双膦酸盐对NFATc1及相关因子p-NF-κB、p-c-Jun在破骨细胞分化过程中的影响
董伟,彭宏峰,梁永强,冯晓洁,廖囡囡,戚孟春
论 著
双膦酸盐对NFATc1及相关因子p-NF-κB、p-c-Jun在破骨细胞分化过程中的影响
董伟,彭宏峰,梁永强,冯晓洁,廖囡囡,戚孟春
目的研究双膦酸盐(ALN)对破骨细胞(OC)分化过程中关键因子NFATc1及其信号分子p-NF-κB、p-c-Jun的影响。方法采用小鼠单核巨噬细胞RAW264.7诱导培养OC,细胞培养于培养皿,分A组(对照组)、B组(ALN组)。培养第2d采用Western blotting检测NFATc1、p-NF-κB、p-c-Jun基因表达变化情况,培养第4d采用免疫荧光方法检测NFATc1表达,培养第7d检测各组OC生成及数目。采用牙本质磨片接种RAW264.7细胞,分组及处理方法同上述培养皿培养过程,第9d检测骨吸收功能。结果两组细胞均有TRAP阳性多核OC生成,并在牙本质磨片上形成吸收陷窝;但A组TRAP阳性多核细胞数目、吸收陷窝数目及陷窝面积均大于B组。免疫荧光检测NFATc1表达A组高于B组;Western blotting检测NFATc1、p-NF-κB、p-c-Jun表达B组较A组低,两组有显著性差异(P<0.01)。结论双膦酸盐通过下调p-NF-κB、p-c-Jun的表达,最终抑制NFATc1的表达,从而抑制OC生成和骨吸收功能。
阿仑膦酸盐;破骨细胞;NFATc1;NF-κB;基因,jun
破骨细胞的功能分化过程是当今研究的重点[1],许多骨骼疾病如骨质疏松症[2]、类风湿性关节炎、Paget's病、多发性骨髓瘤、恶性肿瘤的溶骨性骨转移等,都与破骨细胞(osteoclasts,OC)增殖、骨吸收功能亢进密切相关[3]。阿仑膦酸盐(alendronate,ALN)作为第三代含氮双膦酸盐,是现阶段治疗上述疾病的首选药物,除此之外还被用作预防和治疗骨密度降低及成骨不全症等疾病[4]。ALN主要通过抑制破骨细胞骨吸收功能、诱导破骨细胞凋亡来发挥作用,但确切机制及涉及的胞内信号通路目前知之甚少,特别是对破骨细胞分化过程中关键信号分子NFATc1及其重要通路如NF-κB通路、MKK通路是否产生影响等。本研究在OC培养过程中加入ALN并检测NFATc1的表达,分析影响其表达的p-c-Jun、p-NF-κB,以初步揭示双膦酸盐抑制OC的分子机制,为ALN的临床应用提供理论基础。
1 材料与方法
1.1 实验材料 DMEM培养基(Gibco,美国),RANKL(receptor activator of nuclear factor κB ligand) (Biovision,美国),阿仑膦酸盐(Sigma,美国),兔抗鼠NFATc1、p-c-Jun、p-NF-κB多克隆抗体(Santa Cruz,美国),羊抗兔辣根过氧化物酶标记IgG和RIPA(武汉博士德,中国),抗酒石酸酸性磷酸酶(TRAP)染色试剂盒(Sigma,美国),RAW264.7小鼠单核巨噬细胞系(中国科学院细胞库)。
1.2 OC的培养及实验分组 RAW264.7细胞以1×105/孔的密度接种于48孔培养板中,DMEM培养基(含15%胎牛血清、100U/ml青霉素、100μg/ ml链霉素)培养,细胞贴壁后分为两组,对照组加入100ng/ml RANKL;处理组加入100ng/ml RANKL+5×10-7mol/L ALN,定期于倒置相差显微镜下观察。
1.3 抗酒石酸酸性磷酸酶(TRAP)染色 接种于48孔板的细胞于处理后第7d收获,按TRAP染色试剂盒操作步骤进行染色,显微镜100倍放大倍数下随机选取5个视野计数TRAP染色阳性细胞(细胞核≥3 个)数目,5个视野计数取平均值为该细胞爬片的破骨细胞数目。
1.4 牙本质磨片制备及吸收陷窝检测 将人离体牙制备成直径约0.5cm,厚度约0.2mm的牙本质磨片,打磨抛光表面,消毒灭菌后将RAW264.7细胞接种其上,细胞贴壁后分为两组(同1.2细胞处理方法)。取处理后第9天的牙本质磨片,2.5%戊二醛4℃固定7min,1mol/L氢氧化胺中以50Hz超声清洗5min,再经蒸馏水超声清洗5min×3次;随后2.5%戊二醛固定2h,1%锇酸固定2h,乙醇逐级脱水,醋酸异戊酯置换,CO2临界点干燥,镀金,扫描电镜(HITACHIS-4800)观察吸收陷窝。在500倍放大倍数下每孔牙本质磨片上随机选取5个视野,用医学数码图像分析系统Med6.0测量五个视野吸收陷窝总数目及总面积。
1.5 免疫荧光法检测NFATc1表达 取培养至第4d的RAW264.7细胞,PBS冲洗,4%多聚甲醛固定10min,0.5%Triton穿孔15min,1%BSA封闭30min,PBS冲洗5min×2次。加入5%BSA稀释的兔抗鼠NFATc1多克隆一抗(1:25)过夜。PBS冲洗,加入5%BSA稀释的FITC标记的羊抗兔IgG,37℃孵育2h,5μg/ml DAPI染色2min,于共聚焦显微镜下观察。
1.6 Western blotting检测NFATc1、p-NF-κB、p-c-Jun蛋白表达 两组细胞培养第2天,提取细胞总蛋白BCA法定量。上样量35μg,采用12%SDS-PAGE凝胶电泳分离,采用湿法转膜至PVDF膜,5%BSA室温封闭1h,分别加入1μg/ml兔抗鼠NFATc1、p-NF-κB、p-c-Jun多克隆一抗孵育过夜,羊抗兔辣根过氧化物酶标记IgG二抗结合室温孵育1h。TBST洗膜后按化学发光试剂盒说明书制备反应液,室温孵育5min。用Image J分析软件对条带吸光度值(A)进行半定量分析,以目的蛋白条带(A)值/内参GAPDH蛋白条带(A)值的比值表示目的蛋白的相对量。
1.7 统计学处理 采用SPSS 11.0软件进行统计分析。计量数据以表示,组间比较采用SNK-q检验,P<0.05为差异有统计学意义。
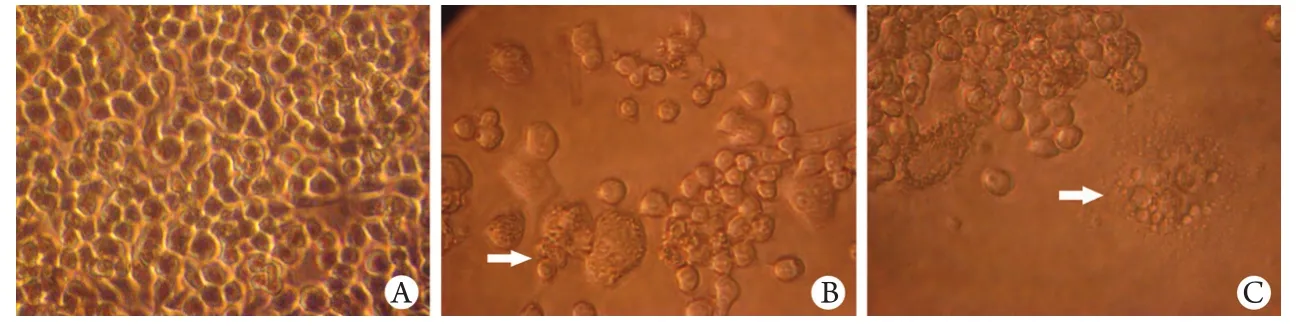
图1 倒置相差显微镜下观察RAW264.7细胞生长情况(×400)Fig.1 RAW264.7 cell growth observed under inverted phase contrast microscope (×400) A. Incubated for 1 day; B. Incubated for 3 days; C. Incubated for 7 days
2 结 果
2.1 倒置相差显微镜观察OC生成情况 培养开始时,贴壁的RAW264.7呈圆形铺于培养板底壁(图1A)。培养至第3天,在诱导因子RANKL的作用下,RAW264.7开始相互融合(图1B)。培养至第7d,细胞融合成多核OC(图1C)。
2.2 TRAP染色及定量分析 对照组OC生成数量显著高于处理组(P<0.01),提示ALN可显著抑制OC的生成(图2)。
2.3 牙本质吸收陷窝观察及定量分析 两组比较,对照组OC吸收陷窝数目较多,陷窝总面积较大,两组间吸收陷窝数目及总面积均有显著性差异(P<0.01,图3,表1)。上述结果表明,ALN可有效抑制OC的骨吸收功能。
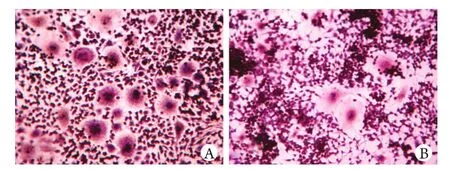
图2 细胞培养第7天的TRAP染色比较(×200)Fig. 2 TRAP staining of osteoclasts on the 7th day (×200)A. Control group; B. ALN-treated group

图3 细胞培养第9天骨吸收陷窝形成情况(SEM×500)Fig. 3 Resorption lacunaes formed on dentin slices after culture for 9 days (SEM×500) A. Control group; B. ALN-treated group
2.4 免疫荧光检测NFATc1表达 两组细胞培养至4d,免疫荧光检测见NFATc1表达(图4),对照组NFATc1荧光强度(5.42±0.63)明显大于处理组(1.26±0.15)(P<0.01)。
2.5 Western blotting检测NFATc1,p-NF-κB,p-c-Jun蛋白的表达 Western blotting检测两组细胞NFATc1、p-NF-κB、p-c-Jun蛋白含量变化,处理组NFATc1、p-NF-κB、p-c-Jun蛋白表达较对照组明显减弱,条带灰度值分别下降47.1%、39.3%、18.4%;结果显示,ALN可抑制OC细胞NFATc1、 p-NF-κB、p-c-Jun的表达。

表1 TRAP阳性破骨细胞计数及牙本质磨片吸收陷窝计数和陷窝面积Tab.1 The number of TRAP(+) osteoclasts, number and size of resorption lacunaes formed on dentin slices in each group
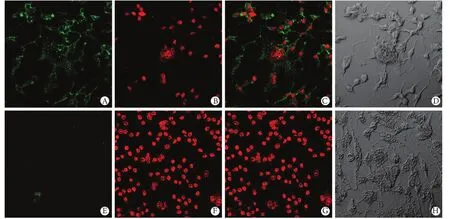
图4 免疫荧光化学法检测两组NFATc1的表达(×400)Fig. 4 Detection of NFATc1 expression by immunofluorescent chemistry (×400) A, E. NFATc1 expression labelled by FITC; B, F. Cell nucleus labelled by DAPI; C, G. Integrated image; D, H. Normal vision without fluorescence

图5 Western blotting检测两组NFATc1、p-NF-κB、p-c-Jun蛋白表达Fig.5 Detection of NFATc1, p-NF-κB, p-c-Jun protein expression in two groups of cells by Western blotting
3 讨 论
OC是骨吸收时唯一的效应细胞,由多功能造血干细胞在诱导因子(RANKL)的诱导下相互融合形成的多核巨细胞。在OC形成过程中,众多因子及信号通路发挥重要作用[5-7],其中活化T细胞核因子NFATc1为破骨细胞分化的重要信号分子[8],而NF-κB及MKK是影响NFATc1较为关键的信号通路分子[9-10]。
在OC分化中,当RANKL与其受体即OC前体上的RANK相互作用后,引起了下游一系列信号因子的级联作用[11]。首先,引发钙/钙调磷酸酶/活化T细胞核因子(calcium/calcineurin/NFAT)活化,导致胞质内Ca2+波动,Ca2+/钙调蛋白依赖的钙调磷酸酶被激活,使胞质内NFATc1蛋白异位进入细胞核[12];同时,NF-κB参与作用的经典途径(classical pathway)和替代途径(alternative pathway)被激活。在经典途径中,由IKKβ和NEMO/IKKγ组成的IKK复合物是关键信号因子,它的作用是诱导在细胞质中与无活性NF-κB形成复合物的抑制蛋白IκB磷酸化,随后NF-κB被释放,形成二聚体p65/cRel[13];而替代途径的关键蛋白是NF-κB诱导激酶NIK(NF-κB-inducing kinase),其激活IκB激酶IKKα,促使NF-κB释放并形成二聚体RelB/p52[13]。上述两途径形成的NF-κB二聚体转移至细胞核中与NFATc2共同作用于NFATc1基因启动子,诱发NFATc1基因初始表达,随后NFATc1基因表达进入自发扩增阶段;与此同时,在RANKL刺激下,另一通路MKK信号通路启动,JUN被激活,NF-κB磷酸化,最终c-Jun磷酸化,c-Jun与c-Fos形成催化蛋白-1(AP-1),c-Fos被激活,选择性地募集到NFATc1基因启动子上,与NF-κB、NFATc2共同促进NFATc1基因的表达,进而促使多核OC前体的形成[14-15]。随后,OC前体活化形成成熟的破骨细胞,发挥其骨吸收功能。
ALN是现阶段治疗骨质疏松等疾病的常用临床药物。众多学者通过动物实验及细胞实验两水平均证实了其对OC分化及骨吸收功能的抑制作用,但其是否对NFATc1表达产生抑制,并影响相关通路的作用,国内外研究并未给出确切结论。本研究结果表明,ALN通过抑制p-NF-κB、p-c-Jun的表达,影响NF-κB参与通路及MKK信号通路,最终下调NFATc1基因表达,从而起到抑制OC生成及骨吸收功能的作用。本实验部分揭示了双膦酸盐对OC生成抑制作用的分子学机制,将更好地为双膦酸盐在临床中的应用提供实验依据。然而,ALN是否会通过影响钙/钙调磷酸酶/活化T细胞核因子(calcium/ calcineurin/NFAT)通路,导致胞质内Ca2+波动受阻,从而阻碍NFATc1发生核异位还需要进一步实验验证。
[1]Yang FX, Yang DZ, Zhou JP,et al. Effect of ERK1/2 signal pathway on the expression of OPG/RANKL in cementoblasts under stress stimulation[J]. Med J Chin PLA, 2014, 39(12): 941-945. [杨凤雪, 杨冬珍, 周建萍, 等. 应力刺激下ERK1/2信号通路对成牙骨质细胞OPG/RANKL表达的影响[J]. 解放军医学杂志, 2014, 39(12): 941-945.]
[2]Ma XJ, Kong XL, Meng XJ,et al. Study on the related factors of affecting the adult female osteoporosis[J]. Chin J Pract Intern Med, 2014, 34(S1): 78-81. [马小静, 孔祥雷, 孟祥菊, 等. 影响成年女性骨质疏松的相关因素研究[J]. 中国实用内科杂志, 2014, 34(S1): 78-81.]
[3]Ikeda F, Nishimura R, Matsubara T,et al. Critical roles of c-Jun signaling in regulation of NFAT family and RANKL-regulated osteoclast differentiation[J]. J Clin Invest, 2004, 114(4): 475-484.
[4]Zhu HM. Bisphosphonates and osteoporosis[J]. Chin J Pract Intern Med, 2011, 31(7): 517-520. [朱汉民. 双膦酸盐和骨质疏松[J]. 中国实用内科杂志, 2011, 31(7): 517-520.]
[5]Kobayashi, N, Kadono Y, Naito A,et al. Segregation of TRAF6-mediated signaling pathways clarifies its role in osteoclastogenesis[J]. EMBO J, 2001, 20(6): 1271-1280.
[6]Kim JH, Kim K, Youn BU,et al. MHC class Ⅱ transactivator negatively regulates RANKL-mediated osteoclast differentiation by downregulating NFATc1 and OSCAR[J]. Cell Signal, 2010, 22(9): 1341-1349.
[7]Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation[J]. Bone, 2007, 40(2): 251-264.
[8]Yuroda Y, Hisatsune C, Nakamura T,et al. Osteoblasts induce Ca+ oscillation-independent NFATc1 activation during osteoclastogenesis[J]. Proc Natl Acad Sci USA, 2008, 105(25): 8643-8648.
[9]Franzoso, G, Carlson L, Xing L,et al. Requirement for NF-kappaB in osteoclast and B-cell development[J]. Genes Dev, 1997, 11(24): 3482-3496.
[10] Miyazaki M, Fujikawa Y, Takita C,et al. Tacrolimus and cyclosporine A inhibit human osteoclast formationviatargeting the calcineurin-dependent NFAT pathway and an activation pathway for c-Jun or MITF in rheumatoid arthritis[J]. Clin Rheumatol, 2007, 26(2): 231-239.
[11] Huang H, Ryu J, Ha J,et al. Osteoclast differentiation requires TAK1 and MKK6 for NFATc1 induction and NF-κB transactivation by RANKL[J]. Cell Death Differ, 2006, 13(11), 1879-1891.
[12] Komarova SV, Pilkington MF, Weidema AF,et al. RANK ligand-induced elevation of cytosolic Ca2+accelerates nuclear translocation of NF-kB inosteoclasts[J]. J Biol Chem, 278(10): 8286-8293.
[13] Deborah V N. Role of NF-κB in the skeleton[J]. Cell Research, 2011, 21(1): 169-182.
[14] Asagiri M, Sato K, Usami T,et al. Autoamplification of NFATc1 expression determines its essential role in bone homeostasis[J]. J Exp Med, 202(9): 1261-1269.
[15] Matsuo K, Galson DL, Zhao C,et al. Nuclear factor of activated Tcells (NFAT) rescues osteoclasto- genesis in precursors lacking c-Fos[J]. J Biol Chem, 279(25): 26475-26480.
Effect of bisphosphonates on NFATc1 and correlators p-NF-κB and p-c-Jun in osteoclast differentiation
DONG Wei, PENG Hong-feng, LIANG Yong-qiang, FENG Xiao-jie, LIAO Nan-nan, QI Meng-chun*
School of Stomatology, North China University of Science and Technology, Tangshan 063000, China
*< class="emphasis_italic">Corresponding author, E-mail: qimengchun@163.com
, E-mail: qimengchun@163.com
This work was supported by the National Natural Science Foundation of China (81270965) and the Natural Science Foundation of Hebei Province (C2011401044)
ObjectiveTo study the effect of alendronate (ALN) on NFATc1 and correlated signaling factors p-NF-κB and p-c-Jun in osteoclast differentiation.MethodsOsteoclasts were inductively cultivated with mouse mononuclear macrophage RAW264.7, and they were divided into 2 groups: group A (control group) and group B (ALN-treated group). The protein expressions of NFATc1, p-NF-κB and p-c-Jun were determined with Western blotting at the 2nd day of cultivation; the expression of NFATc1 was assessed by immunofluorescent assay on the 4th day; and the osteoclast formation was examined on the 7th day of cultivation. RAW264.7 cells were inoculated on abrasive dentine disk, and divided into 2 groups and treated as mentioned above. The resorption function of osteoclast was observed on the 9th day of inoculation.ResultsTRAP positive multinuclear osteoclasts were observed, and resorption lacunaes formed in the abrasive dentine disks of the 2 groups. More TRAP positive multinuclear cells and resorption lacunaes in large size were found in group A than those in group B. Immunofluorescence assay showed the expression of NFATc1 was higher in group A than in group B. The gene expressions of NFATc1, p-NF-κB and p-c-Jun were lower in group B than in group A (P<0.01) as determined with Western blotting.ConclusionBy down-regulating the expressions of p-NF-κB and p-c-Jun, ALN may strongly inhibit the osteoclast formation and its resorption function, thus inhibiting NFATc1 expression.
alendronate; osteoclast; NFATc1; NF-κB; genes, jun
R329.24
A
0577-7402(2015)10-0778-04
10.11855/j.issn.0577-7402.2015.10.02
2014-11-25;
2015-08-20)
(责任编辑:张小利,沈宁)
国家自然科学基金(81270965);河北省自然科学基金(C2011401044)
董伟,医学硕士,讲师。主要从事骨组织工程方面的研究
063000 河北唐山 华北理工大学口腔医学院(董伟、彭宏峰、梁永强、冯晓洁、廖囡囡、戚孟春)
戚孟春,E-mail: qimengchun@163.com

