UPLC/MS/MS检验尿液中的扎来普隆和5-氧-扎来普隆
张蕾萍,黄 霜,舒翠霞,任昕昕,崔冠峰,栾玉静,杜鸿雁
(1.公安部物证鉴定中心 北京市现场物证检验工程技术研究中心,北京 100038;2.泰州市公安局,江苏 泰州225300;3.苏州市公安局,江苏 苏州 215000)
UPLC/MS/MS检验尿液中的扎来普隆和5-氧-扎来普隆
张蕾萍1,黄 霜2,舒翠霞3,任昕昕1,崔冠峰1,栾玉静1,杜鸿雁1
(1.公安部物证鉴定中心 北京市现场物证检验工程技术研究中心,北京 100038;2.泰州市公安局,江苏 泰州225300;3.苏州市公安局,江苏 苏州 215000)
目的 建立尿液中扎来普隆和5-氧-扎来普隆的液相色谱-串联质谱检验法。方法 尿液用乙腈直接沉淀蛋白并通过96孔板去磷酯后,选用ZORBAX Eclipse Plus C18色谱柱,以0.1%甲酸水(A相)和乙腈(B相)作为流动相,进行梯度洗脱分离。采用液相色谱-串联质谱仪的电喷雾电离,正离子模式扫描、多反应监测(MRM)模式检测扎来普隆及5-氧-扎来普隆,并用外标法定量。结果 该方法可有效分离尿液中的扎来普隆及5-氧-扎来普隆,保留时间分别为2.48min和1.96min,样品检验时间仅需4min。尿液中扎来普隆及5-氧-扎来普隆分别在0.1~50ng/mL和0.25~50ng/mL范围内线性关系良好,回归方程分别为y=70393x+33700 和y= 34491x+16854,检出限分别为0.05ng/mL和0.1ng/mL。扎来普隆及5-氧-扎来普隆的回收率均在90%以上,日内与日间精密度均小于10%。结论 本文所建方法简便、快速、分离度好,适用于尿液中的扎来普隆和5-氧-扎来普隆检测。
法医毒物分析;扎来普隆;5-氧-扎来普隆;液相色谱-串联质谱
DOΙ: 10.16467/j.1008-3650.2015.02.009
扎来普隆(zaleplon),商品名曲宁,是非苯二氮卓类催眠药的代表药物之一,由于吸收完全且迅速,催眠效果好,使用人群不断增加。近年来法庭科学检验鉴定中出现此类毒物的频率越来越多,由于其具有滥用性,2007年国际法庭毒理协会会议将扎来普隆列为新型滥用药物之一。扎来普隆口服治疗量为10~20mg,作用持续6h。其进入体内后,迅速分布在所有组织,经CYP3A4 酶代谢,转化为非活性5-氧-扎来普隆等代谢产物,有极少部分(<0.1%)以原药形式从尿液排出[1]。在一些麻醉抢劫案中,由于案发至报案的时间间隔较长,原体已从体内排出而使检验结果呈阴性,如果检验代谢物则能提高检出率,还原案件的客观情况。国外可见扎来普隆及其代谢物的液相色谱及免疫法检验研究[2],国内可见生物样品中扎来普隆原体的液相色谱[3]、液相色谱-质谱检验法[4,5],国内外均未见到扎来普隆及其代谢物液相色谱-质谱检验方法的报道。
在麻醉抢劫案件中,尿液是最常用的检材样品之一,由于毒物在尿液中具有更长的检验时间窗口,因此尿液比血液或血清等更具有特殊优势。本文针对办案需求,研究建立了尿样中扎来普隆及其代谢物5-氧-扎来普隆的液相色谱-质谱定性定量检验方法,以提高案件中扎来普隆的检出率。该方法灵敏度高,检出限低,可满足日常检案的需要。扎来普隆分子式为C17H15N5O,分子量为305,分子结构见图1。5-氧-扎来普隆分子式为C17H15N5O2,分子量为321,分子结构见图2。
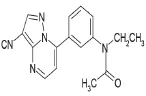
图1 扎来普隆分子结构Fig.1 Molecular structure of zaleplon

图2 5-氧-扎来普 隆分子结构Fig.2 Molecular structure of 5-O-zaleplon
1 材料与方法
1.1 试剂、材料和仪器
标准溶液的配制:扎来普隆和5-氧-扎来普隆对照品,含量 98%,购于加拿大Toronto化学公司,配制成1mg/mL乙腈溶液作为储备液。精确量取标准储备液1.0mL于10mL容量瓶中,用乙腈定容至刻度,配制成100.0µg/mL的标准工作液。实验中所用其它浓度的标准溶液均从上述标准工作溶液中用乙腈稀释制备。
主要材料:96孔去磷酯板ISOLUTE®PLD+ 50mg 96孔板(瑞典Biotage公司)。
主要仪器:串联质谱仪API 5500 QTRAP(美国Applied Biosystems公司),配以离子源ESI,三重四极杆质量分析器;液相色谱仪Shimadzu 30A-LC(日本Shimadzu公司);Shimadzu 30A自动进样器(日本Shimadzu公司)。
空白样品:尿液,采自未服用过药物的健康志愿者。
1.2 样品及处理
取尿液0.2mL,加入0.8mL乙腈,混匀振荡10min,8000rpm离心10min,取上清液400µL经0.22µm有机膜过滤,滤液供仪器分析,剩余上清液过PLD+板,负压抽真空过膜,滤液供仪器分析。
1.3 UPLC/MS/MS检测
液相色谱条件 色谱柱:ZORBAX Eclipse Plus C18(2.1mm×100mm,1.8µm) 柱;进 样 量:2μL;流速:0.4mL/min;流动相A: 含0.1%甲酸水;流动相B:乙腈。梯度洗脱,洗脱条件见表1。
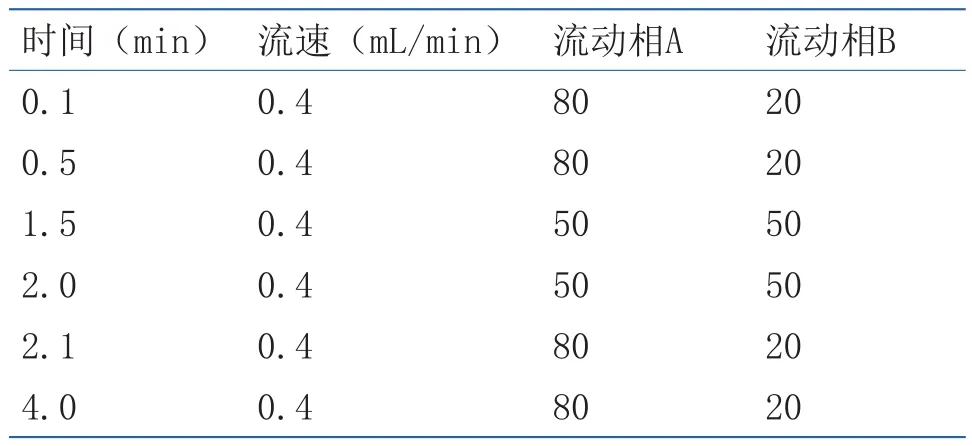
表1 液相色谱的梯度洗脱条件Table1 Gradient elution conditions of liquid phase
质谱条件 电喷雾离子源(ESI);离子化模式:ESI+,MRM扫描模式;离子源电压:5500V;源温度:600℃;气帘气:30psi,雾化气:55psi,辅助气:50 psi。
2 实验部分
2.1 液质条件的优化选择
扎来普隆和5-氧-扎来普隆用乙腈分别配制成50ng/mL的标准溶液,用针泵ESI+直接进样,确定母离子的质量数。在此基础上选择强度最大的前3个子离子,对碰撞能量CE值进行比较优化,通过逐渐改变碰撞能量,使母离子强度约占最大子离子强度的1/3~1/4。最终选定母离子与强度最高的2个子离子,组成2对母/子离子对作为定性分析的指标,以最强离子对作为定量分析指标。在此基础上逐步优化去簇电压、碰撞能量等参数。检测离子对,去簇电压,碰撞能量、保留时间见表2。
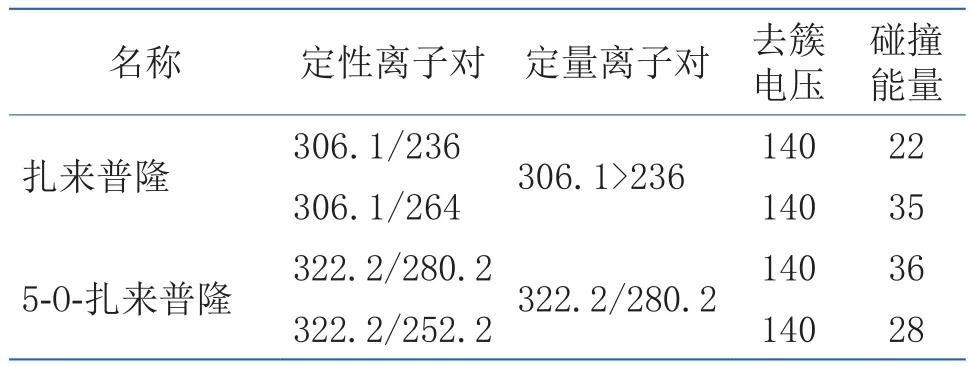
表2 扎来普隆及5-氧-扎来普隆的质谱参数Table2 Mass spectrum parameters of zaleplon and 5-O-zaleplon
在液相条件的优化中,本文比较了Waters ACQUITY UPLC®BEH C18(2.1 mm×100mm,1.7µm)和ZORBAX Eclipse Plus C18(2.1mm×100mm,1.8µm)色谱柱,前者的5-氧-扎来普隆的峰形较宽且有拖尾,后者峰形陡峭良好,灵敏度较高(见图3和图4)。

图3 Waters ACQUITY UPLC®BEH C18柱色谱图Fig.3 Chromatogram of Waters BEH C18 column
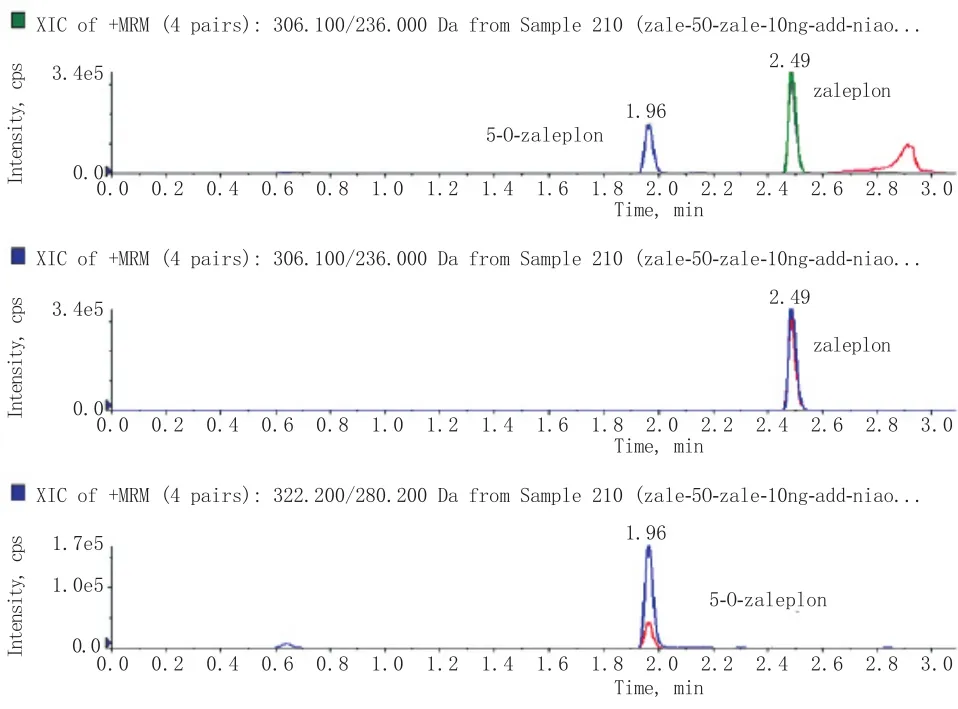
图4 ZORBAX Eclipse Plus C18柱色谱图Fig.4 Chromatogram of Eclipse Plus C18 column
在流动相方面,本文比较了水(A)和乙腈(B)、0.1% 甲酸水(A)和0.1% 甲酸乙腈(B)、0.1% 甲酸水(A)和乙腈(B)三种流动相对分离检测的影响。结果表明,使用第一种流动相时,溶剂效应对5-氧-扎来普隆的影响较大导致色谱峰变形,且保留时间不稳定易飘移;采用第二种流动相0.1% 甲酸水(A)和0.1% 甲酸乙腈(B)时,两目标物保留时间较近,不易完全分离。而采用乙腈-0.1% 甲酸水作为流动相,不仅两目标物分离良好,而且可消除溶剂效应带来的色谱峰变形和保留时间飘移现象,使色谱峰峰形良好,保留时间一致(见图4)。此外,本文还比较了不同流动相梯度条件(流动相B的比例:①初始体积10%保持1min后,增至90%保持1min,下降至10%并保持2min;②初始体积10%保持1min后,增至50%保持1min,下降至10%并保持2min;③初始体积20%保持0.5min后,缓慢增至50%保持0.5min,下降至10%并保持2min)。最终确定梯度③的条件,确保两目标物分离良好,且保留时间稳定。
在选定的仪器条件下,扎来普隆及5-氧-扎来普隆具有良好的色谱峰,保留时间分别为2.48min和1.96min(见图7和图8)。在该色谱条件下分析尿样无干扰,表明该方法具有较好专属性,色谱峰的峰形及分离良好。

图5 采用第一种流动相的色谱图Fig.5 Chromatogram of 1st mobile phase

图7 扎来普隆的提取离子流图(RT 2.48min)Fig.7 Extract ion chromatograph of zaleplon (RT2.48min)

图8 5-氧-扎来普隆的提取离子流图(RT 1.96min)Fig.8 Extract ion chromatograph of 5-O-zaleplon (RT1.96min)
2.2 方法学考察
2.2.1 前处理方法PLD+96孔板的作用是去磷酯作用,本文比较了乙腈沉淀蛋白后和过PLD+96孔板后的样品回收率。结果显示,过PLD+96孔板后的平均回收率略高于乙腈沉淀蛋白的平均回收率 (见表3),说明乙腈沉淀蛋白后再过PLD+96孔板有助于降低生物检材中基质对目标物的影响,可提高扎来普隆和5-氧-扎来普隆回收率。

表3 乙腈沉淀蛋白和过PLD+96孔板的平均回收率Table3 Average recovery rate of acetonitrile protein precipitation and PLD + 96-well plates
2.2.2 标准曲线与灵敏度 将标准储备液用初始流动相稀释成0.01、0.05、0.10、1.00、10.00、50.00ng/ mL的扎来普隆和5-氧-扎来普隆系列标准液,仪器进样2µL分析。以标准溶液浓度为横坐标,色谱峰面积为纵坐标做标准线性回归,得到回归方程。扎来普隆和5-氧-扎来普隆回归方程分别为:y=85260x+16713,y=47139x-15106;相关系数分别为R2= 0.9996,R2= 0.9993。扎来普隆和5-氧-扎来普隆标准线性范围为0.01~50ng/mL、0.05~50ng/mL,灵敏度分别为0.01ng/ mL和0.05ng/mL。
2.2.3 工作曲线与检出限 取空白尿样品各0.2mL 5份,分别加入扎来普隆和5-氧-扎来普隆系列标准液制备成添加尿液样品,使浓度分别为0.1、0.25、1、10、50ng/mL。按1.2处理并进行仪器分析。以浓度对相应的峰面积进行线性回归,得到回归方程分别为:y=70393x+33700,y=34491x+16854;扎来普隆在0.1~50ng/mL线性范围内,相关系数R2=0.9984, 5-氧-扎来普隆在0.25~50ng/mL。线性范围内相关系数R2=0.9987;按信/噪比(S/N)≥3,计算得尿液中扎来普隆和5-氧-扎来普隆定量检测限为0.1ng/mL和0.25ng/mL,检出限为0.05ng/mL和0.1ng/mL。
2.2.4 回收率与精密度 取空白尿样品0.2mL,分别加入扎来普隆和5-氧-扎来普隆系列标准液制备成添加尿液样品,使浓度为0.1、1、50ng/mL,每个浓度平行操作5份。按1.2处理并进行仪器分析。测得的峰面积和对应浓度的标准品峰面积的比率作为回收率,平均回收率分别为91.4%和101.3%。
按照以上方法配制高、中、低3种浓度的样品进行萃取检测,按1.2处理并检测。每一浓度平行5份,在同一天内三个不同时间进行检测,计算目标物峰面积的相对标准偏差,得到日内精密度,两目标物结果均小于5%。按照同样方法配制三种浓度的样本进行萃取检测,每天测一批,连续测5d,计算目标物峰面积相对标准偏差,得到日间精密度,两目标物结果均小于10%(见表4)。

表4 扎来普隆和5-氧-扎来普隆的精密度表Table4 Precision of zaleplon and 5-O-zaleplon
3 结果与讨论
通过以上实验研究,本文建立的扎来普隆和5-氧-扎来普隆的直接沉淀蛋白-过磷脂板-液相色谱-质谱检验方法,操作简单,检出限低,回收率高,选择性强,可用于尿液中扎来普隆的染毒检验鉴定。该检验方法的广泛应用一方面可提高中毒事件和刑事案件的检验中扎来普隆的检出率,另一方面也可用于医疗检验部门对药物使用监管进行检测。
[1] 张蕾萍,于忠山,何毅,等.法庭科学中的新型催眠药[J].刑事技术,2008(5): 37-40.
[2] Kawashima K, Hosoi Ki, Maruke T, et al.Aldehyde oxidasedependent marked species difference in hepatic metabolism of the sedative-hypnoticzaleplon between monkeys and rats[J].Drug Metabolism and Disposition, 1999, 27(3): 422-428.
[ 3] 张蕾萍,于忠山,何毅,等.固相萃取/气相色谱法检测全血中的扎来普隆[J].中国法医学杂志,2011, 2 6(1): 30-32.
[4] 张蕾萍,于忠山,何毅,等.采用气质联用负化学源分析痕量催眠药扎来普隆[J].分析实验室,2010, 29: 55-56.
[5] 吴海,晏晓军,高汨,等.HPLC-MS/MS同时检测全血中佐匹克隆、唑吡坦和扎来普隆[J].刑事技术,2013(2): 22-24.
引用本文格式:张蕾萍,黄霜,舒翠霞,等.UPLC/MS/MS检验尿液中的扎来普隆和5-氧-扎来普隆[J].刑事技术,2015,40(2):122-126.
Determination of Zaleplon and 5-O-zaleplon in Urine by UPLC/MS/MS
ZHANG Lei-ping1, HUANG Shuang2, SHU Cui-xia3, REN Xin-xin1, CUΙ Guan-feng1, LUAN Yu-jing1, DU Hong-yan1
(1.Beijing Engineering Research Center of Crime Scene Evidence Examination, Ιnstitute of Forensic Science, Ministry of Public Security, Beijing 100038, China; 2.Taizhou Public Security Bureau, Jiangsu Taizhou 225300, China; 3.Suzhou Public Security Bureau, Jiangsu Suzhou 215000, China.)
Objective Zaleplon, a pyrazolopyrimidine drug, is one of non-benzodiazepine sedative hypnotic drugs.As a new kind of hypnotic, it is an improvement of traditional benzodiazepines in treatment of insomnia.Ιn 2007, the Ιnternational Association of Forensic Toxicologists (TΙAFT) listed zaleplon as one of the new type of abuse drugs since it was often used in drug-facilitated cases.Zaleplon acted rapidly, and victims were less able to recall the circumstances under which the offence occurred due to its amnesic properties.The hypnotic acts within 0.5h and has short half-life (0.9~1.1h).Because its ultra-short half-life, low frequency of use, and short window of detection, zaleplon has been detected in few clinical and forensic cases.Urine samples are most likely to be useful in cases of drug-facilitated crimes as its detection window is longer than that of blood or plasma samples.5-O-zaleplon is a main metabolite of zaleplon in biological body.Ιn order to improve detectable rate of zaleplon poisoning cases, we established an ultra-performance liquid chromatography tandem mass spectrometry (UPLC/MS/ MS) assay for simultaneous determination of zaleplon and its main metabolite 5-O-zaleplon in urine samples.Methods Aliquots of 0.2mL urine samples were used for the analysis.Zaleplon and its main metabolite 5-O-zaleplon were extracte d with twostep method using acetonitrile to precipitate protein and 96-well plates to deplete phospholipid.The separation was performed on a ZORBAX Eclipse Plus C18 (2.1mm×100mm, 1.8μm) analytical column by Shimadzu 30A-LC ultra-performance liquid chromatography.The MS/MS analysis was performed on APΙ 5500 QTRAP tandem mass spectrometry.The column temperature was maintained at 40, while the sample plate was maintained at 4.By comparing different chromatographic column, different mobile phase, different gradient, and optimization conditions of mass spectrometry, we established the liquid chromatographytandem mass spectrometry method.The mobile phases consisted of acetonitrile (mobile phase B) and water containing 0.1% formic acid (mobile phase A), and the fl ow rate was 0.4mL/min.MS-MS in the multiple reaction monitoring (MRM) mode via positive electrospray ionization mode (ESΙ+) was used.The retention times and the two parent/daughter ion pairs were used for qualitative analysis, and the fi rst parent/daughter ion pair was used for quantitative analysis.Results Zaleplon and 5-O-zaleplon in urine samples were separated well.There were no interferences of endogenous impurity.The chromatographic separation time was 4 min.Calibration curve of zaleplon was linear within the range of 0.1~50ng/mL and calibration curve of 5-O-zaleplon was linear within the range of 0.25~50ng/mL.Regression equations were y=70393x+33700 and y=34491x+16854, respectively.The limits of detection for zaleplon and 5-O-zaleplon were 0.05ng/mL and 0.1ng/mL.The recoveries of zaleplon and 5-O-zaleplon were more than 90%.The inter-day and intra-day precisions were less than 10%.Conclusions This method is rapid, sensitive and effective.Ιt is suitable for determination of zaleplon and its main metabolite 5-O-zaleplon in urine sample.Ιt can be a solution to prolong the window of detection and applied to forensic toxicological analysis.
forensic toxicological analysis; zaleplon; 5-O-zaleplon; UPLC/MS/MS
DF795.1
A
1008-3650(2015)02-0122-05
公安部重点研究计划项目(No.201302ZDYJ002);公安技术交流培训计划项目(No.B2014004A)
张蕾萍(1974—),女,山西太原人,副研究员,硕士,研究方向为毒物分析。 E-mail:zlpbjft@sohu.com
2014-11-24

