Implications for directionality of nanoscale forces in bacterial attachment
Jan J.T.M.Swartjes,Deepak H.VeeregowdaUniversity of Groningen and University Medical Center Groningen,Department of Biomedical Engineering, Antonius Deusinglaan 1,9713 AV Groningen,the Netherlands
2Ducom Instruments Europe B.V,Center for Innovation,9713 GX Groningen,the Netherlands
MINI-REVIEW
Implications for directionality of nanoscale forces in bacterial attachment
Jan J.T.M.Swartjes1✉,Deepak H.Veeregowda21University of Groningen and University Medical Center Groningen,Department of Biomedical Engineering, Antonius Deusinglaan 1,9713 AV Groningen,the Netherlands
2Ducom Instruments Europe B.V,Center for Innovation,9713 GX Groningen,the Netherlands
Received:5 November 2015/Accepted:11 January 2016/Published online:22 February 2016
Adhesion and friction are closely related and play a predominant role in many natural processes.From the wall-clinging feet of the gecko to bacteria forming a bioflm,in many cases adhesion is a necessity to survive.The direction in which forces are applied has shown to infuence the bond strength of certain systems tremendously and can mean the difference between adhesion and detachment.The spatula present on the extension of the feet of the gecko can either attach or detach,based on the angle at which they are loaded.Certain proteins are known to unfold at different loads,depending on the direction at which the load is applied and some bacteria have specifc receptors which increase their bond strength in the presence of shear.Bacteria adhere to any man-made surface despite the presence of shear forces due to running fuids,air fow,and other causes.In bacterial adhesion research,however,adhesion forces are predominantly measured perpendicularly to surfaces,whereas other directions are often neglected.The angle of shear forces acting on bacteria or bioflms will not be at a 90°angle,as shear induced by fow is often along the surface.Measuring at different angles or even lateral to the surface will give a more complete overview of the adhesion forces and mechanism,perhaps even resulting in alternative means to discourage bacterial adhesion or promote removal.
Bacteria,Bacterial adhesion,Friction,Anisotropy,Shear
Both friction and adhesion play a key role in many natural phenomena.Along with the important role in all kinds of processes,the notion that both friction and adhesion can depend on the applied direction and angle, has intrigued scientists.One well-known example is the occurrence of high and low friction and adhesion cycles in the attachment and detachment of the gecko toe (Tian et al.2006).Containing millions of small extensions,called spatula,all exerting nanoscale forces to the surface,the gecko can climb even upside down.By rolling its toe,the gecko changes the angle between its spatula and the surface,allowing it to shift between increasing the normal adhesion force and the frictional component(Autumn et al.2006).At a molecular level, these changes in the angle of the spatula infuence the Van der Waals forces in such a way that the attractive force between the spatula and the surface is altered to switch between high and low values(Tian et al.2006). Simplifed,by changing the direction of loading,either the normal adhesion force is high and the friction is low, or the frictional component is high and the normal adhesion force is low.
Whereas geckos can actively choose the loading angle,allowing them to either stay attached or detached, less autonomous systems like molecules and proteins do not have this option.Nevertheless,these systems display forces that highly depend on direction as well. The E2lip3 protein,for example,which is high in betasheet content,displays a resistance to pulling that strongly depends on the angle of the applied force(Brockwell et al.2003).Similar behavior is found in the unfolding of Ubiquitin by mechanical stretching (Carrion-Vazquez et al.2003).The direction of the applied force determines to a large extent the protein’s stability.In these cases,the different angles in which the force is applied are believed to cause a change in the way the hydrogen bonds of inner beta-sheets rupture. As in the case of the gecko,the angle of the force determines whether bonds are broken by shearing or peeling(Brockwell et al.2003).The regulation of bond organization by mechanical force has been simplifed by describing it either as parallel distribution of forces, where each bond aids in resisting a mechanical force,or a zipper-like distribution(Fig.1)in which one bond after another is required to oppose detachment(Albrecht et al.2003;Hess 2006;Isabey et al.2013).Based on the organization of the bonds,changing the loading direction will shift the distribution of forces,switching from parallel to zipper-like,or the other way around.In the parallel scenario,the collective bond behavior is able to resist much larger forces as the contribution of each bond is added up.A popular example of this is Velcro,which is easily loosened when pulled up from one side,but displays a highly increased resistance to detachment when pulled sideways(Matouschek and Bustamante 2003).
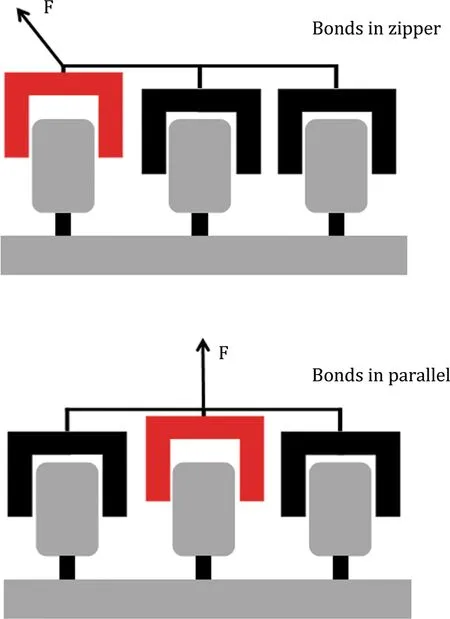
Fig.1 Distribution of forces over multiple bonds.In the zipperlike distribution(top)each bond is loaded consecutively,passing the load on to the next bond after breaking.While in the parallel distribution(bottom)the load is distributed over all included bonds and after breaking of one bond,the load is redistributed over the remaining ones.Adapted with permissions from Isabey et al.(2013)
Unzipping of proteins is,for example,also observed in the amyloid-like interactions within clusters of adhesins called Als,which contribute in the adhesion of Candida albicans(Alsteens et al.2010,2012).Force analysis of the adhesion shows mechanical unzipping of β-sheet interactions between Als proteins upon being pulled from an amyloid coated surface at 90°. One step beyond the scale of inter-protein bindings, shear,or lateral,force dependency of adhesion is observed in ligand-receptor complexes.The FimHadhesin expressed by Escherichia coli binds to mannose and is found to enhance adhesion under high shear conditions(Thomas et al.2004;Aprikian et al. 2007).Whereas the behavior of the previously mentioned proteins is generally ascribed to bond organization,it has been suggested that in the case of E.coli the typical behavior stems from allosteric regulation, causing bond enhancementby mechanicalforce (Yakovenko et al.2008).
From animals to proteins it is clear that the direction of an applied force can make the difference between adhesion or detachment,structural integrity or unfolding.For certain microorganisms,like bacteria,surface attachment is the preferred mode of survival,as stable surface bound communities offer protection to antibiotics and mechanical removal(O’Toole et al.2000; Dunne 2002;Vlamakis et al.2013).At the same time, whether the surface comprises the inner-lining of the human body,or an implant,the formation of these bioflm communities is often highly unwanted(Cegelski et al.2008;Lo¨fing et al.2011;Foster et al.2014).For decades,researchers are trying to deal with bacterial adhesion by an almost endless effort to create nonfouling surfaces which can withstand adhesion of bacteria.On the other hand,there is also a tremendous amount of work put into new strategies of effective removal of adhered bacteria.In both cases,fundamental knowledge of bacterial adhesion and the mechanisms behind it are of crucial importance,and since this knowledge is limited,new information can hold the key to breakthroughs in either feld.
Especially in the medical feld where bacterial adhesion and the forthcoming bioflms cause life-threatening infections that are continuing to be more diffcult to treat with antibiotics,detachment as well as adhesion prevention strategies are well sought after(Busscher et al.2012;Campoccia et al.2013;Swartjes et al.2013; Swartjes et al.2014a).To fnd out more about the mechanisms of bacterial adhesion,atomic force microscopy(AFM)has proven to be the tool of preference inorder to determine the forces by which bacteria attach to surfaces and keep themselves adhered(Dufrene 2002;Dorobantu and Gray 2010;Mu¨ller and Dufreˆne 2011;Dorobantu et al.2012).The vertical motion of the AFM cantilever is often used to determine the force necessary to pull a bacterium from a cell or surface.This force,which has a magnitude of several nano Newton,is considered as the adhesion force(Dorobantu and Gray 2010).Whenever AFM is used to measure bacterial adhesion,the angle of the direction in which the bacterial adhesion force is measured and the substrate is approximately 90°.However,the amount of work(W)to overcome adhesion is a function of the pull-off angle θ, i.e.,W=F·d·cos(θ),which indicates that the force required for detachment might change for different pulloff angles.Additionally,bacteria in most situations adhere from a fowing condition,in which the angle of approach leads to friction between a bacterium and the surface(Swartjes et al.2014b).In fact,there is a relationship between adhesion and friction at nanoscale often used to describe the contact between two solid bodies,Ff=μ(Fn+Fadh),stating that the friction force Ff,equals the coeffcient of friction(μ)multiplied by the sum of the normal force Fnand the adhesion force Fadh(Gao et al.2004).In relation to these directional infuences on bacterial adhesion,several methods have been applied to determine the lateral forces between bacteria and surfaces.
A distinction can be made between two types of lateral forces;frst,the shear adhesion force depending on the strength of the bond between an adhered bacterium and a surface,which breaks by moving the bacterium along the surface after it has adhered,and second,the lateral force arising between a bacterium and a surface when initial contact is made by a bacterium approaching the surface at an angle,representing the friction(Swartjes et al.2014b).By challenging the shear strength of the adhesion bond using different fow rates of the liquid carrying the bacteria(Gazzola et al.2015),or by detachment induced by passage of a liquid-air interface(Perera-Costa et al.2014),estimations of the frst type of lateral force have been made. However,since perpendicular to the surface the adhesion force of a single bacterium can be measured directly using the AFM,it is desirable to achieve a similar mode of action to measure the adhesion force at a different angle.Several attempts have recently been made to determine the lateral forces occurring between bacteria and surfaces using AFM(Verran et al.2010; Zhang et al.2011;Swartjes et al.2014b).Quantifcation of the shear strength of bacterial adhesion has been achieved by imaging of bacteria;as the AFM cantilever moves along the surface in contact mode,the lateral movement of the cantilever can displace bacteria by pushing them away(Verran et al.2010;Zhang et al. 2011).To measure lateral forces more directly,singlecell force spectroscopy(SCFS),in which a single bacterial cell is attached to the AFM cantilever,can be applied to probe the forces between this single bacterium and a surface.Kweon et al.modifed an AFM cantilever with a bacterial spore and rubbed the spore against a silica surface to retrieve the values of occurring friction forces (Kweon et al.2011).Even though this only involved a bacterial product,rather than an actual bacterium,the principle has also been performed using bacteria instead of a spore.The friction between polymer brushmodifed surfaces and bacteria attached to a cantilever showed that friction was correlated to the amount of bacteria adhering to the surface,suggesting that friction forces play a role in attachment(Swartjes et al.2014b). Interestingly,the friction and adhesion forces did not relate to each other as per the previously stated equation describing friction forces,indicating that bacterial friction and adhesion is more complex and challenging.
Most of these studies on lateral forces involved whole bacteria,however,based on the behavior of single proteins when subjected to forces at different angles, direction-dependent adhesion can also be studied by looking at components of bacterial adhesion complexes. SCFS has taken a fight over the last years and has expanded the insights about bacterial adhesion mechanisms considerably(Helenius et al.2008;Mu¨ller and Dufreˆne 2011;Isabey et al.2013;Beaussart et al.2014). Additionally,the technique has been extended to the use of single molecules,offering the possibility of isolating specifc adhesion structures of bacteria and identifying their sole contribution in adhesion(Benoit et al.2000; Sullan et al.2015).Interestingly,single-molecule force spectroscopy(SMFS)using specifc bacterial adhesion complexes reveals peaks in the force-distance curves due to breakage of multiple bonds,suggested to be caused by unfolding of the protein(Fig.2C,D)and closely resembling the unzipping of previously mentioned proteins displaying anisotropic behavior(Fig.2A, B)(El-Kirat-Chatel et al.2014;Sullan et al.2015). Additionally,measurements of a whole Streptococcus mutans cell suggest the presence of up to 10 ligandreceptor complexes being responsible for the binding of a single bacterial cell(Sullan et al.2015).
Even though there are many examples showing that bond strength and adhesion phenomena in certain cases display properties highly depending on the direction of the applied force,direct measurements in the case of bacteria are scarce.In the quest for solutions to the bacterial adhesion problem,attention for shear and friction forces is present,as based on the previouslymentioned examples and studies developing antifouling super-slippery surfaces(Wong et al.2011; Epstein et al.2012;Li et al.2013;MacCallum et al. 2015),however,the fundamental role it plays in adhesion is not known.As the use of bacterial probes in AFM is increasing,unraveling the role of directionality could reveal completely new information on the adhesion mechanism of bacteria.Besides the added value it could have in non-specifc adhesion,the example of a sheardependent specifc adhesion mechanism in E.coli suggests that specifc interactions between ligands and receptors,which are present in the majority of bacteria, are able to exhibit different strengths based on how their unity is challenged.Given that specifc patterns of unzipping and unfolding of individual proteins involved in bacterial adhesion are observed(Table 1),it is very well possible that the same directional dependent behavior seen in multiple types of proteins would also apply in the case of proteins associated with adhesion of bacteria.Additionally,for several pathogens it is suggested that zipper-like sequences are involved in host cell invasion,implicating that structures known to show directional dependent strength are partly responsible not only for general adhesion,but also for bacterial pathogenesis(Schwarz-Linek et al.2003).Altogether, anisotropic adhesion behavior could not only stem from an array of adhesion complexes acting as individual bonds that can be loaded in a parallel or a zipper-like fashion,but also from within adhesion proteins or complexes where unfolding of single proteins might depend on the loading direction.
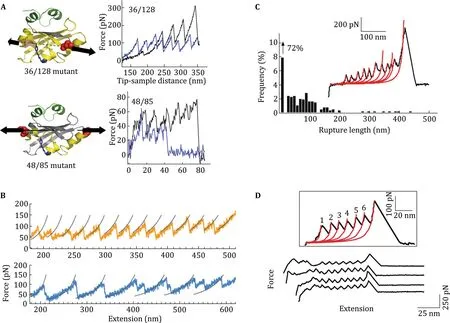
Fig.2 Unfolding behavior of proteins shown to have anisotropic responses to loading(A,B)and bacterial adhesion proteins displaying similar force patterns(C,D).A The distinct differences in force curves upon stretching of PYP by pulling at different axis.B Forceextension curves of unfolding of GFP displaying a distinct unzipping pattern for different directions of loading.C Force curves for the interaction between S.mutans adhesin P1 and fbronectin-coated solid substrates,exhibiting similar peaks observed for anisotropic proteins.D Unfolding force patterns of Als5p adhesion proteins closely resemble those of proteins known to respond differently to different loading directions.Adapted with permissions from Dietz et al.(2006),Nome et al.(2007),Alsteens et al.(2009)and Sullan et al. (2015)
As shear is present almost everywhere inside the human body,e.g.,in the oral cavity,blood vessels, intestine,and lungs,it suggests that frm attachment byresisting shear forces is mandatory for bacteria in order to persist in an adhered state.Proteins highly involved in the adhesion of different bacterial strains have shown to exhibit similar unfolding patterns compared to other proteins,which are highly anisotropic in their unfolding. Additionally,the shear strengthening of FimH in E.coli supports the implications for the possibility of direction-dependent adhesion mechanisms in bacteria, similar to those suggested for mammalian cells(Isabey et al.2013).By probing friction forces and the adhesion forces lateral to the surface,specifc information can be obtained that possibly provide new clues for antiadhesive,or easy to clean surfaces.It is impossible to say which type of lateral force has more impact on bacterial adhesion,and the frictional forces probably contribute most to the transitions from unbound to surface attached,while it is likely that shear adhesion forces are most important in remaining an adhered state.As such,the frictional forces seem most interesting for design of non-fouling surface,while the shear adhesion force could help in designing strategies for bacterial removal.Nano-topographic surfaces could perhaps alter the direction in which bacteria experience shear forces,making them less likely to adhere,or easier to be removed.
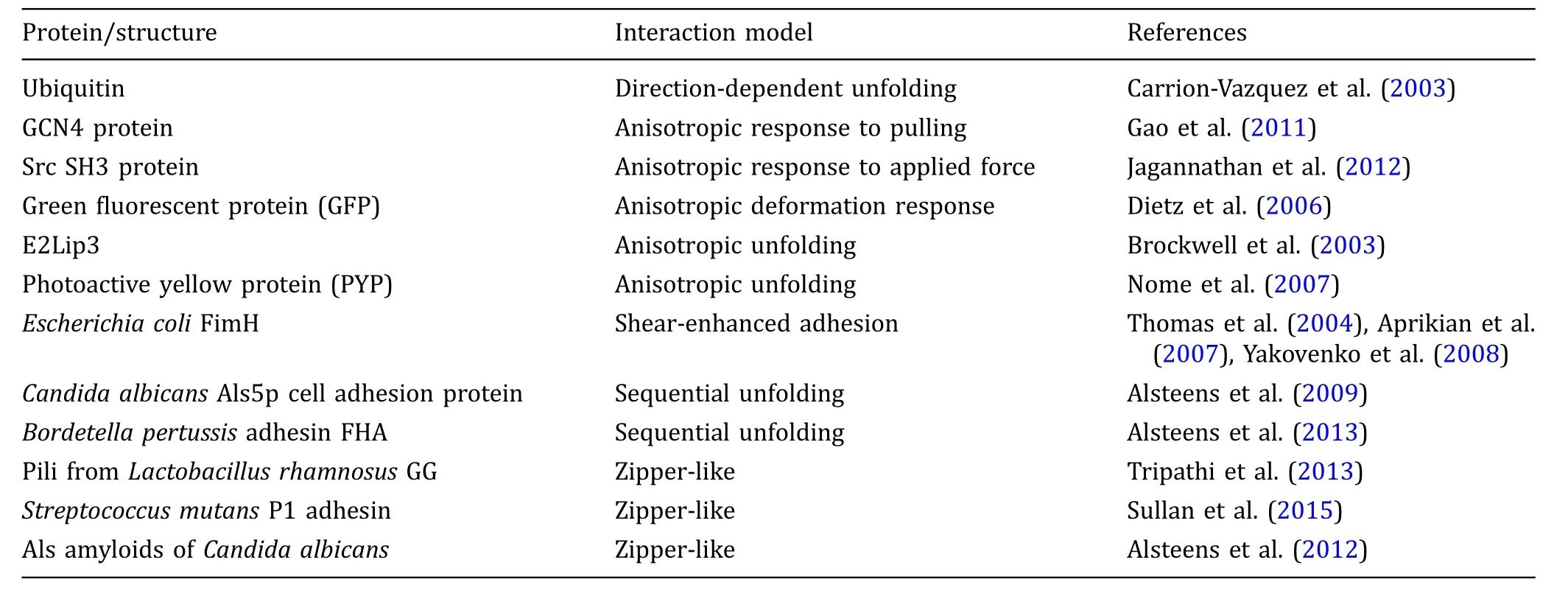
Table 1 Overview of different proteins whose behavior depends on the loading axis and of bacterial associated proteins whose unfolding characteristics imply the possibility of similar anisotropic behavior
Bacteria have outsmarted mankind by adapting resistance to a major part of our antibiotic spectrum, resulting in an increase in infections which are extremely hard to resolve(Spellberg et al.2013;Wellington et al.2013).In order to prevent infection there are many aspects of bacterial adhesion and bioflm formation requiring our utmost attention.The many suggestions for anisotropy of bacterial adhesion forces therefore imply that studying forces between bacteria and surfaces in multiple directions are desirable,as it might reveal precious information that can help in making crucial steps toward the development of new and more effcient anti-bacterial strategies.
Compliance with Ethical Standards
Confict of Interest Jan J.T.M.Swartjes and Deepak H.Veeregowda declare that they have no confict of interest.Deepak H. Veeregowda is a manager of Ducom Instruments in the Netherlands and also a research scientist at the University Medical Center Groningen.
Human and Animal Rights and Informed ConsentThis article does not contain any studies with human or animal subjects performed by any of the authors.
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License(http:// creativecommons.org/licenses/by/4.0/),which permitsunrestricted use,distribution,and reproduction in any medium,provided you give appropriate credit to the original author(s)and the source,provide a link to the Creative Commons license,and indicate if changes were made.
Albrecht C,Blank K,Lalic-Mu¨lthaler M,Hirler S,Mai T,Gilbert I, Schiffmann S,Bayer T,Clausen-Schaumann H,Gaub HE (2003) DNA:a programmable force sensor.Science 301:367-370
Alsteens D,Dupres V,Klotz SA,Gaur NK,Lipke PN,Dufreˆne YF (2009)Unfolding individual Als5p adhesion proteins on live cells.ACS Nano 3:1677-1682
Alsteens D,Garcia MC,Lipke PN,Dufreˆne YF(2010)Force-induced formation and propagation of adhesion nanodomains in living fungal cells.Proc Natl Acad Sci USA 107:20744-20749
Alsteens D,Ramsook CB,Lipke PN,Dufreˆne YF(2012)Unzipping a functional microbial amyloid.ACS Nano 6:7703-7711
Alsteens D,Martinez N,Jamin M,Jacob-Dubuisson F(2013) Sequential unfolding of beta helical protein by single molecule atomic force microscopy.PLoS One 8:e73572
Aprikian P,Tchesnokova V,Kidd B,Yakovenko O,Yarov-Yarovoy V, Trinchina E,Vogel V,Thomas W,Sokurenko E(2007) Interdomain interaction in the FimH adhesin of Escherichia coliregulates the affnity to mannose.JBiolChem 282:23437-23446
Autumn K,Dittmore A,Santos D,Spenko M,Cutkosky M(2006) Frictional adhesion:a new angle on gecko attachment.J Exp Biol 209:3569-3579
Beaussart A,El-Kirat-Chatel S,Sullan RMA,Alsteens D,Herman P, Derclaye S,Dufreˆne YF(2014)Quantifying the forces guiding microbial cell adhesion using single-cell force spectroscopy. Nat Protoc 9:1049-1055
Benoit M,Gabriel D,Gerisch G,Gaub HE(2000)Discrete interactions in cell adhesion measured by single-molecule force spectroscopy.Nat Cell Biol 2:313-317
Brockwell DJ,Paci E,Zinober RC,Beddard GS,Olmsted PD,Smith DA,Perham RN,Radford SE(2003)Pulling geometry defnes the mechanical resistance of a beta-sheet protein.Nat Struct Biol 10:731-737
Busscher HJ,van der Mei HC,Subbiahdoss G,Jutte PC,van den Dungen JJAM,Zaat SAJ,Schultz MJ,Grainger DW(2012) Biomaterial-associated infection:locating the fnish line in the race for the surface.Sci Transl Med 4:153rv10
Campoccia D,Montanaro L,Arciola CR(2013)A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials 34:8533-8554
Carrion-Vazquez M,Li H,Lu H,Marszalek PE,Oberhauser AF, Fernandez JM(2003)The mechanical stability of ubiquitin is linkage dependent.Nat Struct Biol 10:738-743
Cegelski L,Marshall GR,Eldridge GR,Hultgren SJ(2008)The biology and future prospects of antivirulence therapies.Nat Rev Microbiol 6:17-27
Dietz H,Berkemeier F,Bertz M,Rief M(2006)Anisotropic deformation response of single protein molecules.Proc Natl Acad Sci USA 103:12724-12728
Dorobantu LS,Gray MR(2010)Application of atomic force microscopy in bacterial research.Scanning 32:74-96
Dorobantu LS,GossGG,BurrellRE (2012)Atomicforce microscopy:a nanoscopic view of microbial cell surfaces. Micron 43:1312-1322
Dufrene YF(2002)Atomic force microscopy,a powerful tool in microbiology.J Bacteriol 184:5205-5213
Dunne WM(2002)Bacterial adhesion:seen any good bioflms lately?Society 15:155-166
El-Kirat-Chatel S,Beaussart A,Boyd CD,O’Toole GA,Dufreıˆne YF (2014)Single-cell and single-molecule analysis deciphers the localization,adhesion,and mechanics of the bioflm adhesin LapA.ACS Chem Biol 9:485-494
Epstein AK,Wong TS,Belisle RA,Boggs EM,Aizenberg J(2012) Liquid-infused structured surfaces with exceptional antibiofouling performance. Proc Natl Acad Sci USA 109:13182-13187
Foster TJ,Geoghegan JA,Ganesh VK,Ho¨o¨k M(2014)Adhesion, invasion and evasion:the many functions of the surface proteins of Staphylococcusaureus.NatRev Microbiol 12:49-62
Gao J,Luedtke WD,Gourdon D,Ruths M,Israelachvili JN,Landman U(2004)frictional forces and amontons’law:from the molecularto themacroscopicscale.JPhysChem B 108:3410-3425
Gao Y,Sirinakis G,Zhang Y(2011)Highly anisotropic stability and folding kinetics of a single coiled coil protein under mechanical tension.J Am Chem Soc 133:12749-12757
Gazzola G,Habimana O,Murphy CD,Casey E(2015)Comparison of biomass detachment from bioflms of two different Pseudomonas spp.under constant shear conditions.Biofouling 31:13-18
Helenius J,Heisenberg C-P,Gaub HE,Muller DJ(2008)Single-cell force spectroscopy.J Cell Sci 121:1785-1791
Hess H(2006)Self-assembly driven by molecular motors.Soft Mater 2:669
Isabey D,Fe´re´ol S,Caluch A,Fodil R,Louis B,Pelle G(2013)Force distribution on multiple bonds controls the kinetics of adhesion in stretched cells.J Biomech 46:307-313
Jagannathan B,Elms PJ,Bustamante C,Marqusee S(2012)Direct observation of a force-induced switch in the anisotropic mechanical unfolding pathway of a protein.Proc Natl Acad Sci USA 109:17820-17825
Kweon H,Yiacoumi S,Tsouris C(2011)Friction and adhesion forces of Bacillus thuringiensis spores on planar surfaces in atmospheric systems.Langmuir 27:14975-14981
Li J,Kleintschek T,Rieder A,Cheng Y,Baumbach T,Obst U, Schwartz T,Levkin PA(2013)Hydrophobic liquid-infused porous polymer surfaces for antibacterial applications.ACS Appl Mater Interfaces 5:6704-6711
Lo¨fing J,Vimberg V,Battig P,Henriques-Normark B(2011) Cellular interactions by LPxTG-anchored pneumococcal adhesins and their streptococcal homologues.Cell Microbiol 13:186-197
MacCallum N,Howell C,Kim P,Sun D,Friedlander R,Ranisau J, Ahanotu O,Lin JJ,Vena A,Hatton B,Wong TS,Aizenberg J (2015)Liquid-infused silicone as a biofouling-free medical material.ACS Biomater Sci Eng 1:43-51
Matouschek A,Bustamante C(2003)Finding a protein’s Achilles heel.Nat Struct Biol 10:674-676
Mu¨ller DJ,Dufreˆne YF(2011)Atomic force microscopy:a nanoscopic window on the cell surface.Trends Cell Biol 21:461-469
Nome RA,Zhao JM,Hoff WD,Scherer NF(2007)Axis-dependent anisotropy in protein unfolding from integrated nonequilibrium single-molecule experiments,analysis,and simulation. Proc Natl Acad Sci USA 104:20799-20804
O’Toole GA,Kaplan HB,Kolter R(2000)Bioflm formation as microbial development.Annu Rev Microbiol 54:49-79
Perera-Costa D,Bruque JM,Gonza´lez-Martı´n ML,Go´mez-Garcı´a AC,Vadillo-Rodrı´guez V(2014)Studying the infuence of surface topography on bacterial adhesion using spatially organized microtopographic surface patterns.Langmuir 30:4633-4641
Schwarz-Linek U,Werner JM,Pickford AR,Gurusiddappa S,Kim JH,Pilka ES,Briggs JAG,Gough TS,Ho¨o¨k M,Campbell ID,Potts JR(2003)Pathogenic bacteria attach to human fbronectin through a tandem beta-zipper.Nature 423:177-181
Spellberg B,Bartlett JG,Gilbert DN (2013)The future of antibiotics and resistance.N Engl J Med 368:299-302
Sullan RMA,Li JK,Crowley PJ,Brady LJ,Dufreˆne YF(2015) Binding forces of Streptococcus mutans P1 Adhesin.ACS Nano 9:1448-1460
Swartjes JJTM,Das T,SharifS,Subbiahdoss G,Sharma PK,Krom BP,Busscher HJ,Van der Mei HC(2013)A functional DNase I coating to prevent adhesion of bacteria and the formation of bioflm.Adv Funct Mater 23:2843-2849
Swartjes JJTM,Sharma PK,van Kooten T,Van der Mei HC, Mahmoudi M,Busscher HJ,Rochford ETJ(2014a)Current developments in antimicrobial surface coatings for biomedical applications.Curr Med Chem 21:2116-2129
Swartjes JJTM,Veeregowda DH,Van der Mei HC,Busscher HJ, Sharma PK(2014b)Normally oriented adhesion versus friction forcesin bacterialadhesion to polymer-brushfunctionalized surfaces under fuid fow.Adv Funct Mater 24:4435-4441
Thomas WE,Nilsson LM,Forero M,Sokurenko EV,Vogel V(2004) Shear-dependent‘stick-and-roll’adhesion of type 1 fmbriated Escherichia coli.Mol Microbiol 53:1545-1557
Tian Y,Pesika N,Zeng H,Rosenberg K,Zhao B,McGuiggan P, Autumn K,Israelachvili J(2006)Adhesion and friction in gecko toe attachment and detachment.Proc Natl Acad Sci USA 103:19320-19325
Tripathi P,Beaussart A,Alsteens D,Dupres V,Claes I,Von Ossowski I,De Vos WM,Palva A,Lebeer S,Vanderleyden J, Dufreıˆne YF(2013)Adhesion and nanomechanics of pili from the probiotic LactobacillusrhamnosusGG.ACS Nano 7:3685-3697
Verran J,Packer A,Kelly PJ,Whitehead KA(2010)Use of the atomic force microscope to determine the strength of bacterial attachment to grooved surface features.J Adhes Sci Technol 24:2271-2285
Vlamakis H,Chai Y,Beauregard P,Losick R,Kolter R(2013) Sticking together:building a bioflm the Bacillus subtilis way. Nat Rev Microbiol 11:157-168
Wellington EMH,Boxall ABA,Cross P,Feil EJ,Gaze WH,Hawkey PM,Johnson-Rollings AS,Jones DL,Lee NM,Otten W,Thomas CM,Williams AP(2013)The role of the natural environment in the emergence of antibiotic resistance in Gram-negative bacteria.Lancet Infect Dis 13:155-165
Wong TS,Kang SH,Tang SKY,Smythe EJ,Hatton BD,Grinthal A, Aizenberg J(2011)Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity.Nature 477:443-447
Yakovenko O,Sharma S,Forero M,Tchesnokova V,Aprikian P,Kidd B,Mach A,Vogel V,Sokurenko E,Thomas WE(2008)FimH forms catch bonds that are enhanced by mechanical force due to allosteric regulation.J Biol Chem 283:11596-11605
Zhang T,Chao Y,Shih K,Li XY,Fang HHP(2011)Quantifcation of the lateral detachment force for bacterial cells using atomic force microscope and centrifugation.Ultramicroscopy 111:131-139
✉Correspondence:janjtmswartjes@gmail.com(J.J.T.M.Swartjes),
- Biophysics Reports的其它文章
- Visualizing the Ensemble Structures of Protein Complexes Using Chemical Cross-Linking Coupled with Mass Spectrometry
- E2-2,a novel immunohistochemical marker for both human and monkey plasmacytoid dendritic cells
- Radiolig and saturation binding for quantitative analysis of ligand-receptor interactions
- Pseudomonas sp.LZ-Q continuously degrades phenanthrene under hypersaline and hyperalkaline condition in a membrane bioreactor system
- Thermodynamics of GPCR activation