Molecularly imprinted polymers combination with deep eutectic solvents for solid-phase extraction of caffeic acid from hawthorn
LI Guizhen,TANG Weiyang,CAO Weimin,WANG Qian,ZHU Tao*
(1.Tianjin Key Laboratory of Organic Solar Cells and Photochemical Conversion,School of Chemistry and Chemical Engineering,Tianjin University of Technology,Tianjin 300384,China;2.Shijiazhuang Yiling Pharmaceutical Co.,Ltd.,Shijiazhuang 050035,China)
Hawthorn,a member of the Rosaceae family,has been used as food and medicine around the world[1-4].Hawthorn is known as a valuable medicinal plant which contains a large number of biological active substances (flavonoids [5],chlorogenic acid[6],caffeic acid (Fig.1)[7],and so on).Caffeic acid,as one of the ingredients of hawthorn,has been widely investigatedbecause of biological and pharmacological activities,such as antidiabetic[8],antioxidants[9,10],anti-inflammatory [11-13],and so on.Caffeic acid was extracted with many different methods,such as sonication/ethanol extraction[14,15],capillary zone electrophoresis [16],and so on.Because of the complexity of the plant extracts,asimpleand effectivepretreatment process is necessary to isolate and concentrate the caffeic acid before analysis[17].Until now,the pretreatment methods mainly include solidphase extraction (SPE)[18,19],dispersive liquid-liquid microextraction[20],solid-phase microextraction[21],and hollow fiber-based liquidliquid-liquid microextraction [22,23].Among these methods,SPE is the most widely applied pretreatment technique because of its high recovery,reproducibility,and simple operation,low cost and so on[24,25].
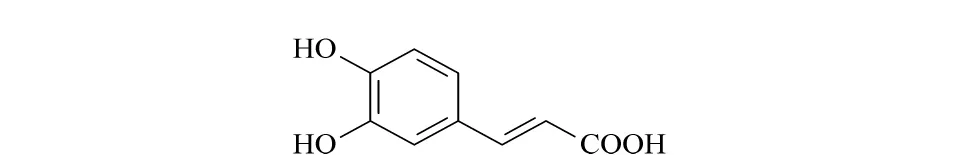
Fig.1 Chemical structure of caffeic acid
Molecularly imprinted polymers(MIPs)exhibiting high selectivity and affinity to the target molecule (template),are attracting a fast growing research[26-29].The special binding sites of MIPs are formed by the self-assembly of the template with functional group and the monomer in a co-polymerization process.So MIPs can selectively rebind the template in the presence of other closely related structures[30-34].Because of its features of high selectivity,low cost for preparation and workability under different conditions,SPE involving MIPs have been proved to be successful applications[35-39].Furthermore,elution solutions in the MIPs-SPE procedures are important in the elution capability[40].Deep eutectic solvents (DESs)are green designer solvents composed of quaternary ammonium salts,hydrogen donors and show some good properties,such as low volatility,low toxicity,low cost,and high biodegradability[41-43].DESs have attracted considerable attentions in the area of synthesis,electrochemistry,materials,biochemistry and separation [44-49] in recent years.DES with addition of methanol can have significant benefits in terms of a decrease in viscosity,stronger basicity and lower cost for extracting the target compounds[50].
In this work,MIPs with caffeic acid as template molecule and non-imprinted polymers (NIPs)were prepared,and they were characterized using field emission scanning electron microscopy(FESEM)and adsorption capacity test.MIPs,NIPs and C18were used for rapid purification of caffeic acid from hawthorn using SPE.To optimize the MIPs-SPE procedures,methanol was mixed with the two kinds of DESs (glycerol-based DESs,urea-based DESs)in different ratios (0.5∶1,1∶1,2∶1,3∶1,4∶1,5∶1,v/v),and they were used as elution solutions in the MIPs-SPE procedures.
1 Experimental
1.1 Reagents and material
Hawthorn was bought from a market in Tianjin,China.Caffeic acid was bought from Jinsui Bio-Technology Co.,Ltd.(Shanghai,China).Choline chloride (ChCl)was bought from Guangfu Chemical Reagent Co.,Ltd.(Tianjin,China).Glycerol and urea were bought from Beichen Chemical Reagent Co.,Ltd.(Tianjin,China).Ethylene glycoldimethacrylate (EDMA)was bought from Hengshan Sci-Tech Co.,Ltd (Tianjin,China).2-Methylpropionitrile(AIBN)was bought from Bodi Chemical Industry Co.,Ltd.(Tianjin,China).Acrylamide(AM)was bought from Guangfu Chemical Research Institute(Tianjin,China).Acetic acid was bought from Zhiyuan Chemical Reagent Co.,Ltd.(Tianjin,China).Methanol was bought from Jinke Chemical Research Institute (Tianjin,China).Empty SPE cartridges(with gaskets)were bought from Agilent Technologies Inc.(Tianjin,China).C18cartridges(with 200 mg packed materials)were bought from Agilent Technologies Inc.(Tianjin,China).All the other solvents used in the experiment were HPLC or analytical grade,and all the samples were filtered before injection into the HPLC system.
1.2 Apparatus
The chromatography system consisted of LC-10ATVP pump and SPD-10AVP UV-Vis detector(Shimadzu,Suzhou,China),with the injector(20-μL sample loop).The analysis was performed on an Optima Pak C18column(150 mm×4.6 mm,5 μm,RS tech Corporation,Daejeon,Korea).DF-101 heating magnetic agitator (Yuhua,Gongyi,China) and a Soxhlet extractor(Hengshan,Tianjin,China)were used for preparation of MIPs.Distilled water(18.2 MΩ·cm)was filtered with a vacuum pump (Yukang,Shanghai,China)and a filter before use.
1.3 Preparation of DESs
The glycerol-based DESs were formed by choline chloride (ChCl)-glycerol (1/2,n/n)in a conical flask,heating to 80℃ with constant stirring for 2 h until a homogeneous liquid formed.The urea-based DESs were prepared with choline chloride-urea (1/2,n/n)in the same procedure.
1.4 Preparation of imprinted polymers
Template molecule (caffeic acid,0.180 6 g)and AM functional monomers (0.280 4 g)were added to a clean,dry round bottom flask containing a magnetic stirring bar,and then dissolved in appropriate 4.0 mL methanol-water (90/10,v/v)solvents.The solution was ultrasonicated for 30 min and sparged with nitrogen for 5 min to remove oxygen.EDMA (3.79 mL)and AIBN (0.04 g)were then added into the solution,and they were kept in oil bath at 60℃ for 48 h.The synthesis diagram of MIPs is shown in Fig.2.After polymerization,polymers were ground into particles and sieved.A total of 200 mg MIP polymer was ground and washed with methanol-acetic acid(90/10,v/v)to remove the templates,porogenic solvents and other compounds.Finally,the obtained particles were purified with deionized water and methanol for 2 h by Soxhlet extraction,and dried at 60℃ under vacuum for 48 h.The NIPs(without template molecule)were prepared in the same procedure.

Fig.2 Synthesis diagram of MIPs
1.5 Characterization of MIPs and NIPs
The morphological microstructures of the dried MIPs and NIPs particles were observed by FESEM (MERLIN Compact,ZEISS,Germany).
1.6 Rebinding experiment
For static adsorption experiment,20.0 mg each of MIPs and NIPs particles was mixed with 2.0 mL of caffeic acid solutions of mass concentrations of 5.0-200.0 mg/L in centrifuge tubes.After shaking for 10 h,the mixtures were centrifuged,and the upper solutions were determined to calculate the adsorption capacities.
For dynamic adsorption experiment,2.0 mL of 50.0 mg/L caffeic acid aqueous solution was mixed with 20.0 mg each of MIPs and NIPs in 10 mL of centrifuge tubes,and they were shaken for 60-450 min.After centrifuging the mixture,the upper solution in each tube was determined to calculate the adsorption capacities.
1.7 Preparation of standard solutions and chromatographic separation
The standard caffeic acid was dissolved in methanol to give a mass concentration of 1 000.00 mg/L.For method development, a series of standard solutions containing caffeic acid were prepared at five mass concentration levels over the range of 5.00-100.00 mg/L.The standard curve of caffeic acid was linear by assaying five data points and each sample was determined for three times.
The analysis was performed on an HPLC system with a C18column,and the mobile phase was methanol-water-acetic acid (18/82/0.5,v/v/v).The flow rate was 0.8 mL/min,and the chromatogram was monitored at a wavelength of 330 nm.
1.8 SPE by MIPs,NIPs and C18
Hawthorn was washed by water,dried in an oven at 50℃,and ground into powder.A portion of 0.5 mg hawthorn powder was added into 5.0 mL ethanol,and the mixture was ultrasonicated for 30 min.After centrifugation,the extract was filtered and collected as a stock sample solution.The corresponding MIPs and NIPs (200.0 mg)were packed in empty SPE cartridges,and two gaskets were put at both ends to avoid the loss of adsorbent.After each SPE cartridge was preconditioned sequentially by methanol(1.0 mL)and deionized water(3.0 mL),1.0 mL of the extract solution was loaded on the cartridge,followed by deionized water(1.0 mL)as the washing solution and methanol(2.0 mL)as the elution solution.The effluents at every step were collected by a 1.0 mL syringe,which was connected to the bottom of SPE cartridge to ensure a suitable and constant flow rate.The obtained effluents were transferred to reagent bottles for further HPLC analysis.
1.9 DESs for optimization of MIPs-SPE procedure
Methanol was mixed with two kinds of DESs(glycerol-based DESs,urea-based DESs)in different ratios (0.5∶1,1∶1,2∶1,3∶1,4∶1,5∶1,v/v),and they were used as elution solutions (2.0 mL)in the MIPs-SPE procedures.
2 Results and discussion
2.1 Morphological characteristics of imprinted polymers
FE-SEM has successfully been used to observe the morphologies of the MIPs and NIPs (Fig.3),which were important parameters used to evaluate polymerization stability and reproducibility.According to the figure,there were no significant difference between the MIPs and NIPs.Moreover,many macropores and flow-through channels were inlaid in the network skeletons of these polymers.Additionally,the shapes of the two polymers were analogously globular with diameters ranging from 1 μm to 4 μm,which indicated their fine macropores structure and suitability as SPE adsorbents.
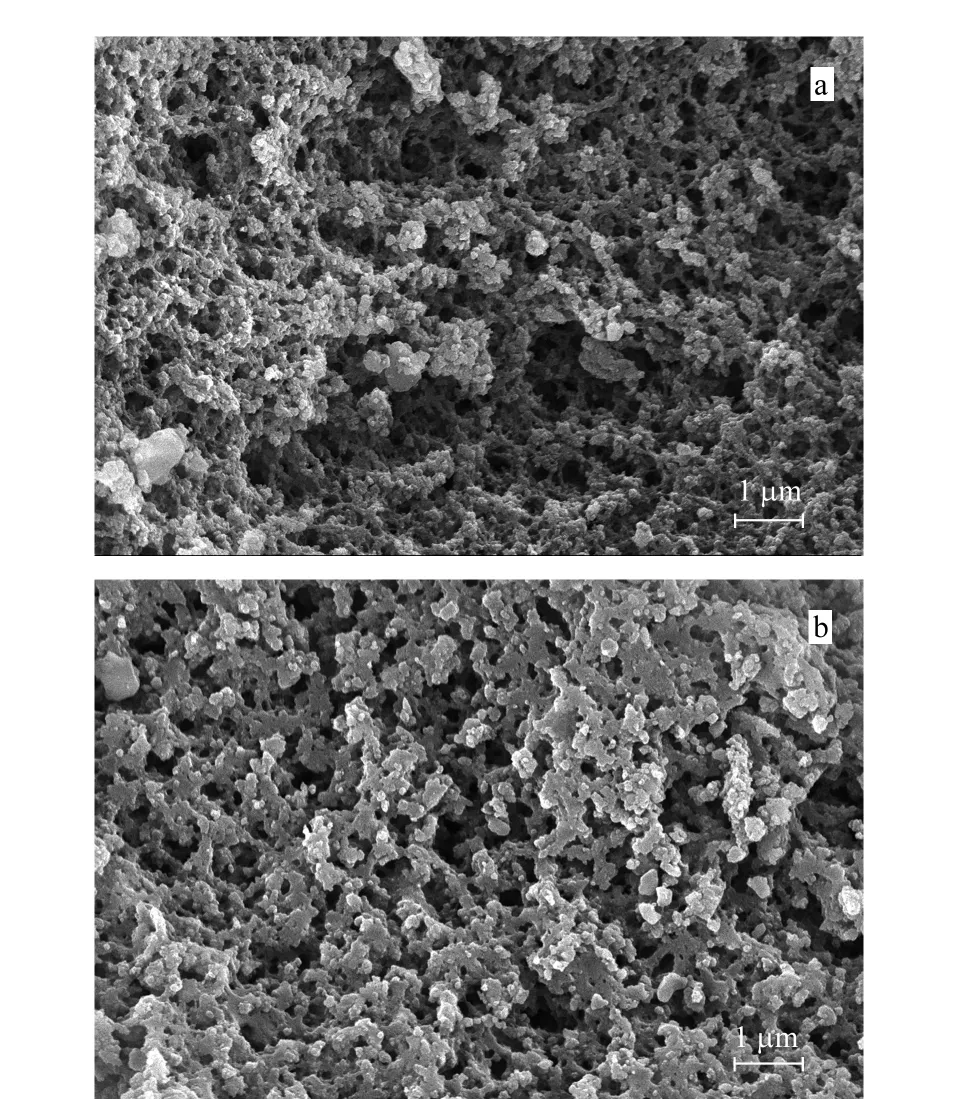
Fig.3 FE-SEM images of the (a)MIPs and (b)NIPs
2.2 Adsorption characteristics of the imprinted polymers
The adsorption equilibrium showed the mass transfer rate of polymers.To evaluate the binding property of the polymers(MIPs and NIPs),static adsorption and dynamic adsorption experiments were performed at room temperature.The results of the static absorption and dynamic adsorption are shown in Fig.4.Fig.4a shows that caffeic acid adsorption increased with an increase in initial concentration,and the adsorption capacities gradually tended to be saturated when the caffeic acid mass concentration was more than 150.0 mg/L.Fig.4b showed that the adsorptions of MIPs and NIPs increased slightly but tended to balance at approximately 390-450 min.The results of dynamic equilibrium adsorption showed that the adsorptions of both MIPs and NIPs were the greatest at nearly 390 min,which indicatedthat the interaction time of aggregation and adhesion of NIPs was similar to that of MIPs.Compared with NIPs,MIPs revealed higher adsorption capacity owing to its specific imprinted recognition.
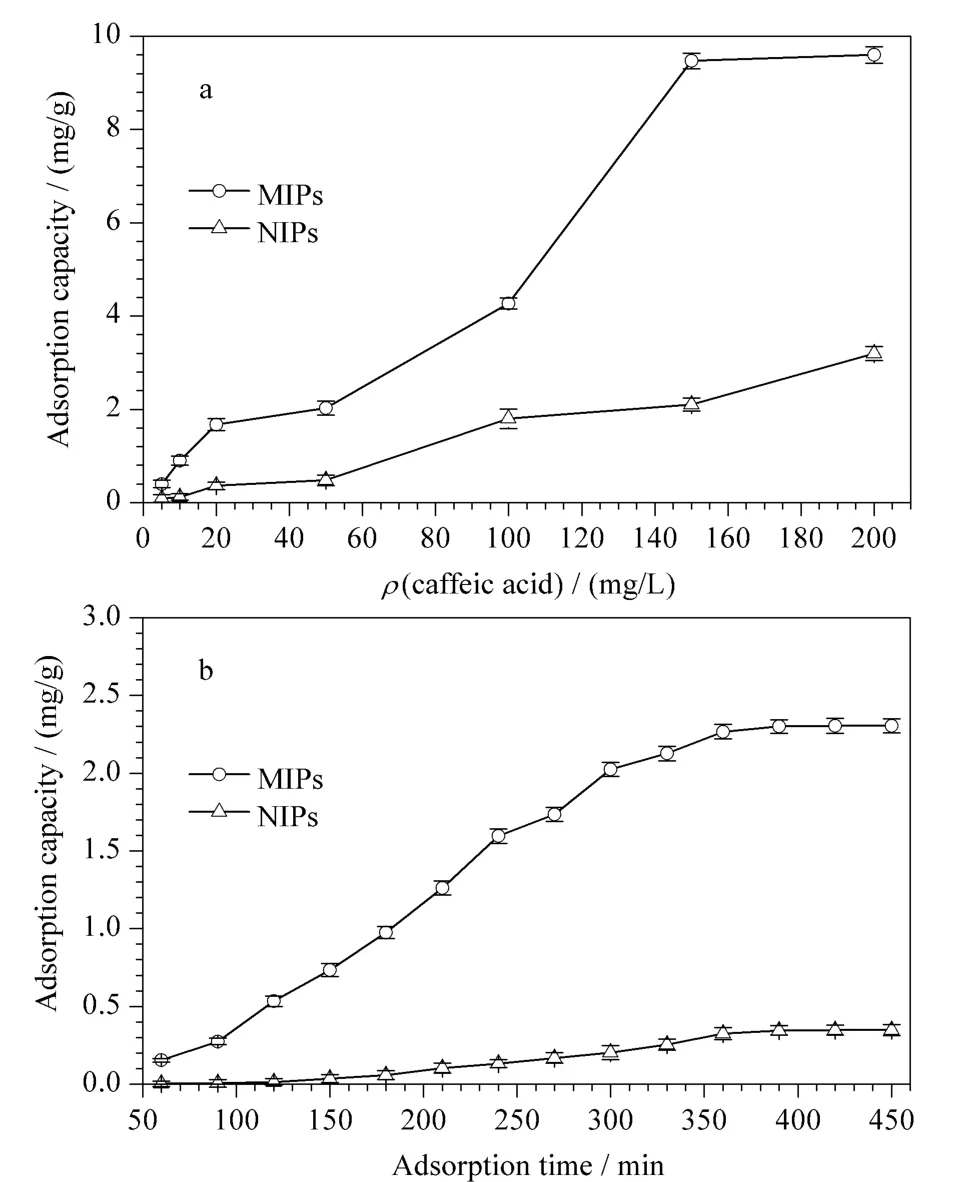
Fig.4 Adsorption capacity of MIPs and NIPs to caffeic acid (n=3)
2.3 Optimization of the chromatographic conditions
The chromatographic conditions were optimized in order to improve the separation efficiency.The chromatograms of the standard caffeic acid samples (50 mg/L)with different volume percentages of acetic acid in the mobile phase are shown in Fig.5.The methanol-water-acetic acid (18/82/0.5,v/v/v)system was tested as the solution to simplify the operation,and the volume percentages of acetic acid in the mobile phase were changed from 0.1% to 0.5%.At last,the optimum mobile phases were methanol-water-acetic acid (18/82/0.5,v/v/v)at a flow rate of 0.8 mL/min.
2.4 Method validation
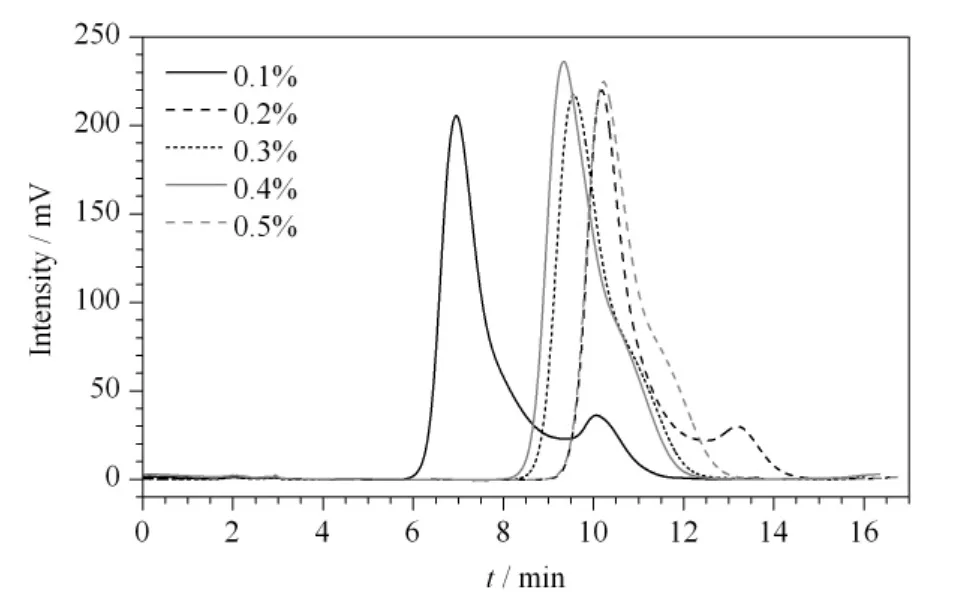
Fig.5 Chromatograms of the standard caffeic acid samples(50 mg/L)with different volume percentages of acetic acid in the mobile phase
The peak areas(Y)of the caffeic acid were measured and plotted against its mass concentrations (X)after HPLC analysis.The standard curve of caffeic acid was linear over the range of 5.00-100.00 mg/L by assaying seven data points and the two quality control samples in triplicate on three separate occasions,and the regression equations were Y=38 696.53X-47 002.27(R2=0.999 9,n=5).
According to Table 1,the precision and accuracy of this method were expressed by performing five replicate analyses for the quality control samples at three different concentrations of caffeic acid on the same day and on consecutive days.The intra-and inter-day relative standard deviations(RSDs)of the proposed method were not more than 5.92%and 5.52%,respectively.
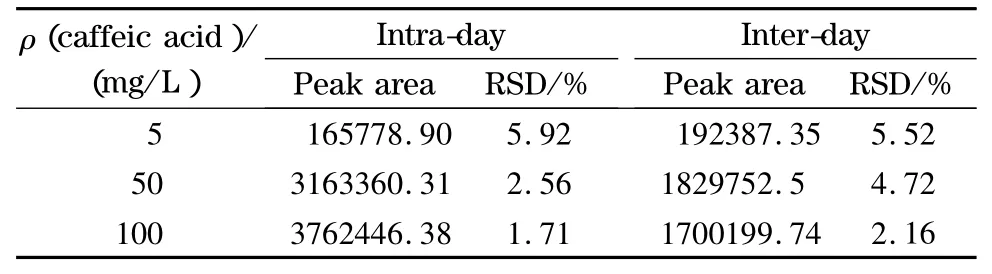
Table 1 Intra-day and inter-day accuracies of caffeic acid by HPLC (n=5)
2.5 Extraction of caffeic acid from hawthorn
MIPs,NIPs,and C18were used for rapid purification of caffeic acid from hawthorn with SPE and the chromatograms are shown in Fig.6.With this established method,the extract yields for caffeic acid were 3.46 μg /g,1.01 μg /g and 1.17 μg/g, respectively. This indicated thatSPE process with the MIPs played an important role in this experiment,and the MIPs had good selectivityfor the caffeic acid.These results were conductive to the quantitative analysis of caffeic acid.
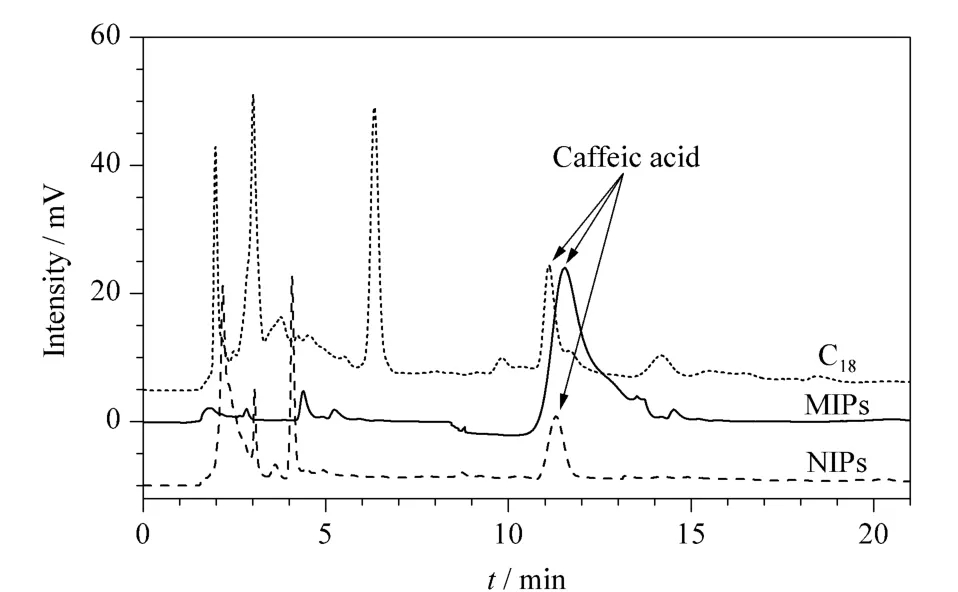
Fig.6 Extraction chromatograms of caffeic acid in hawthorn samples with MIPs,NIPs,and C18SPE cartridge
2.6 DES for optimization of MIPs-SPE procedures
Methanol was mixed with two kinds of DESs(glycerol-based DESs,urea-based DESs)in different ratios (0.5∶1,1∶1,2∶1,3∶1,4∶1,5∶1,v/v),and they were used as elution solutions (2.0 mL)in the MIPs-SPE procedures.Elution capabilitiesofmethanol/glycerol-based DESs and methanol/urea-based DESs in the MIPs-SPE procedures are shown in Fig.7.As the decrease of addition ratio of the two kinds of DESs into methanol,the recoveries increased at first and then decreased. Methanol/glycerol-based DESsobtained better elution capability than methanol/urea-based DESs and pure methanol(72.18% )in the MIPs-SPE procedures,and methanol/glycerol-based DESs (3∶1,v/v)had the best elution capability with the recovery of 82.32%.
The type of DESs is important for the elution capabilities in the MIPs-SPE procedures due to the following effects:diffusion,solubility,viscosity,surface tension,polarity and physicochemical interactions.The DESs with the addition of methanol have significant benefits in terms of a decrease in viscosity,and a lower viscosity solvent is preferred due to the better penetration of pores in the sample matrix.However,an excessive concentration of methanol can decrease the inter-
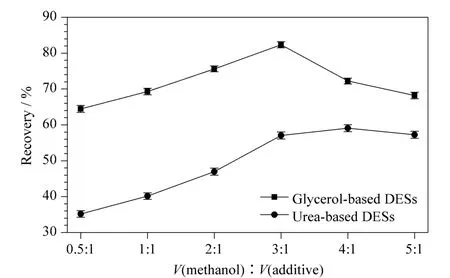
Fig.7 Elution capabilities of methanol/glycerol-based DESs and methanol/urea-based DESs in the MIPs-SPE procedures (n =3)
actions between the DESs and caffeic acid,and increase the polarity of the mixture.The examined viscosities of glycerol-based DESs were greater than the examined viscosities of urea-based DESs,so the elution capabilities of glycerol-based DESs were better than those of urea-based DESs.
The target compounds were adsorbed on the sample matrix by physical adsorption and chemical interactions,such as van der Waals forces,hydrogen bonding,dipole moment and electrostatic interactions.According to the similar dissolve mutually theory,the target compounds can be dissolved easily by solvents with similar polarities,and the order of polarity of the DESs was glycerol-based DESs>urea-based DESs.In addition to the above mentioned factors,the effect of the positioning of the glycerol groups should be considered.The target compound caffeic acid can be considered as a type of hydrogen-bond donor(HBD).Therefore,the glycerol-based HBDs and caffeic acid interact competitively with a chloride anion.If one molecule of glycerol-based HBD has sufficient space between the hydrogen bond donor groups,it can combine the chloride anion so much that only one molecule can complex around it.Another consideration is that excess branches of glycerol-based HBD also resulted in considerable steric hindrance that prevented the interactions between the flavonoids and chloride anions.Therefore,suitable glycerol-based HBD should have proper space between the HBD groups and fewer branches.As a result,glycerol-based DESsshowed better elution capability than urea-based DESs and methanol in the MIPs-SPE procedures.
3 Conclusions
In this work,MIPs and NIPs were prepared in the same procedure,and the FE-SEM and adsorption capacity test were used to evaluate the characteristics of the polymers.The polymers were applied to the rapid purification of caffeic acid from hawthorn.With this established method,the extract yields of caffeic acid from hawthorn with the proposed MIPs,NIPs and C18SPE were 3.46,1.01 and 1.17 μg/g,respectively.To optimize the MIPs-SPE procedures,methanol was mixed with the two kinds of DESs(glycerol-based DESs,urea-based DESs)in different ratios (0.5∶1,1∶1,2∶1,3∶1,4∶1,5∶1,v/v)and they were used as elution solutions.The results showed that MIPs were potential SPE materials,and methanol/glycerol-based DESs (3∶1,v/v)had the best elution capability with the recovery of 82.32%.
[1] Chang W T,Dao J,Shao Z H.Am J Chin Med,2005,33(1):1
[2] Rigelsky J M,Sweet B V.Am J Health Syst Pharm,2002,59(5):417
[3] Jurikova T,Sochor J,Rop O,et al.Molecules,2012,17(12):14490
[4] Eaton L J,Kinkade S.J Fam Pract,2003,52(10):753
[5] Fu J H,Zheng Y Q,Li P,et al.Chinese Journal of Integrative Medicine,2013,19(8):582
[6] Geng C H,Lin M,Wang W Y,et al.Chinese Journal of Analytical Chemistry,2008,63(1):75
[7] Gundogdu M,Ozrenk K,Ercisli S,et al.Biol Res,2014,47:21
[8] Celik S,Erdogan S,Tuzcu M.Pharmacol Res,2009,60(4):270
[9] Maurya D K,Devasagayam T P A.Food Chem Toxicol,2010,48(12):3369
[10] Rajendra Prasad N,Karthikeyan A,Karthikeyan S,et al.Mol Cell Biochem,2011,349(1/2):11
[11] Miles E A,Zoubouli P,Calder P C,et al.Nutrition,2005,21(3):389
[12] Bose J S,Gangan V,Jain S K,et al.Clin Immuno,2009,29:90
[13] Michaluart P,Masferrer J L,Carothers A M,et al.Cancer Res,1999,59:2347
[14] Xing Y,Peng H Y,Zhang M X,et al.J Zhejiang Univ-Sci B:Biomed & Biotechnol,2012,13:487
[15] Iranshahi M,Amanzadeh Y.Chem Nat Comp,2008,44(2):190
[16] Zhao Y K,Cao Q E,Liu H T,et al.Chromatographia,2000,51:483
[17] Zhang L,Lu Y Y,Jiang Y.West China Journal of Pharmaceutical Sciences,2013,28:92
[18] Yan H,Wang F,Han D,et al.Analyst,2012,137:2884
[19] Yan H Y,Wang F,Wang H,et al.J Chromatogr A,2012,1256:1
[20] Gupta V,Kumar M,Brahmbhatt H,et al.Plant Physiol Bioch,2011,49:1259
[21] Zhang Y,Li Y W,Hu Y L,et al.J Chromatogr A,2010,1217:7337
[22] Wu L,Hu B.J Chromatogr A,2009,1216:7657
[23] Wu Q,Wu D P,Duan C F,et al.J Chromatogr A,2012,1265:17
[24] Yang J J,Li Y,Wang J C,ea al.Chinese Journal of Chromatography,2015,33(5):468
[25] Jung S Y,Park J S,Chang M S,et al.Food Sci Biotechnol,2013,22:241
[26] Chen L X,Liu Y X,He X W,et al.Chinese Journal of Chromatography,2015,33(5):481
[27] Yue C Y,Ding G S,Tang A.Chinese Journal of Chromatography,2013,31(1):10
[28] Zhang K G,Hu Y L,Hu Y F,et al Chinese Journal of Chromatography,2012,30(12):1220
[29] Wang C L,Ma F,Zheng H Y,et al.Fine Chemicals,2007,24(8):730
[30] Zheng N,Li Y Z,Chang W B,et al.Anal Chim Acta,2002,452:277
[31] Nicholls I A,Ramstrom O,Mosbach K.J Chromatogr A,1995,691:349
[32] Zhou J,He X,Li Y.Anal Chim Acta,1999,394:353
[33] Takeuchi T,Sunayama H. Encyclopedia of Polymeric Nanomaterials,2014,1:5
[34] Owens P K,Karlsson L,Lutz E S M,et al.TrAC-Trends Anal Chem,1999,18:146
[35] Zhu T,Yoon C H,Row K H.Chin J Chem,2011,29:1246
[36] Blomgre A,Berggren C,Holmberg A,et al.J Chromatogr A,2002,975:157
[37] Li J H,Wen Y Y,Chen L X.Chinese Journal of Chromatography,2013,31(3):181
[38] Sun L,Du F Y,Ruan G H.Chinese Journal of Chromatography,2013,31(4):392
[39] Jodlbauer J,Maier N M,Lindner W.J Chromatogr A,2002,945:45
[40] Wang L H,Wang M Y,Yan H Y,et al.J Chromatogr A,2014,1368:37
[41] Gupta S,Manohar C S.Struct Saf,2004,26:123
[42] Lobo H R,Singh B S,Shankarling G S.Green Chem Lett Rev,2012,5:487
[43] Maugeri Z,Domínguez de María P.RSC Adv,2012,2:421
[44] Durand E,Lecomte J,Villeneuve P.Eur J Lipid Sci Technol,2013,115:379
[45] Tang B,Row K H.Mon Chem,2013,144:1427
[46] Qi L,Zhang J,Zhang Z Q.Chinese Journal of Chromatography,2013,31(3):249
[47] Baldelli S.Acc Chem Res,2008,41:421
[48] Bonhote P,Dias A P,Papageorgiou N,et al.Inorg Chem,1996,35(5):1168
[49] Li J H,Shen Y F,Zhang Y J,et al.Chem Commun,2005,3:360
[50] Bi W T,Tian M L,Row K H.J Chromatogr A,2012,1232:37

