.1 J/g,相变温度为 30℃。通过添加这种添加剂,它可以在亚热带春夏季时期承受长达五个月以上的冷热循环。通过扫描电子显微镜和傅里叶变换红外光谱检测发现,实验制备的硅胶是无定型结构,并有一些小的晶体分布其中。实验推断,硅胶的添加提供了一种多孔结构,有助于硫酸钠水合盐晶体的生长,因此,它可以抑制过冷和相分离的发生。
相变材料;水合盐;硅胶;过冷;相分离;晶体
1 Introduction
In recent years, as the energy cost is skyrocketing,phase change material (PCM) is attracting much renewed attention. PCMs use latent heat to store energy and hence shall have high energy storage density and small temperature variation in the process of storing and releasing heat energy. Various organic, inorganic, polymeric and eutectic PCMs have been studied[1-3]. Among them, salt hydrate is one of the most attractive PCMs[4]owing to its moderate cost, easy production, high volumetric energy storage density, high thermal conductivity,and environment safety. However, its applications have been limited by the problems of supercooling and phase segregation[5].
In order to solve the problems of supercooling and phase segregation, many studies have been carried out. Biswas[6], for example, suggested the use of extra water to prevent the formation of the heavy anhydrous salt. Telkes[7]introduced borax as a nucleating agent to minimize subcooling. The use of some thickening agents[8], such as polymer and gel[9], has been suggested to overcome the phase segregation. In general, at least two additives are needed to diminish the supercooling and phase separation. It is possible to use direct contact heat transfer between an immiscible heat transfer fluid and the salt hydrate solution without additives[10,11]. The agitation caused by the heat transfer fluid minimized the supercooling and prevented phase segregation. However, several controlling systems were needed in the above process. Until now, there is no effective method to solve the problems of supercooling and phase segregation. Moreover, the preventing supercooling mechanics of amorphous nucleating agent for hydrate salt is not well understood.
In this paper, we investigate the phase transition of salthydrate with silica gel. A temperature recording device and the differential scanning calorimeter were used to study the phase change characteristics of salt hydrate in silica gel. The results showed that silica gel was effective in preventing the supercooling and phase segregation of salt hydrate.
2 Experimental Methods
In our experiments, the raw materials include Na2SO4, Na2SiO3and H2SO4.Na2SiO3was dissolved into saturated Na2SO4solution at 40℃ (All the raw solutions used in our experiments are saturated Na2SO4solution). Through chemical reaction of Na2SiO3with acid, silica sol was obtained. After ripening it for a moment, homogeneous silica gel[12]was obtained and saturated Na2SO4solution could be dispersed in the silica gel uniformly.
Two methods were used to test the long-time cycling stability of silica gel. One was natural heating-cooling process which worked by the temperature change of a day in the subtropical spring and summer in the lab (The lab is located in Shenzhen, Guangdong, China, a typical subtropic region where the temperature ranges from 18℃to 35℃). The other was forcible heating-cooling process which worked by two prepared constant temperature water baths (One was at 10℃ and the other was at 40℃).
A simple device was made to record the temperature timely. Firstly, samples on test tubes with a thermal couple in the center were initially heated to 40℃. Secondly, the sampleswere immersed into a 10℃ water cooler with the temperature changing recorded using a multipoint recorder (IDAQ-8018+) and stored in a computer via an RS-232 port.
Differential scanning calorimeter (DSC) was used with nitrogen atmosphere to analyze the phase transition in microscopic level. Samples about 15.00 mg were sealed in an aluminum pan and was placed in DSC analyzing from 10℃ to 60℃ with a linear heating rate of 7℃/min and the nitrogen flow rate at 50 mL/min. The accuracy of the DSC is ±0.02℃and the latent heat was calculated as the total area under the peaks of transitions process.
The scanning electron microscopy (SEM) and Fourier transformation infrared (FTIR) spectroscopy were used to study the microstructure of the samples. For SEM analysis, a thin layer of gold was coated in the surface of sample, and images were acquired on a HITACHI S-4700 microscope with a constant ambient temperature at 22℃. For FTIR spectroscopy,viscous samples were mixed with KBr at 10℃. Then the functional groups of samples were obtained.
3 Results and Discussions
3.1 The Time-temperature Characteristics of the Sodium Sulfate Hydrate
Fig. 1 shows two time-temperature (t-T) curves. Curve “1” corresponds to the saturated solution of sodium sulfate without silica gel. The phase change temperature is about 28℃ and however,the supercooling to 13℃ is obvious. Curve “2”corresponds to the saturated sodium sulfate solution with silica gel. The phase change phenomenon is clearly seen at 30℃ and there is no supercooling. It is also found that after 30 heating-cooling cycles,the silica gel is still homogeneous and stable without phase separation.

Fig. 1 The t-T curves of saturated sodium sulfate solution with and without silica gel
Table 1 summarizes the phase change temperature and latent heat of the 7 samples. It shows that except sample 1, the characteristics of the other samples are similar.
To test the long time stability of saturated sodium sulfate solution with silica gel, the same sample using forcible heating-cooling method was tested in many cycles. Fig. 2 is the t-T curves of a sample. Curves 1, 2 and 3 are the t-T curves after 10, 20 and 30 heating-cooling cycles, respectively. Each10 heating-cooling cycles was completed in one day. Then, the next 10 heating-cooling cycles were done after 10 days. It is seen that the phase change temperature is stabilized at 30℃, and the process is very stable.
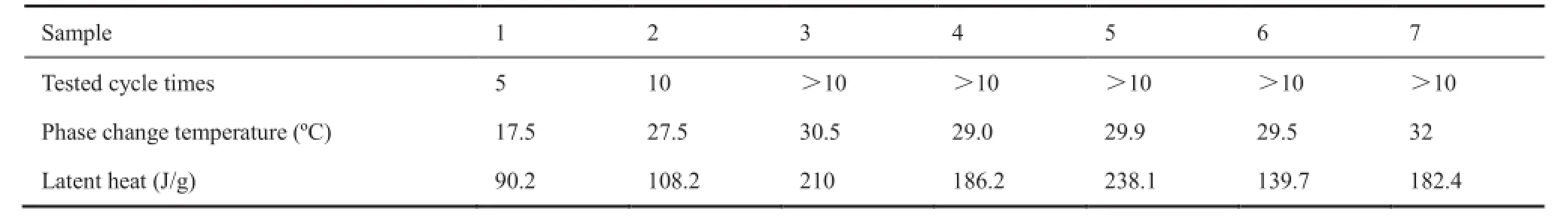
Table 1 Summary of the experiment results

Fig. 2 The t-T cooling curves of saturated sodium sulfate with silica gel in different cycles
To further improve the long-time recycling stability of silica gel, span 80 as a surfactant was used. Fig. 3 is the t-T curve of a sample. It can be seen that the surfactant does not affect the phase change characteristics.
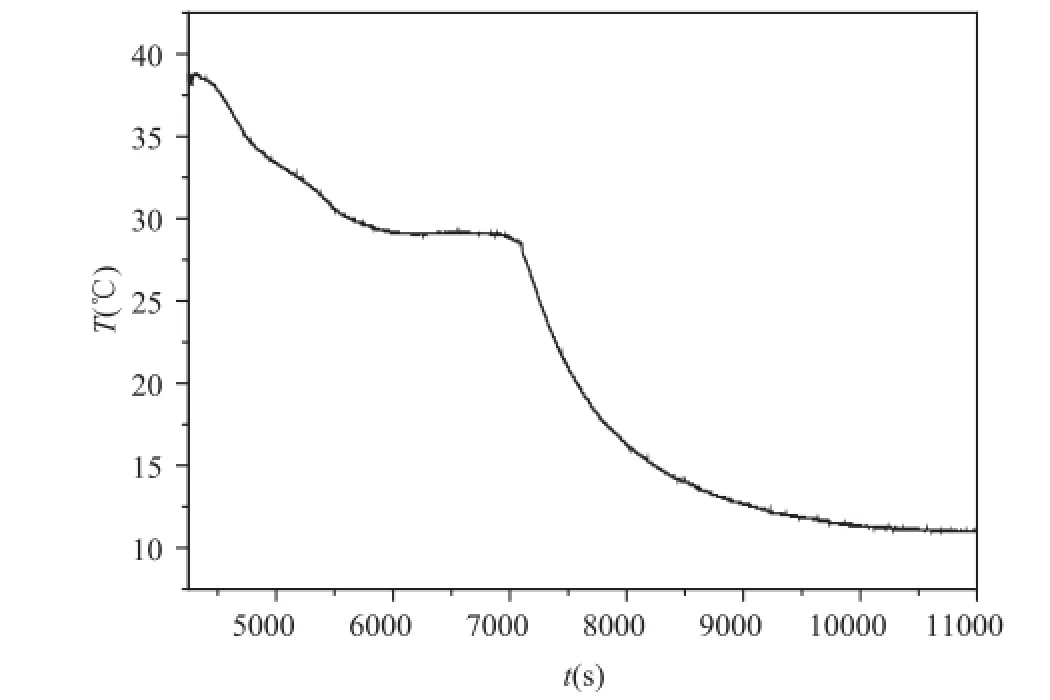
Fig. 3 The t-T curve of saturated sodium sulfate hydrate with silica gel and surfactant
Fig. 4(a) is the picture of saturated sodium sulfate hydrate without additives. The sample has clear phase segregation and bulky crystals can be observed at the bottom of the test tube. Fig. 4(b) is the saturated sodium sulfate hydrate in silica gel with surfactant. The natural heating-cooling method was used to test the long-time stability of the sample. After five months, it has been found that the sample still shows no phase segregation, indicating good stability.

Fig. 4 Pictures of sodium sulfate hydrate
3.2 The DSC Results
Fig. 5 shows the DSC curves of samples with and without additives. The curve with solid line depicts the peak of heat absorption of saturated hydrate salt without additives. The latent heat can be measured by the area of the peak. It is only 96.7 J/g and the phase change temperature is 25.4℃. The curve with dashed line shows the peak of heat absorption of saturated hydrate salt with additives (silica gel). As is shown in Fig. 5, the latent heat is increased to 238.1 J/g and the phase change temperature is 29.9℃. This indicates that the addition of silica gel improves the heat energy storage capability.
3.3 SEM Results

Fig. 5 DSC curve of a sample hydrate salts in silica gel

Fig. 6 SEM images of a sample hydrate salt with silica gel
Fig. 6 shows the SEM images of sodium sulfate hydrate in silica gel, magnified by 10000, 30000 and 200000 times, respectively. Fig. 6(a) shows the micro structure is complex. The crystals were randomly packed with many pores about several micrometers in between. Fig. 6(b) shows a couple of typical needle-shaped crystals and their lengths are about 10 µm. Fig. 6(c) zooms in a pore, which is full of tiny holes about 20 nm.
3.4 FTIR Spectroscopy Results
Fig. 7 shows the FTIR spectroscopy of a sample hydrate salt with silica gel in solid phase. The absorption bands at 470.63 and 1099.43 cm-1are associated with the bending, stretching of amorphous SiO2[13]. The bands at 617.22 and 796.60 cm-1are assigned to α-cristobalite[14]. The bending of the absorbed H2O molecules is at 1618.22 cm-1. The band at 1448.54 cm-1is attributed to the stretching(S=O) groups. The band at 3477.66 cm-1is stretching (OH) groups[15]. No other bonds are formed. So, it can be concluded that sodium sulfate hydrate should be lying in the matrix without any interaction with silica gel.

Fig. 7 FTIR spectrum of the salt hydrate with silica gel
The above analysis indicates that there should be two kinds of components in silica gel with hydrate salts: the amorphous silicon dioxide and the crystallized silicon dioxide. This would be the reason that with the silica gel, the bond strength is improved.
3.5 Discussions
Based on the testing results, it is clear that adding silica gel can suppress the supercooling and phase segregation of hydrated salt at the same time, which can omit the adding of thickening agents. The main reasons are: firstly, the complex structure of silica gel can act as a seed of the saturated solution of sodium sulfate during the solidification and aided effective crystallization; secondly, the existence of the stable micro-regions between the crystals can hinder convection and hence, decrease dynamic resistance of the saturated solution of sodium sulfate; thirdly,as Rosa[16]reported, the Gibbs energy necessary for heterogeneous nucleation was diminished by the presence of foreign substances. Therefore, the phase change process can occur homogeneously with less impetus.
In addition, silica gel is a good thickening agent. The saturated hydrate salt solution is dispersed homogeneously in the micro regions, which effectively reduces the effect of gravity. Thus, phase segregation can be reduced. It may be argued that those micro regions are connected. Though, the densely packed structure of silica gel minimizes the connection channels between the adjacent micro regions. In other words, the hydrate salt in each micro region can be considered as a single system. Thus, silica gel can hinder crystal growth. The small crystal of hydrate salt with large surface area can contact the water around and hence, improve the stability. Therefore, the porous structure of silica gel is effective. In fact, compared with the results obtained by other researchers, the gel-salt hydrate system is more stable and the results are comparable.
4 Conclusions
In this paper, a new process for making sodium sulfate hydrate with silica gel was proposed. Based on the obtained results and discussions, the following conclusions can be made:
(a)Adding the silica gel helps to suppress the supercooling and phase separation.
(b)The latent heat of the sodium sulfate hydrate with silica gel is about 238 J/g, and its phase transition temperature is around 30℃ and its life is more than 5 months.
(c)The silica gel can act as a seed of the saturated sodium sulfate solution during the solidification process. Its stable pores structure generates many micro regions which can hinder convection, reduce thermal resistance and improve the uniformity of the hydrate salt. Therefore, it is effective in preventing the supercooling and phase segregation.
The future work includes the study of the mechanics of the phase change and the development of an effective process to produce stable PCM.
References
[1] Tyagi VV, Buddhi D. PCM thermal storage in buildings: A state of art [J]. Renewable &Sustainable Energy Reviews, 2007, 11: 1146-1166.
[2] He Q, Zhang WN. A study on latent heat storage exchangers with the high-temperature phasechange material [J]. International Journal of Energy Research, 2001, 25: 331-341.
[3] Memon SA. Phase change materials integrated in building walls: A state of the art review [J]. Renewable & Sustainable Energy Reviews, 2014,31: 870-906.
[4] Canbazoglu S, Sahinaslan A, Ekmekyapar A, et al. Enhancement of solar thermal energy storage performance using sodium thiosulfate pentahydrate of a conventional solar water-heating system [J]. Energy and Buildings, 2005, 37: 235-242.
[5] Garcia-Romero A, Diarce G, Ibarretxe J, et al. Influence of the experimental conditions on the subcooling of Glauber’s salt when used as PCM [J]. Solar Energy Materials and Solar Cells, 2012, 102: 189-195.
[6] Biswas DR. Thermal-energy storage using sodiumsulfate decahydrate and water [J]. Solar Energy,1977, 19: 99-100.
[7] Telkes M. Nucleation of supersaturated inorganic salt solutions [J]. Industrial and Engineering Chemistry, 1952, 44: 1308-1310.
[8] Ryu HW, Woo SW, Shin BC, et al. Prevention of supercooling and stabilization of inorganic salt hydrates as latent heat storage materials [J]. Solar Energy Materials and Solar Cells, 1992, 27: 161-172.
[9] Zhang SM, Swarthout D, Noll T, et al. Silicone phase change thermal interface materials: properties and applications [J]. Advances in Electronic Packaging, 2003, 2: 167-170.
[10] Mulyono P. Direct contact thermal energy storage system using Na2CO3·10H2O solution [J]. Energy,2004, 29(12-15): 2573-2583.
[11] Farid MM, Khalaf AN. Performance of directcontact latent-heat storage units with 2 hydrated salts [J]. Solar Energy, 1994, 52: 179-189.
[12] Gao Y, Choudhury NR, Dutta N, et al. Organicinorganic hybrid from ionomer via sol-gel reaction [J]. Chemistry of Materials, 2001, 13: 3644-3652.
[13] Bruni S, Cariati F, Casu M, et al. IR and NMR study of nanoparticle-support interactions in a Fe2O3-SiO2nanocomposite prepared by a sol-gel method [J]. Nanostructured Materials, 1999, 11: 573-586.
[14] Chen HS, Ji SF, Niu JZ, et al. Vibration spectroscopy on transformation of amorphous silica to alpha-cristobalite [J]. Acta Physico-Chimica Sinica, 1999, 15: 454-457.
[15] Wu XL, Wang YH, Sun R, et al. The antisupercooling effect of surface-modified nano-scaled SiO2in hydrated salts phase transition system [J]. Journal of Physics Conference Series, 2009, 188(1): 012046-012049.
[16] Espinosa RM, Franke L, Deckelmann G. Phase changes of salts in porous materials: Crystallization,hydration and deliquescence [J]. Construction and Building Materials, 2008, 22(8): 1758-1773.
Phase Change Mechanism of Confined Sodium Sulfate Hydrates in Micro Network Regions
WU Xiaolin1SHI Jian1FU Xianzhu1SUN Rong1,2
1( Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen 518055, China )
2( The Chinese University of Hong Kong, Hong Kong 999077, China )
Hydrate salt is an attractive solid-liquid phase change material because of its high energy storage density. However, its applications have been limited owing to the supercooling and phase segregation. In order to solve these problems, we propose to make the silica gel in the saturated solution of sodium sulfate by means of in-situ polymerization. According to our experiments, the latent heat of saturated solution of sodium sulfate in silica gel is about 238.1 J/g and the phase change temperature is 30℃. With some surfactant, it can endure more than five months of heating-cooling cycles stably in subtropical spring and summer. Using scanning electron microscope and Fourier transformation infrared spectroscopy, we found that the microstructure of silica gel was mainly amorphous with some small crystals distributed in it. We believe that adding the silica gel produces a porous structure, which helps the crystal growth of sodium sulfate hydrate and hence, suppresses the supercooling and phase segregation.
phase change material; hydrated salt; silica gel; supercooling; phase segregation; crystal
2014-07-18 Revised: 2014-11-12
Wu Xiaolin (corresponding author), Ph.D., Research Assistant. Her research interest is materials science, E-mail:xl.wu@siat.ac.cn; Shi Jian, Master’s degree candidate. His research interests include electronic & packaging materials and applied electrochemistry; Fu Xianzhu, Ph.D.,Associate Researcher. His research interest is electrochemistry; Sun Rong (corresponding author), Ph.D., Researcher. Her research interest is materials science, E-mail:rong.sun@siat.ac.cn.
O 611.4
A
Foundation:Shenzhen Foundamental Research Program (JCYJ20120831180118531);Guangdong Innovative Research Team Program (2011D052);Shenzhen Peacock Pragram (KYPT20121228160843692);Shenzhen Electronic Packaging Materials (The Development and Reform Commission of Shenzhen【2012】372)

