赖氨大黄酸对氧化应激引起的小鼠动脉血管损伤的保护作用及其机制
冯秋生,阚 泉,吕翠平,李 冉,魏静波,赵毓芳,甄永占,2
(1. 华北理工大学基础医学院组织学与胚胎学系,河北 唐山063000;2. 华北理工大学基础医学院河北省慢性疾病重点实验室 河北省唐山市慢性病临床基础研究重点实验室,河北 唐山 063000)
赖氨大黄酸对氧化应激引起的小鼠动脉血管损伤的保护作用及其机制
冯秋生1,阚泉1,吕翠平1,李冉1,魏静波1,赵毓芳1,甄永占1,2
(1. 华北理工大学基础医学院组织学与胚胎学系,河北 唐山063000;2. 华北理工大学基础医学院河北省慢性疾病重点实验室 河北省唐山市慢性病临床基础研究重点实验室,河北 唐山 063000)
目的:观察赖氨大黄酸(RHL)对氧化应激引起的小鼠动脉血管损伤的保护作用,并探讨其作用机制。方法:采用腹腔注射百草枯方法建立活性氧引起血管损伤小鼠模型。将30只C57小鼠随机分为对照组(n=10)、百草枯模型组(n=10)和RHL干预组(n=10)。RHL干预组小鼠在造模前1周灌胃给予RHL,对照组和百草枯模型组灌胃给予等体积蒸馏水。百草枯模型组和RHL干预组腹腔注射百草枯,对照组腹腔注射等体积生理盐水,每周注射1次,持续2周。造模2周后检测血清丙二醛(MDA)水平、超氧化物歧化酶(SOD)和谷胱甘肽过氧化物酶(GSH-Px)活性;DCFH-DA染色观察血管活性氧水平;HE染色观察腹主动脉病理表现;Western blotting 法检测血管损伤相关基因的表达。结果:与对照组比较,百草枯模型组小鼠血管组织小鼠血清SOD和GSH-Px活性下降(P<0.05),MDA水平升高(P<0.05);与百草枯模型组比较,RHL干预组MDA水平下降(P<0.05),SOD和GSH-Px活性升高(P<0.05);DCFH-DA和HE染色,百草枯模型组小鼠血管组织血管组织活性氧水平升高(P<0.05),血管结构破坏, RHL干预组血管活性氧水平下降(P<0.05),病理变化明显减轻。Western blotting法检测,与对照组比较,百草枯模型组小鼠血管组织内皮型一氧化氮合酶(eNOS)和caspase-3表达水平降低(P<0.05),caspase-3裂解片段表达水平升高(P<0.05);与模型组比较,RHL干预组小鼠血管组织 eNOS和caspase-3 表达水平升高 (P<0.05),caspase-3裂解片段表达水平降低(P<0.05)。结论:百草枯能够诱导体内活性氧水平升高引起血管细胞损伤,RHL通过清除活性氧,上调eNOS表达,下调caspase-3裂解片段表达来拮抗百草枯的作用。
赖氨大黄酸;百草枯;活性氧;细胞凋亡
随着人口老龄化,慢性疾病(如糖尿病和高血压)的发病率日益增加。内皮功能损伤在动脉粥样硬化、糖尿病和原发性高血压等慢性疾病的发生发展过程中扮演着重要角色。体内氧化物质(如氧化型低密度脂蛋白和一些化合物)参与了内皮功能损伤的病理过程。百草枯(1-1-二甲基-4-4-联吡啶二氯化物,paraquat)是一种快速灭生性除草剂,其毒性主要是来源于其代谢产物和氧化还原作用产生的活性氧(ROS),因此其常被用来诱导体内ROS生成,建立动物体内氧化损伤模型[1-6]。赖氨大黄酸(rhein lysinate,RHL)是本室对大黄酸进行结构改造获得的具有较好水溶性的化合物,体外研究[7]表明:低剂量(10 μmol·L-1)RHL能够保护过氧化氢诱导的脐静脉血管内皮细胞衰老,RHL延缓内皮细胞衰老的作用是通过清除活性氧自由基实现的。本课题组前期研究[8]还发现:RHL能够延长快速老化小鼠(SAMP10)的寿命,其延长寿命的作用也与清除活性氧自由基作用有关。但RHL对体内活性氧直接引起的血管内皮细胞损伤是否具有保护作用尚未见报道。本研究采用百草枯建立活性氧引起血管损伤模型,观察RHL对血管氧化损伤的保护作用,旨在为RHL应用于临床提供一定的实验依据。
1 材料与方法
1.1实验动物、主要试剂和仪器30只C57小鼠,雄性,体质量24~28 g,动物合格证号:SCXK(京)2013-0020,购自中国医学科学院动物所,在恒温(22℃)、相对湿度65%~70%、光照周期12 h∶12 h 环境中适应饲养1周后用于实验。RHL由本室合成,分子式为C21H22N2O8,相对分子质量430,纯度98%; 百草枯(上海Sigma公司);超氧化物歧化酶(SOD)测定试剂盒、谷胱甘肽过氧化物酶(GSH-Px)和丙二醛(MDA)测定试剂盒(南京建成生物工程研究所);HE染色试剂盒(北京经科宏达生物技术有限公司);DCFH-DA活性氧检测试剂盒(上海Sigma公司);eNOS 和caspase-3抗体(Cell Signaling 公司);β-actin(SC-16)、辣根过氧化物酶偶联二抗(北京中杉金桥公司)。可见光成像系统(日本Olympus公司),FAITH-4060全自动生化分析仪(中国南京劳拉电子有限公司),Spectra Max 190酶标仪(美国Molecular Device 公司),伯乐电泳转印系统和Quantily One分析软件(美国Bio-Rad 公司),Chemilmager 5500 凝胶成像系统(美国Alpha Innotech 公司)。
1.2实验动物分组及处理将小鼠随机分为对照组(n=10)、百草枯模型组(n=10)和RHL干预组(n=10)。RHL干预组小鼠在造模前1周灌胃给予RHL(50 mg·kg-1),每日1次,对照组和百草枯模型组灌胃给予等体积蒸馏水。百草枯模型组和RHL干预组腹腔注射百草枯(10 mg·kg-1),对照组腹腔注射等体积生理盐水,每周注射1次,持续2周。
1.3标本收集小鼠造模2周后,用10%水合氯醛麻醉,右心房采血,分离血清用于测定其MDA水平、SOD和GSH-Px活性。处死小鼠取腹主动脉(主动脉入横膈处向下1 cm),一部分置于10% 中性甲醛溶液中固定,用于HE 和DCFH-DA染色,剩余腹主动脉置于液氮中,冻透后转入-80℃冰箱中用于测定相关蛋白的表达。
1.4血清MDA水平、SOD和GSH-Px 活性测定MDA 水平检测采用硫代巴比妥酸法,SOD 活力检测采用联苯三酚自氧化法,GSH-Px 活性检测采用NADPH 偶联法。上述检测均严格按照试剂盒说明书操作。
1.5腹主动脉活性氧水平检测取一段腹主动脉在4℃ 预冷的生理盐水中反复清洗,置于OCT中包埋,放入液氮上冷却,-80℃冻存,经冰冻切片机切片后,按照DCFH-DA活性氧检测试剂盒说明书进行染色,在荧光显微镜下观察红色活性氧荧光强度,并拍照。
1.6腹主动脉病理学表现10% 中性甲醛固定腹主动脉,常规石蜡包埋,石蜡切片HE 染色后,显微镜下观察并拍照。
1.7Western blotting法检测血管损伤相关基因表达水平冻存的腹主动脉加入新鲜配制的组织裂解液(137 mmol·L-1NaCl,20 mmol·L-1Tris,pH 7.4,1% NP40,20% glycerol,10 mmol·L-1PMSF,1 mmol·L-1Na3VO4,10 mmol·L-1NaF,2.5 mg·L-1aprotinin,2.5 mg·L-1leupetin,phosphotase inhibitor cocktail),4℃下匀浆,冰上裂解15 min, 4℃、12 000 r·min-1离心20 min,取上清,BCA法检测蛋白浓度。等量蛋白(40 μg)于10% 十二烷基磺酸钠-聚丙烯酰胺凝胶电泳(SDS-PAGE)分离后,电转至聚偏二氟乙烯(PVDF)膜,5%脱脂牛奶 37℃封闭1 h,分别加入抗SIRT1(1∶1 000)、抗p21 (1∶1 000)、抗p16 (1∶1 000)及抗β-actin(1∶2 000)抗体,4℃孵育过夜。洗涤10 min×4次,用含辣根过氧化物酶(HRP) 标记的相应二抗(1∶5 000) 孵育1 h,洗膜15 min×4次,膜上滴加化学发光增强剂(Santa Cruz biotechnology SC-2048),按照试剂盒说明书操作,结果在凝胶成像仪上照相并使用Quantity One分析软件测量条带吸光度(A)值,目的条带与β-actin条带A值比值即为该目的蛋白的相对表达量。
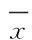
2 结 果
2.1小鼠一般情况和体质量与对照组比较,百草枯模型组小鼠活动减少,进食量减少。RHL干预组小鼠活动量和进食量较正常对照组虽有所下降,但高于百草枯模型组。与对照组[(28.5±1.8)g]比较,百草枯模型组[(25.3±2.8)g]和RHL干预组[(26.2±2.7)g]小鼠体质量下降(P<0.05)。
2.2各组小鼠血清中MDA 水平、SOD 和GSH-Px 的活性与对照组比较,百草枯模型组小鼠血清中SOD 和GSH-Px 活性明显降低(P<0.05),MDA 水平明显升高(P<0.05)。与百草枯模型组比较, RHL干预组小鼠血清中SOD 和GSH-Px 活性明显升高(P<0.05),MDA水平明显降低(P<0.05)。 RHL干预组小鼠血清中SOD水平升高,但与对照组比较差异无统计学意义(P>0.05)。见表1。
2.3DCFH-DA染色检测各组小鼠血管活性氧水平与对照组比较,百草枯模型组小鼠血管活性氧水平明显升高。与百草枯氧化损伤模型组比较, RHL干预组小鼠血管活性氧水平明显下降。见图1(插页三)。
2.4各组小鼠腹主动脉病理学表现与对照组比较,百草枯模型组小鼠血管组织可见明显水肿,内皮细胞中可见气球样变性,部分细胞脱落,细胞质颜色嗜碱性,中膜弹性膜增宽、排列松散、间隙增宽。 RHL干预组小鼠以上所述的病理变化明显减轻。各组小鼠血管组织炎细胞浸润均不明显。见图2(插页三)。
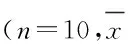
表1 各组小鼠血清MDA水平和SOD、GSH-Px活性
*P<0.05vscontrol group;△P<0.05vsparaquat model group.
2.5各组小鼠血管组织中eNOS和caspase-3表达与对照组比较,百草枯模型组小鼠血管eNOS和caspase-3表达水平明显下降(P<0.05),而剪切后 caspase-3表达水平升高(P<0.05)。与百草枯模型组比较, RHL干预组小鼠血管组织中 eNOS和caspase-3表达水平升高(P<0.05),剪切后caspase-3表达水平降低(P<0.05)。见图3和表2。
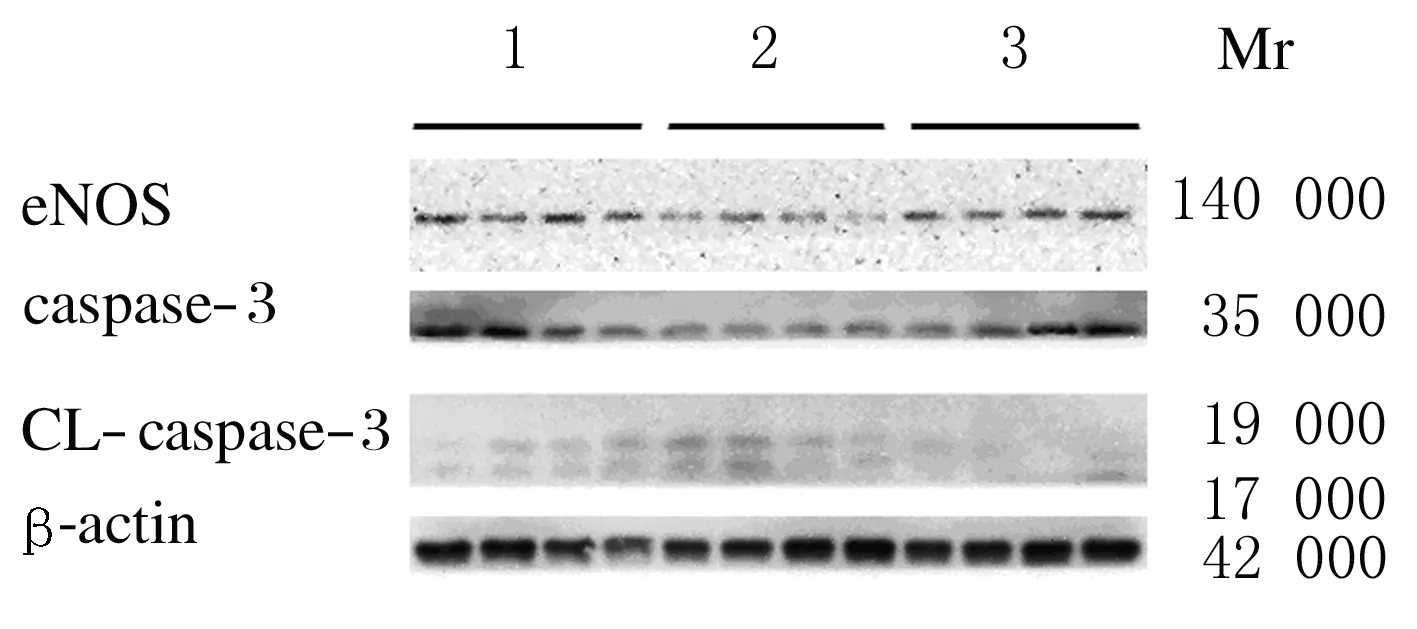
Lane 1:Control group;Lane 2:Paraquat model group;Lane 3:RHL prevention group.
图3各组小鼠血管凋亡相关基因表达检测电泳图
Fig.3Electrophoregram of expressions of apoptosis-related genes in blood vessel of mice in various groups

表2 各组小鼠血管凋亡相关蛋白的表达水平
CL:Cleaved.*P<0.05vscontrol group;△P<0.05vsparaquat model group.
3 讨 论
本课题组前期研究[7]发现:低剂量RHL能够清除活性氧自由基,保护脐静脉内皮细胞免受过氧化氢的损害。目前对于RHL是否能够清除动物体内产生的活性氧尚未见报道。本研究采用百草枯诱导动物体内氧自由基生成模型,探讨RHL是否对活性氧诱发的血管损伤存在保护作用。百草枯是一种快速灭生性除草剂,其毒性主要是来源于其代谢产物和氧化还原作用产生的活性氧[9-15]。本研究结果显示:与对照组比较,百草枯模型组小鼠体质量减轻、血清SOD和GSH-Px活性降低,而MDA水平升高;与百草枯模型组比较,RHL干预组血清SOD和GSH-Px活性升高,MDA水平降低。上述结果表明:RHL能够清除百草枯诱发的体内活性氧自由基生成。百草枯诱导血清活性氧水平升高,但是否也能诱导血管组织活性氧水平升高受到关注。本研究通过DCFH-DA染色观察血管活性氧水平结果表明:百草枯模型组血管活性氧水平明显升高,RHL干预后,活性氧水平明显降低。同时HE染色显示:血管组织活性氧水平升高后,出现部分内皮细胞脱落、弹性膜增宽和排列松散等病理变化,应用RHL干预能够逆转百草枯对血管组织的损伤。
凋亡在氧自由基诱导细胞损伤中发挥着重要作用,百草枯所致的氧化应激可诱导血管细胞凋亡。本研究采用Western blotting法检测在百草枯诱导血管细胞凋亡过程中哪些凋亡信号通路发生改变及RHL是否能够通过抗氧化应激作用阻止血管细胞凋亡,结果显示:百草枯模型组小鼠血管组织中caspase-3表达水平明显下降,而剪切后caspase-3表达升高。与百草枯模型组比较,应用RHL干预后caspase-3表达水平显著上调,剪切后caspase-3表达下调,说明caspase-3凋亡蛋白在百草枯诱导血管细胞凋亡和RHL保护血管免受氧化损伤中起着重要作用。本研究同时还显示:代表血管功能状况的eNOS表达水平在应用百草枯造模后也降低,而应用RHL干预后能够逆转。
总之,RHL能清除活性氧,降低活性氧自由基对血管内皮细胞和弹性膜的损伤。RHL有望成为预防心脑血管疾病的新药。
[1]Amirshahrokhi K.Anti-inflammatory effect of thalidomide in paraquat-induced pulmonary injury in mice [J].Int Immunopharmacol,2013,17(2):210-215.
[2]Harrison FE,Best JL,Meredith ME,et al.Increased expression of SVCT2 in a new mouse model raises ascorbic acid in tissues and protects against paraquat-induced oxidative damage in lung [J].PLoS One,2012,7(4):e35623.
[3]Kobayashi S,Kuwata K,Sugimoto T,et al.Enhanced expression of cystine/glutamate transporter in the lung caused by the oxidative-stress-inducing agent paraquat [J].Free Radic Biol Med,2012,53(12):2197-2203.
[4]Ahmad I,Shukla S,Kumar A,et al.Biochemical and molecular mechanisms of N-acetyl cysteine and silymarin-mediated protection against maneb- and paraquat-induced hepatotoxicity in rats [J].Chem Biol Interact,2013,201(1-3):9-18.
[5]Tan D,Wang Y,Bai B,et al.Betanin attenuates oxidative stress and inflammatory reaction in kidney of paraquat-treated rat [J].Food Chem Toxicol,2015,78:141-146.
[6]Medina-Leendertz S,Paz M,Mora M,et al.Longterm melatonin administration alleviates paraquat mediated oxidative stress in Drosophila melanogaster [J].Invest Clin,2014,55(4):352-364.
[7]Lin YJ,Zhen YZ,Wei J,et al.Effects of Rhein lysinate on H2O2-induced cellular senescence of human umbilical vascular endothelial cells [J].Acta Pharmacol Sin,2011,32(10):1246-1252.
[8]Hu G,Liu J,Zhen YZ,et al.Rhein lysinate increases the median survival time of SAMP10 mice:protective role in the kidney [J].Acta Pharmacol Sin,2013,34(4):515-521.
[9]Lima ME,Colpo AC,Salgueiro WG,et al.Ilex paraguariensis extract Increases lifespan and protects against the toxic effects caused by paraquat in Caenorhabditis elegans [J].Int J Environ Res Public Health,2014,11(10):10091-10104.
[10]Shokrzadeh M,Shaki F,Mohammadi E,et al.Edaravone decreases paraquat toxicity in a549 cells and lung isolated mitochondria [J].Iran J Pharm Res,2014,13(2):675-681.
[11]Podder B,Song KS,Song HY,et al.Cytoprotective effect of kaempferol on paraquat-exposed BEAS-2B cells via modulating expression of MUC5AC [J].Biol Pharm Bull,2014,37(9):1486-1494.
[12]Muralidhara HR.Prophylactic treatment with Bacopa monnieri leaf powder mitigates paraquat-induced oxidative perturbations and lethality in Drosophila melanogaster [J].Indian J Biochem Biophys,2010,47(2):75-82.
[13]Krucek T,Korandová M,erM,et al.Effect of low doses of herbicide paraquat on antioxidant defense in Drosophila [J].Arch Insect Biochem Physiol,2015,88(4):235-248.
[14]Muralidhara HR.Acute exposure of Drosophila melanogaster to paraquat causes oxidative stress and mitochondrial dysfunction [J].Arch Insect Biochem Physiol,2013,83(1):25-40.
[15]Yao R,Zhou Y,He Y,et al.Adiponectin protects against paraquat-induced lung injury by attenuating oxidative/nitrativestress [J].Exp Ther Med,2015,9(1):131-136.
[16]Utkan T,Yazir Y,Karson A,et al.Etanercept improves cognitive performance and increases eNOS and BDNF expression during experimental vascular dementia in streptozotocin-induced diabetes [J].Curr Neurovasc Res,2015,12(2):135-146.
Protective effect of rhein lysinate on blood vessel damage induced by oxidative stress in mice and its mechanism
FENG Qiusheng1,KAN Quan1,LYU Cuiping1,LI Ran1,WEI Jingbo1,ZHAO Yufang1,ZHEN Yongzhan1,2
(1. Department of Histology and Embryology,School of Basic Medical Sciences,North China University of Science and Technology,Tangshan 063000,China;2. Key Laboratory for Chronic Diseases of Hebei Province,Key Laboratory for Preclinical and Basic Research on Chronic Diseases of Tangshan City,School of Basic Medical Sciences,North China University of Science and Technology,Tangshan 063000,China)
ObjectiveTo investigate the protective effects of rhein lysinate (RHL) on the blood vessel damage induced by oxidative stress in the mice,and to explore its mechanism.MethodsThe mouse models of oxidative damage were established by intraperitoneal injection of paraquat.30 C57 mice were randomly divided into control,paraquat model,and RHL prevention groups.The mice in RHL prevention group were given RHL by gavage for one week before performing model.The mice in other two groups were given equal volume of distilled water.For making model,paraquat was intraperitoneally injected in the mice in paraquat model and RHL prevention groups once a week for two weeks.The activities of superoxide dismutase (SOD) and glutathione peroxidase(GSH-Px) and the content of serum malonaldehyde (MDA) of the mice were detected 2 weeks after modeling.The pathological profile of blood vessel was observed by hematoxylin and eosin (HE) staining and the level of reactive oxygen species was observed by DCFH-DA staining.The expressions of genes related to blood vessel damage were detected by Western blotting method.ResultsCompared with control group,the activities of SOD and GSH-Px were decreased and the content of MDA was increased in paraquat model group (P<0.05).Compared with paraquat model group,the activities of SOD and GSH-Px were increased and the content of MDA was decreased in RHL prevention group (P<0.05).The pathological examination indicated the structure of blood vessel of the mice was damaged and the level of reactive oxygen species of blood vessel was increased (P<0.05) in paraquat model group.The pathological changes were significantly improved and the level of reactive oxygen species of blood vessel of the mice was decreased (P<0.05) in RHL prevention group.The Western blotting analysis showed that compared with control group,the expression levels of nitric oxide endothelial synthase (eNOS) and caspase-3 of the mice in paraquat model group were decreased (P<0.05),however the expression level of cleaved fragment of caspase-3 was increased (P<0.05).Compared with paraquat model group,the expression levels of eNOS and caspase-3 of the mice in RHL prevention group were increased (P<0.05) and the expression level of cleaved fragment of caspase-3 was decreased (P<0.05).ConclusionParaquat could induce vascular cell damageinvivothrough increasing the levels of reactive oxygen species,and RHL could antagonize the effects of paraquat by scavenging reactive oxygen species,and up-regulating the eNOS expression and reducing the expression of the cleaved fragment of caspase-3.
rhein lysinate; paraquat; reactive oxygen species; apoptosis
1671-587Ⅹ(2015)06-1171-05
10.13481/j.1671-587x.20150614
2015-02-27
河北省科技厅自然科学基金资助课题(H2012401030);河北省唐山市科学技术研究与发展指导计划项目资助课题(10130267c)
冯秋生(1974-),男,河北省唐山市人,在读医学硕士,主要从事心脑血管疾病发病机制和治疗方面的研究。
甄永占,副教授,硕士研究生导师(Tel:0315-3725754,E-mail:yongzhanzhen@126.com)
R285.5
A

