PS-PNVP嵌段共聚物的合成及其对CaCO3形貌的调控
王 佳,王燕萍,王依民
(东华大学 a.材料科学与工程学院;b.纤维材料改性国家重点实验室,上海 201620)
PS-PNVP嵌段共聚物的合成及其对CaCO3形貌的调控
王佳a,王燕萍b,王依民b
(东华大学 a.材料科学与工程学院;b.纤维材料改性国家重点实验室,上海 201620)
采用原子转移自由基聚合(ATRP)和可逆加成-断裂链转移聚合(RAFT)两步法合成了两亲性嵌段共聚物聚苯乙烯 -b- 聚乙烯基吡咯烷酮(PS-PNVP).经核磁共振氢谱(1H-NMR)、傅里叶变换红外光谱(FTIR)、凝胶渗透色谱(GPC)方法确认了PS-PNVP的结构组成、相对分子质量及相对分子质量分布.采用气相扩散法,研究了PS-PNVP胶束溶液对CaCO3晶体的矿化及调控作用.采用扫描电子显微镜(SEM)表征了矿化所得CaCO3晶体的形貌.通过调整矿化温度和改变CaCl2溶液的浓度,获得了形貌丰富的多级结构CaCO3晶体.研究表明,以PS-PNVP胶束作为模板,可制备出形貌更复杂、结构更具层次的大尺度CaCO3晶体.
碳酸钙粒子; 形貌; 胶束; 生物矿化
无论是在自然界还是在工业上,CaCO3都是一种重要的生物矿物.CaCO3的仿生矿化合成对于深入理解生物矿化的机理具有重要的意义,因此,其一直是仿生矿化研究领域的重要研究方向之一[1].
生物体通过天然的生物大分子(蛋白质、多肽等)超分子组装,控制矿化过程,可形成具有一定结构、尺寸、形态及独特性能的CaCO3.这些生物大分子不仅能提高CaCO3的力学性能,而且还能影响矿化过程,甚至影响其同质多晶型[2].根据生物矿化过程中有机基质调控无机物种生长的原理,近年来,各种有机添加剂被用来调控无机物种的生长,并在温和的条件下获得了具有丰富形貌和跨越多级尺度结构的晶体.这些有机添加剂包括生物大分子[3-5]、小分子有机物[6]、凝胶体系[7]、双亲水嵌段共聚物[8-10]等.
两亲性嵌段共聚物分子结构中含有亲水链段和疏水链段,其作为模板或添加剂使用,可以控制CaCO3的成核、生长和聚集[11-12].两亲性嵌段共聚物自组装形成的胶束溶液有球、棒、囊泡、大复合胶束等多种形态[13-14],以其为模板,有望制备出形貌更为复杂、结构更具层次的大尺度CaCO3晶体.
本文经原子转移自由基聚合(ATRP)和可逆加成-断裂链转移聚合(RAFT)两步法,合成以聚乙烯基吡咯烷酮(PNVP)为亲水链段、聚苯乙烯(PS)为疏水链段的两亲性嵌段共聚物聚苯乙烯-b-聚乙烯基吡咯烷酮(PS-PNVP),并以其胶束为模板,研究CaCl2浓度和矿化温度对CaCO3形貌的影响.通过气相扩散法,在嵌段共聚物胶束的调控下,得到不同形貌多级结构的CaCO3晶体,为仿生矿化制备无机材料提供有效的途径.
1 试 验
1.1原料与试剂
N- 乙烯基吡咯烷酮(NVP,Aldrich),用前减压蒸馏,苯乙烯(用前减压蒸馏),溴化亚铜,溴化苄,2,2- 联吡啶,乙基黄原酸钾,偶氮二异丁腈(AIBN),CaCl2,碳酸氢铵,均来源于国药集团化学试剂有限公司.
1.2PS-PNVP的合成
ATRP法制备大分子引发剂PS-Br:在100 mL烧瓶中依次加入苯乙烯40 mL、 2,2- 联吡啶1.386 g、 溴化亚铜0.698 g、溴化苄0.386 g.体系通氮气30 min除氧,将烧瓶置于110 ℃油浴中反应8 h.产物用四氢呋喃(THF)溶解后,过Al2O3柱去除催化剂络合物等杂质.用甲醇沉淀并洗涤,真空干燥后得到白色固体粉末PS-Br.
PS-Br端基改性:将2 g乙基黄原酸钾加入到含有40 mL丙酮的圆底烧瓶中,在搅拌的同时逐滴加入含10 g PS-Br的丙酮溶液60 mL,于30 ℃反应48 h.经甲醇沉淀、洗涤、真空干燥得大分子链转移剂PS-Xanthate.
RAFT法制备嵌段共聚物PS-PNVP:将5 g大分子链转移剂PS-Xanthate、 5 mL 的NVP、 0.007 g 的AIBN、 10 mL的THF加入到50 mL圆底烧瓶中,通氮气30 min除去体系中的氧,置于60 ℃油浴中反应18 h.产物在石油醚中沉淀,再用甲醇洗涤,真空干燥得两亲性嵌段共聚物PS-PNVP.
1.3CaCO3的矿化
取30 mg两亲性嵌段共聚物PS-PNVP,溶于10 mL N,N′- 二甲基甲酰胺(DMF) 共溶剂中,在室温缓缓搅拌下用微量进样器逐滴加水,直至溶液呈蓝色为止.将上述胶束溶液转移到透析袋中透析3 d.取10 mL的平头胶束溶液转移到50 mL的烧杯中,在搅拌状态下向胶束溶液中加入定量的CaCl2.将烧杯置于干燥器底部,以一定量的NH4HCO3为CO2扩散源.密封干燥器,并转移到恒温培养箱中,保持一定的温度,静置矿化7 d.产物用去离子水和乙醇洗涤,自然干燥.
1.4测试与表征
傅里叶变换红外光谱(FTIR)测试:采用美国Nicolet NEXUS-670型红外光谱仪对聚合物的结构进行表征,KBr压片法测试.
核磁共振氢谱(1H-NMR)测试:采用瑞士Bruker Acance 400型核磁共振波谱仪对聚合物的结构和嵌段比进行表征,溶剂为CDCl3.
凝胶渗透色谱(GPC)测试:采用美国Waters BI-MWA 型凝胶渗透色谱仪对聚合物的相对分子质量和相对分子质量分布进行表征,色谱级THF为流动相,聚苯乙烯校正.
扫描电子显微镜(SEM)测试:采用日本JEOL JSM-5600LV型扫描电镜观察矿化所得CaCO3的形貌.
2 结果与讨论
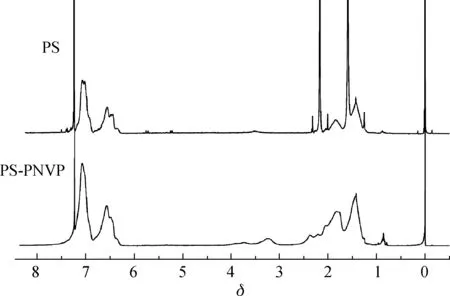
图1 PS和 PS-PNVP嵌段共聚物的1H-NMR谱图Fig.1 1H-NMR spectra of PS and PS-PNVP block copolymer
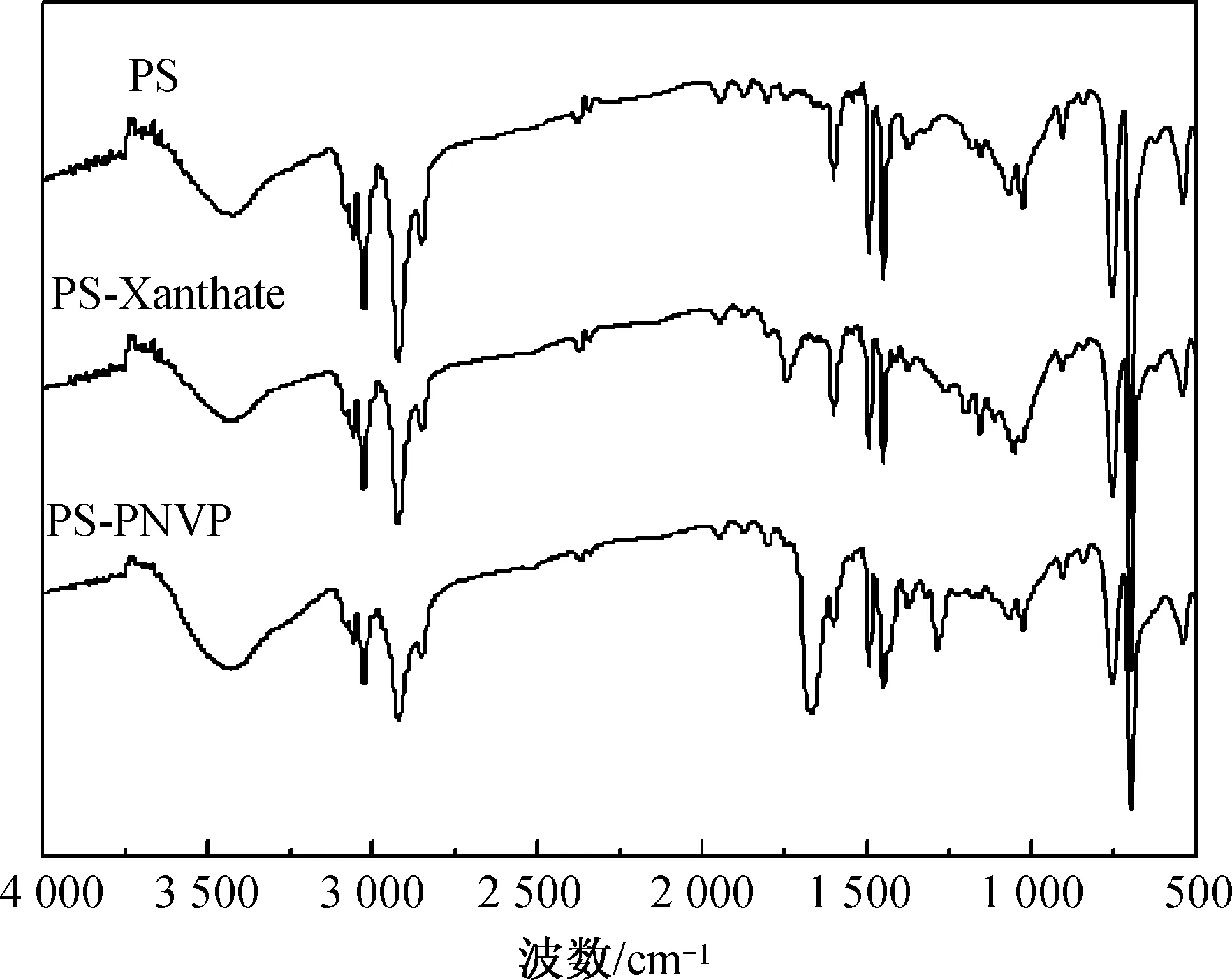
图2 PS,PS-Xanthate,PS-PNVP嵌段共聚物的FTIR谱图Fig.2 FTIR spectra of PS,PS-Xanthate and PS-PNVP block copolymer
当不加任何添加剂时,在纯水溶液中形成的是典型的斜方六面体型方解石相碳酸钙[15-16].温度为30 ℃、胶束质量浓度为1.0 g/L的矿化条件下,不同CaCl2浓度下得到的CaCO3粒子形貌如图3所示.从图3可以看出,PS-PNVP胶束的存在对CaCO3晶体的形貌有影响.在PS-PNVP胶束调控下形成的CaCO3粒子为有序堆积的片层晶体与菱形晶体的混合产物.片层晶体是薄片叠加形成的,菱形晶体则未受到胶束的调控.随着CaCl2浓度的增加,片层结构CaCO3粒子的含量及尺寸也随之增长.
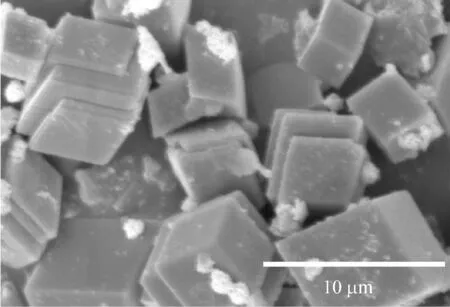
(a) 20 mmol/L
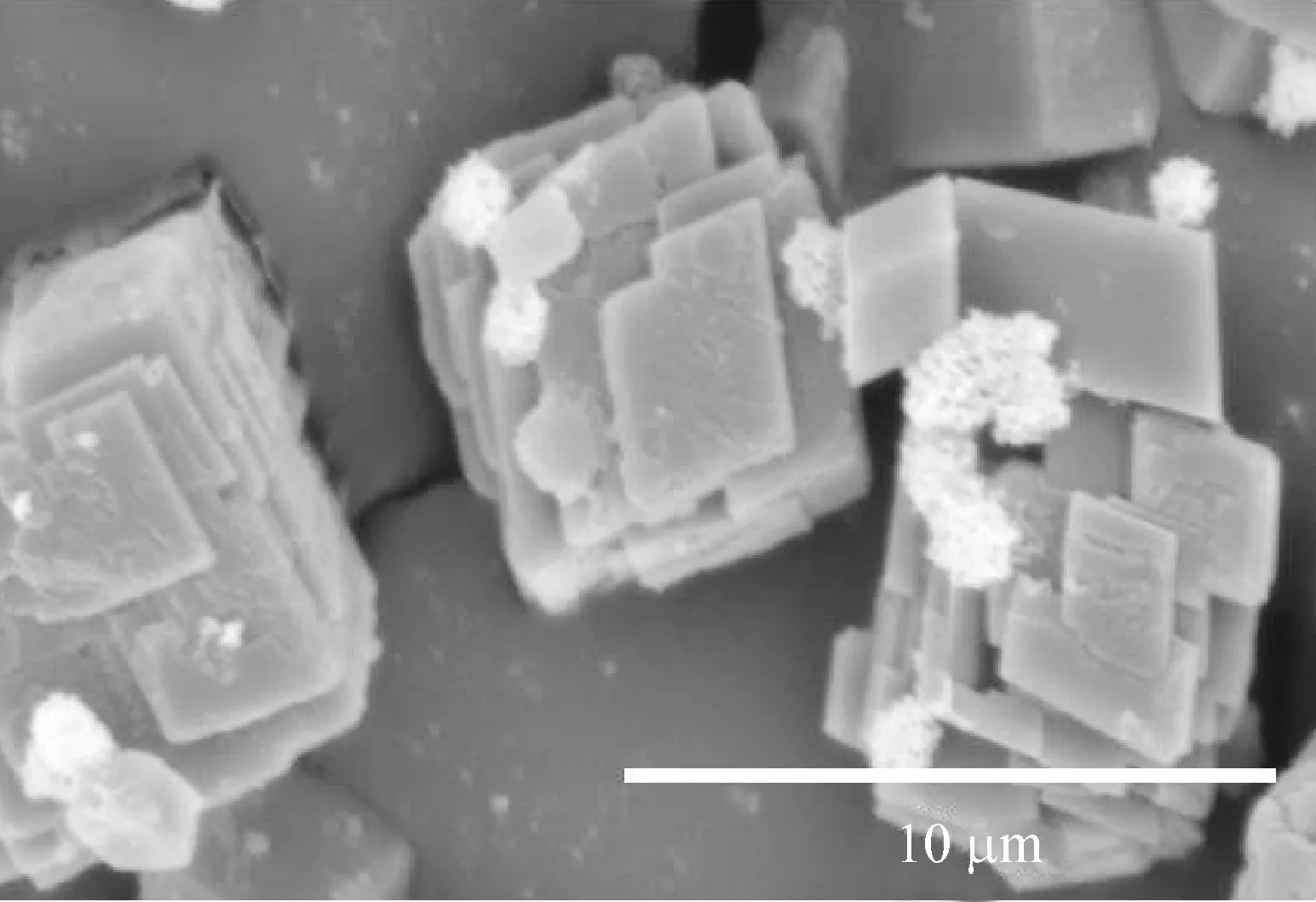
(b) 50 mmol/L
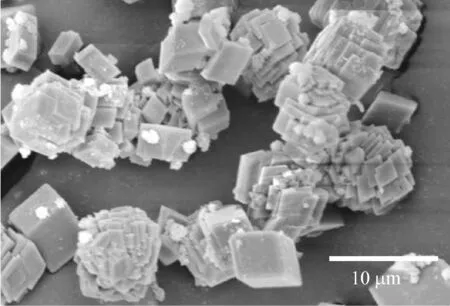
(c) 100 mmol/L

(d) 200 mmol/L图3 30 ℃下不同CaCl2浓度调控下CaCO3粒子的SEM图Fig.3 SEM images of CaCO3 particles obtained with different CaCl2 concentrations at 30 ℃
矿化温度为18 ℃、胶束质量浓度为1.0 g/L时,改变CaCl2浓度调控得到的CaCO3粒子形貌如图4所示.
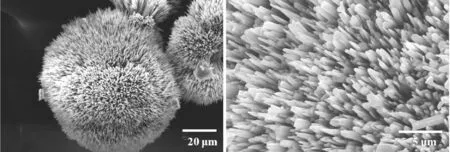
(a) 10 mmol/L
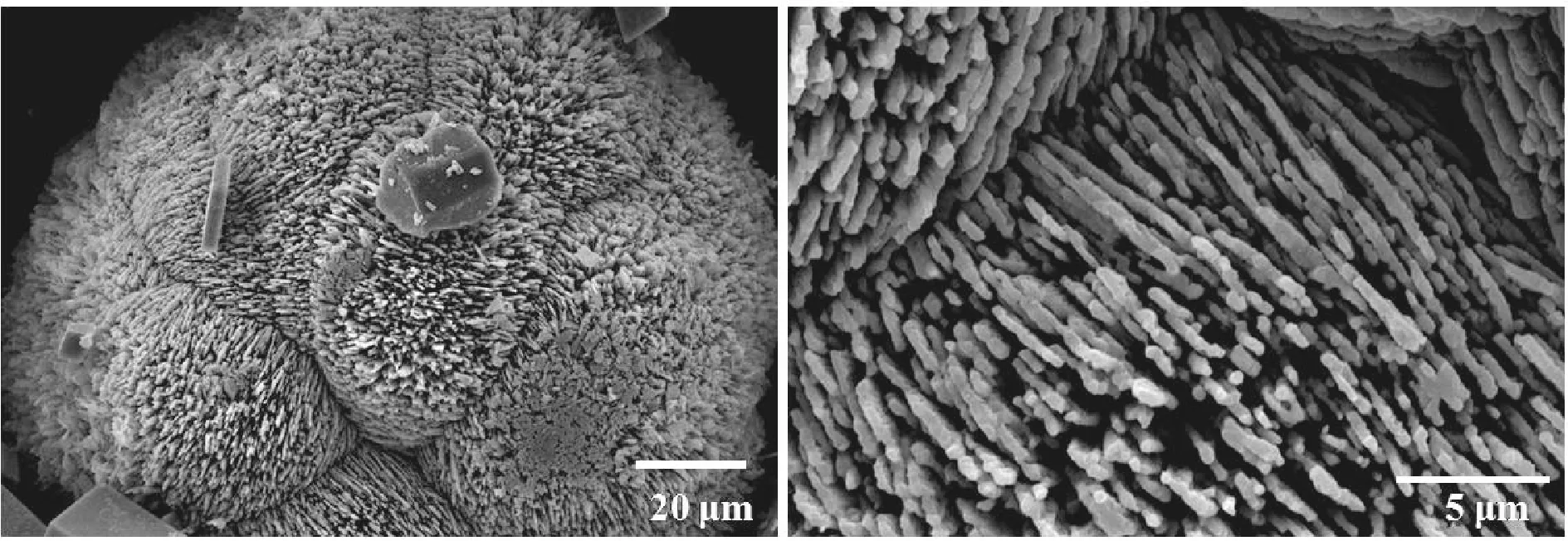
(b) 20 mmol/L

(c) 30 mmol/L
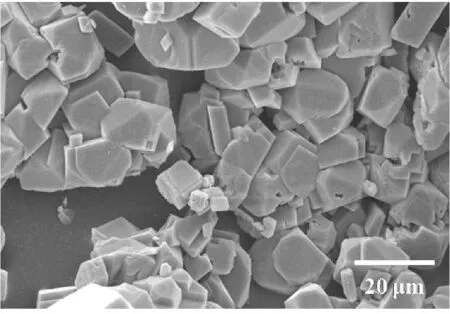
(d) 50 mmol/L
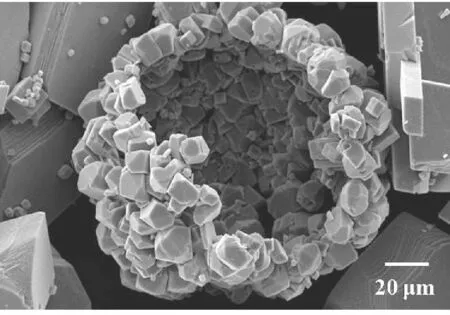
(e) 100 mmol/L图4 18 ℃下不同CaCl2浓度调控下CaCO3粒子的SEM图Fig.4 SEM images of CaCO3 particles obtained with different CaCl2 concentrations at 18 ℃
由图4(a)可知,CaCl2浓度为10 mmol/L时,矿化得到由无数纳米棒组成的直径为70~80 μm的刺球.由图4(b)可知,CaCl2浓度增加到20 mmol/L时,CaCO3粒子的尺寸进一步增长,由取向不同的纳米片的聚集体形成的球状粒子.由图4(c)可知,CaCl2浓度为30 mmol/L时,矿化得到片状多层结构的球形晶体,晶片厚度约为0.5 μm.当CaCl2浓度为50 mmol/L时,矿化所得CaCO3粒子的形貌为多面体粒子形成的聚集体,如图4(d)所示.CaCl2浓度增大到100 mmol/L时,形成了以多面体粒子为结构单元的空心球,如图4(e)所示.对比图3和4可知,与温度为30 ℃条件下矿化时得到的CaCO3粒子相比,在较低的温度(18 ℃)下矿化时,能够得到形貌更为丰富多变的CaCO3粒子.
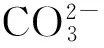
3 结 语
本文经ATRP和RAFT两步法,合成了两亲性嵌段共聚物PS-PNVP.通过气相扩散法,研究了CaCl2浓度和矿化温度对CaCO3粒子形貌的影响,得出以下结论:
(1) 在较高的矿化温度(30 ℃)下,矿化所得CaCO3为有序堆积的片层晶体与菱形晶体的混合形貌;
(2) 在较低的矿化温度(18 ℃)下,通过改变CaCl2的浓度,得到刺球、空心球等多种形貌的CaCO3超结构粒子;
(3) PS-PNVP胶束的存在对CaCO3粒子的形貌有影响,随着矿化温度的降低,胶束对CaCO3粒子形貌的调控作用更显著.
PS-PNVP胶束作为晶体成核促进剂和生长修饰剂,有着自身特殊的优势,为仿生矿化制备无机材料提供了一个有效途径.
[1] SOMMERDIJK N A J M,WITH G D.Biominetic CaCO3mineralization using designer molecules and interfaces [J].Chemical Reviews,2008,108(11): 4499-4550.
[2] YANG X D,XU G Y,CHEN Y J,et al.CaCO3crystallization controlled by (2-hydroxypropyl-3-butoxy) propylsuccinyl chitosan [J].Powder Technology,2012,215/216: 185-194.
[3] ZHANG Z P,GAO D M,ZHAO H,et al.Biomimetic assembly of polypeptide-stabilized CaCO3nanoparticles [J].The Journal of Physical Chemistry B,2006,110(17): 8613-8618.
[4] ROQUE J,MOLERA J,SAZ M V,et al.Crystal size distributions of induced calcium carbonate crystals in polyaspartic acid and mytilus edulis acidic organic proteins aqueous solutions [J].Journal of Crystal Growth,2004,262(1/2/3/4): 543-553.
[5] CAI G B,CHEN S F,LIU L,et al.1,3-Diamino-2-hydroxypropane-N,N,N′,N′-tetraacetic acid stabilized amorphous calcium carbonate: Nucleation,transformation and crystal growth [J].CrystEngComm,2010,12(1): 234-241.
[6] NASSIF N,GEHRKE N,PINNA N,et al.Synthesis of stable aragonite superstructures by a biomimetic crystallization pathway [J].Angewandte Chemie,2005,117(37): 6158-6163.
[7] GAO Y X,YU S H,CONG H P,et al.Block-copolymer controlled growth of CaCO3microrings [J].The Journal of Physical Chemistry B,2006,110(13): 6432-6436.
[8] GUO X H,LIU L,WANG W N,et al.Controlled crystallization of hierarchical and porous calcium carbonate crystals using polypeptide block copolymer as crystal growth modifier in a mixed solutin [J].CrystEngComm,2011,13(6): 2054-2061.
[9] GUO X H,XU A W,YU S H.Crystallization of calcium carbonate mineral with hierarchical sturctures in DMF solution under contrl of poly(ethylene glycol)-b-poly(L-glutamic acid): Effects of crystallization temperature and polymer concentration [J].Crystal Growth & Design,2008,8(4): 1233-1242.
[10] GUO X H,YU S H,CAI G B.Crystallization in a mixture of solvents by using a crystal modifier: Morphology control in the synthesis of highly monodisperse CaCO3microspheres [J].Angewandte Chemie International Eidition,2006,45(24): 3977-3981.
[11] XU A W,ANTONIETTI M,YU S H,et al.Polymer-mediated mineralization and self-similar mesoscale-organized calcium carbonate with unusual superstructures [J].Advanced Materials,2008,20(7): 1333-1338.
[12] SONG R,WANG Y,LIU X,et al.The effect of a novel polyolefine based amphiphilic copolymer on the mineralization of calcium carbonate [J].Colloids and Surfaces A: Physicochemical and Engineering Aspects,2014,446: 50-56.
[13] ZHANG L F,EISENBERG A.Multiple morphologies of “crew-cut” aggregates of polystyrene-b-poly(acrylic acid) block copolymers [J].Science,1995,268(5218): 1728-1731.
[14] YU K,EISENBERG A.Bilayer morphologies of self-assembled crew-cut aggregates of amphiphilic PS-b-PEO diblock copolymers in solution [J].Macromolecules,1998,31(11): 3509-3518.
[15] BUTLER M F,GLASER N,WEAVER A C,et al.Calcium carbonate crystallization in the presence of biopolymers [J].Crystal Growth & Design,2006,6(3): 781-794.
[16] YANG H Y,YAO W G,YANG L,et al.The self-assembly of CaCO3crystals in the presence of protein [J].Journal of Crystal Growth,2009,311(9): 2682-2688.
Synthesis of PS-PNVP Block Copolymer and Its Control on CaCO3Morphology
WANGJiaa,WANGYan-pingb,WANGYi-minb
(a.College of Material Science and Engineering; b.State Key Laboratory for Modification of Chemical Fibers and Polymer Materials,Donghua University,Shanghai 201620,China)
Amphiphilic polystyrene-b-polyvinyl pyrrolidone(PS-PNVP) block copolymer was synthesized by a combination of atom transfer radical polymerization (ATRP) and reversible addition-fragmentation chain transfer (RAFT).The structure,molecular weight and molecular weight distribution index of the resulting copolymer were characterized by1H-NMR,Fourier transform infrared spectroscopy (FTIR),gel permeation chromatography (GPC),respectively.CaCO3particles were mineralized in PS-PNVP micelles solutions by a slow vapor diffusion method.The morphology of the CaCO3crystals was characterized by scanning electron microscope (SEM).By varying the temperature of mineralization and the concentrations of CaCl2,the CaCO3crystals with different morphologies were obtained.The result showed that large scale CaCO3crystals with complicated morphologies and hierarchical structures could be obtained by using PS-PNVP as crystal growth modifier.
CaCO3particles; morphology; micelles; biomineralization
1671-0444(2015)02-0162-05
2014-02-17
王佳(1985—),女,上海人,博士研究生,研究方向为生物矿化材料.E-mail:wjia850219@163.com
王依民(联系人),男,教授,E-mail:ymw@dhu.edu.cn
O 631
A

