Pyrolysis Characteristics and Kinetics of Methyl Oleate Based on TG-FTIR Method
(Department of Military Oil Application & Administration Engineering, Logistical Engineering University, Chongqing 401311)
Pyrolysis Characteristics and Kinetics of Methyl Oleate Based on TG-FTIR Method
Wang Xuechun; Fang Jianhua; Chen Boshui; Wang Jiu; Wu Jiang
(Department of Military Oil Application & Administration Engineering, Logistical Engineering University, Chongqing 401311)
The thermal decomposition characteristics of methyl oleate were preliminarily investigated under nitrogen atmosphere by a thermogravimetric analyzer when the ester was heated at a heating rate of 10 ℃/min from room temperature to 600 ℃. Furthermore, the pyrolytic and kinetic characteristics of methyl oleate were intensively studied at different heating rates. The gaseous species obtained during thermal decomposition were also identified by the TG-FTIR coupling analysis. The results showed that the pyrolysis of methyl oleate proceeded in three stages, viz. the drying stage, the main pyrolysis stage and the residual pyrolysis stage. The initial decomposition temperature, the maximum weight loss temperature, the peak decomposition temperature and the rate of maximum weight loss of methyl oleate increased with the increasing heating rates. Gaseous CO, CO2and H2O were the typical decomposition products from pyrolysis of methyl oleate. In addition, a kinetic model for thermal decomposition of methyl oleate was built up based on the experimental results using the Coats-Redfern integral method and the multiple-linear regression method. The activation energy, the pre-exponential factor, the reaction order and the kinetic equation for thermal decomposition of methyl oleate were obtained. Comparison of the experimental data with the calculated ones and analysis of statistical errors of pyrolysis ratios demonstrated that the kinetic model was reliable for studying the pyrolysis of methyl oleate. Finally, the kinetic compensation effect between the preexponential factors and the activation energy in the pyrolysis of methyl oleate was also confirmed.
methyl oleate; pyrolysis; kinetics; thermogravimetric analysis; biodiesel
1 Introduction
With the continuous depletion of petroleum reserves and the increasing environmental pollution problems brought about by the extensive use of petroleum, the exploitation of alternative fuels and renewable resources has attracted increasing attention worldwide from the perspective of environmental protection and resource strategy[1-5]. Biodiesel, which is referred to as mixtures of fatty acid mono-alkyl esters with relatively high contents of the long-chain, mono- and poly-unsaturated compounds, is produced from vegetable oils and animal fats by transesterification with alcohols of low molecular weights in the presence of a catalyst[6-8]. Fatty acid methyl esters (FAME) such as the soybean methyl ester (SME) are typically mixtures of esters with 16—18 carbon atoms, in which 80%—85% of the total mixture are unsaturated compounds[9]. However, the presence of such mono- and poly-unsaturated compounds makes biodiesel extremely liable to thermal decomposition at elevated temperatures. As we know, during the operation of a biodiesel-powered engine, a small amount of biodiesel will leak into the crankcase by oil seepage flow or gas entrainment. The inleakage of biodiesel into the engine crankcase markedly impairs the quality of the engine oils due to thermal instability of biodiesel. At present, some studies have been made on biodiesel-induced deterioration of engine oil in order to facilitate the development and application of biodiesel as a clean and renewable petro-diesel substitute[10-12]. However, the thermal decomposition characteristics and kinetics of biodiesels, as well as their influence on deterioration of engine oils, have so far not been investigated intensively, partly because of the complexity of its pyrolytic chemical behaviors and mechanisms. In fact, thermal instability of biodiesel is governed by its chemi-cal nature, especially the structures and compositions of unsaturated fatty acid methyl esters. It is therefore essential to investigate the thermal decomposition behaviors of unsaturated FAME so as to better understand the nature of biodiesel-induced deterioration of engine oil. In this paper, the thermal decomposition characteristics and kinetics of unsaturated methyl oleate were preliminarily studied based on thermogravimetric analysis. The present investigation is of significance for further understanding the pyrolytic behaviors of biodiesel and, in the nature of things, its influence on engine oil deterioration.
2 Experimental
2.1 Materials and apparatus
Methyl oleate (abbreviated as MOE): An analytically purified chemical supplied by the Xiya Reagent Research Center. Thermo-gravimetric analyzer: model SDT-Q600, made by the TA Instruments, USA.
2.2 Thermogravimetric analysis
The pyrolysis characteristics of MOE were tested on the thermogravimetric analyzer. In each test run, 7 mg of MOE were put uniformly on the bottom of an alumina crucible, which was placed at the same position of the beam platform of the analyzer. Subsequently, the sample was continuously heated from room temperature to 600 ℃ at different heating rates of 10, 15, 20 and 30 ℃/min, respectively, under a nitrogen carrier gas flowing at a rate of 50 mL/min. The weight loss and heat flow changes in response to temperature were recorded. Finally, the thermogravimetric (TG) curves, the derivative thermogravimetric (DTG) curves and the differential scanning calorimetric (DSC) curves were plotted and the pyrolysis kinetics were studied.
3 Results and Discussion
3.1 Pyrolysis characteristics of MOE
3.1.1 Pyrolysis of MOE at different heating rates
Shown in Figure 1 are the TG-DTG and DSC curves of MOE at a heating rate of 10 ℃/min. Table 1 shows the pyrolysis parameters of MOE. It can be clearly observed from Figure 1 and Table 1 that, with an increasing temperature, the pyrolysis process of MOE in TG curves can be divided into three stages, viz.: the drying stage (<Ts), the main pyrolysis stage (Ts—Tf), and the residual pyrolysis stage (>Tf). The first stage occurred as the temperature increased from room temperature to the initial decomposition temperature Ts. The second stage occurred as the temperature increased from Tsto the maximum weight loss temperature Tf, while the third stage exhibited a further slow loss of weight as the temperature increased from Tfto 600 ℃. To further understand the pyrolysis characteristics of MOE, thermogravimetric tests at different heating rates (β), i.e.: 10 ℃/min, 15 ℃/min, 20 ℃/min and 30 ℃/min, respectively, were also conducted. The pyrolysis curves of MOE at different heating rates are shown in Figure 2. Also shown in Table 2 are the main pyrolysis characteristic parameters of MOE. It can be seen from the TG curves in Figure 2(a) that the decomposition temperatures at different heating rates were slightly different. When the heating rate increased from 10 ℃/min to 30 ℃/min, the initial temperature for main decomposition shifted to a higher one. In the DSC curves shown in Figure 2(b), strong peaks occurring between 213.79 ℃ and 326.03 ℃can be found for each heating rate. Higher heating rate shifted the DSC curve to a higher range of temperatures. The DTG profiles given in Figure 2(c) indicated that the maximum weight loss rate increased and the corresponding peak temperature at maximum rate shifted to higher temperatures with an increasing heating rate. The increase in the initial decomposition temperature, the maximum weight loss temperature, the peak decomposition temperature and the rate of maximum weight loss with increasing heating rates could be attributed to the reduction of the activated energy, as well as to the delay of heat transfer during thermal decomposition of MOE[13-16].

Figure 1 TG/DTG and DSC curves of MOE under N2atmosphere
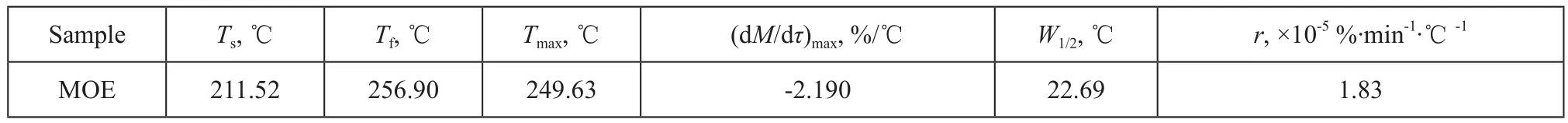
Table 1 Pyrolysis parameters
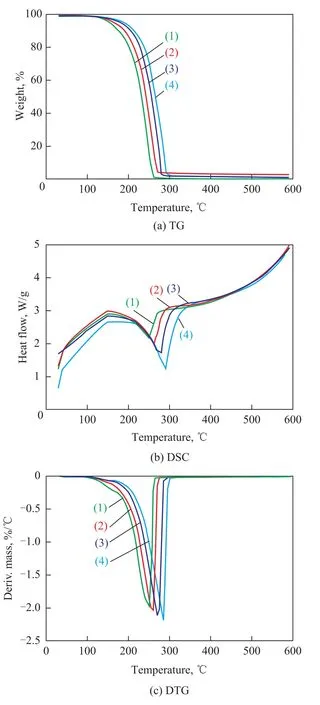
Figure 2 TG/DTG and DSC curves of MOE at different hating rates
3.1.2 TG-FTIR analysis of gaseous products
The gaseous products evolved during pyrolysis of MOE at different thermal decomposition durations at a heating rate of 10 ℃/min were investigated on a three-dimensional TG-FTIR coupling analyzer. A typical 3D plot of FTIR spectrum is shown in Figure 3. Also shown in Figure 4 are the FTIR spectra of the gases evolved at the duration of 9.387 min, 25.554 min, 32.333 min and 48.500 min, respectively. The main gaseous species obtained during thermal decomposition and identified from the spectra cover CO and CO2(2 250—2 375 cm-1, 700 cm-1) and H2O (3 500—3 800 cm-1, 1 200—1 700 cm-1). The peaks at 1 600—1 850 cm-1and 1 250—1 500 cm-1are associated with the stretching vibration of C=O, the bending vibration of C—H and the stretching vibration of C—O and C—C. Besides, very weak peaks of C—H at 2 650—3 000 cm-1correspond to alkanes, aldehydes, ketones, carboxylic acids, alcohols and other macromolecular substances[17-19]. This implies that the thermal reactions of MOE covered dehydration, cracking and polymerization.

Table 2 Pyrolysis parameters of MOE at different heating rat

Figure 3 The 3D FT-IR spectra of MOE pyrolysis gaseous products

Figure 4 FT-IR spectra of the evolved gases from MOE pyrolysis at different durations
3.2 Kinetics of thermal decomposition of MOE
3.2.1 Kinetics modeling
The kinetic parameters obtained from TG and DTG analyses are extremely crucial for efficient evaluation and calculation of the thermal decomposition process of MOE. Assuming that the thermal decomposition of MOE is a non-isothermal process, the decomposition rate equation can be given as follows:

where α is the conversion rate and is defined as: α=(m0-mt)/(m0-m∞); m0is the initial weight of the test sample; mtis the weight after a specified decomposition duration t; while m∞is the weight of the indecomposable residue; n-reaction order; and k-reaction rate constant. Generally, the Arrhenius equation is suitable for the thermal decomposition reaction. Based on the Arrhenius equation, the reaction rate constant k for thermal decomposition of MOE is given below:

where, A—pre-exponential factor, min-1; E—activation energy, kJ/mol; T—reaction temperature, K; R—ideal gas constant, 8.314 J/(mol·K).
Since the mechanism function f(α) in Equation (1) is dependent on the reaction model and the reaction mechanism during pyrolysis process, therefore for a simple reaction, f(α) is suggested as:

Then, Equations (1), (2) and (3) can be combined to give:
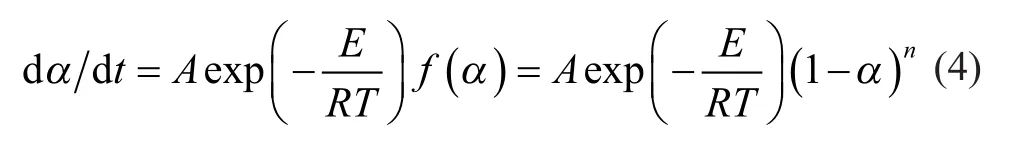
Furthermore, substituting the heating rate, β, into Equation (4) gives

where β=dT/dt.
3.2.2 Determination of kinetic parameters
According to the kinetic model given above, the kinetic parameters such as the pre-exponential factor, the activation energy and reaction order, and the most probable mechanism function for thermal decomposition of MOE were determined based on the Coats-Redfern integral method and the multiple-linear regression method, respectively.
(1) Coats-Redfern integral method
Based on the Coats-Redfern method[20-21], which is suited to different reaction orders, Equation (5) can be rearranged by taking the natural logarithm as given in Equation (6):
通过采用折线滑动法分别计算滑坡各剖面的稳定系数及剩余下滑力,可以得出如下结论:滑坡在自重工况下,处于稳定-基本稳定状态,与宏观分析结果一致;在暴雨工况下,滑坡处于基本稳定-不稳定状态。
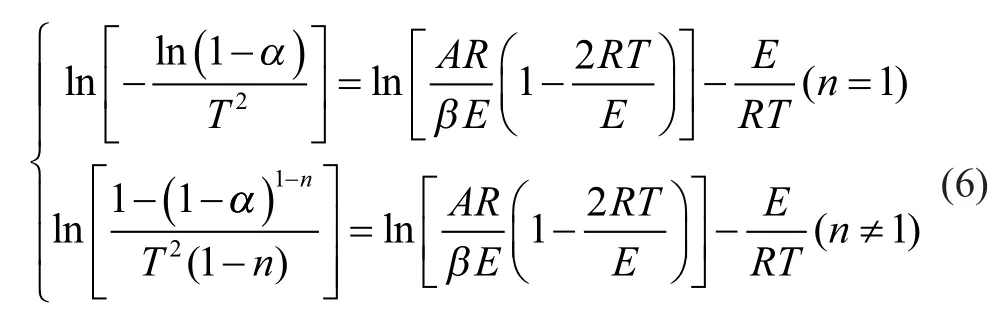

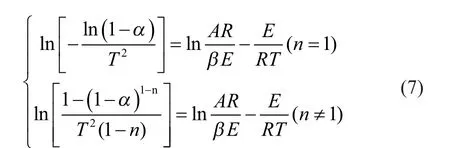
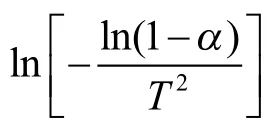
(2) Multiple linear regression method
Taking the natural logarithm of Equation (5) gives:
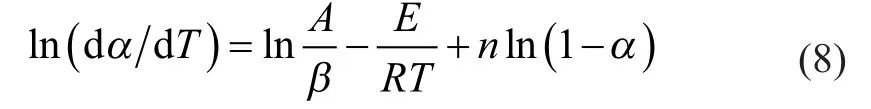
where dα/dT is the ratio of weight changes with temperature. Equation (8) may be further expressed in the linear form as given below:

where Y=ln(dα/dT), X=1/T, Z=ln(1-α), B=ln(A/β), C=-E/R, and D=n.
The constants B, C and D were estimated by the multiple linear regression with the TG-DTG data for pyrolysis of MOE using the Origin 8.0 software. The kinetic parameters, viz.: the pre-exponential factor, the activation energy and the reaction order for each test run, are presented in Table 4.

Figure 5 The plot of ln[-ln(1-α)/T2] and ln[-(1-(1-α)1-n)/(1-n)T2] vs 1/T
3.2.3 Kinetic reliability analysis
The relationship and the numerical statistical errors of conversion rate between the experimental data and the calculated data by the Coats-Redfern integral method and by the multiple linear regression method at different heating rates are shown in Figure 6, Figure 7 and Table 5, respectively. The numerical statistical errors including the root mean square error (RMSE) and the mean absolute percentage (MAPE) were calculated by the following equations:

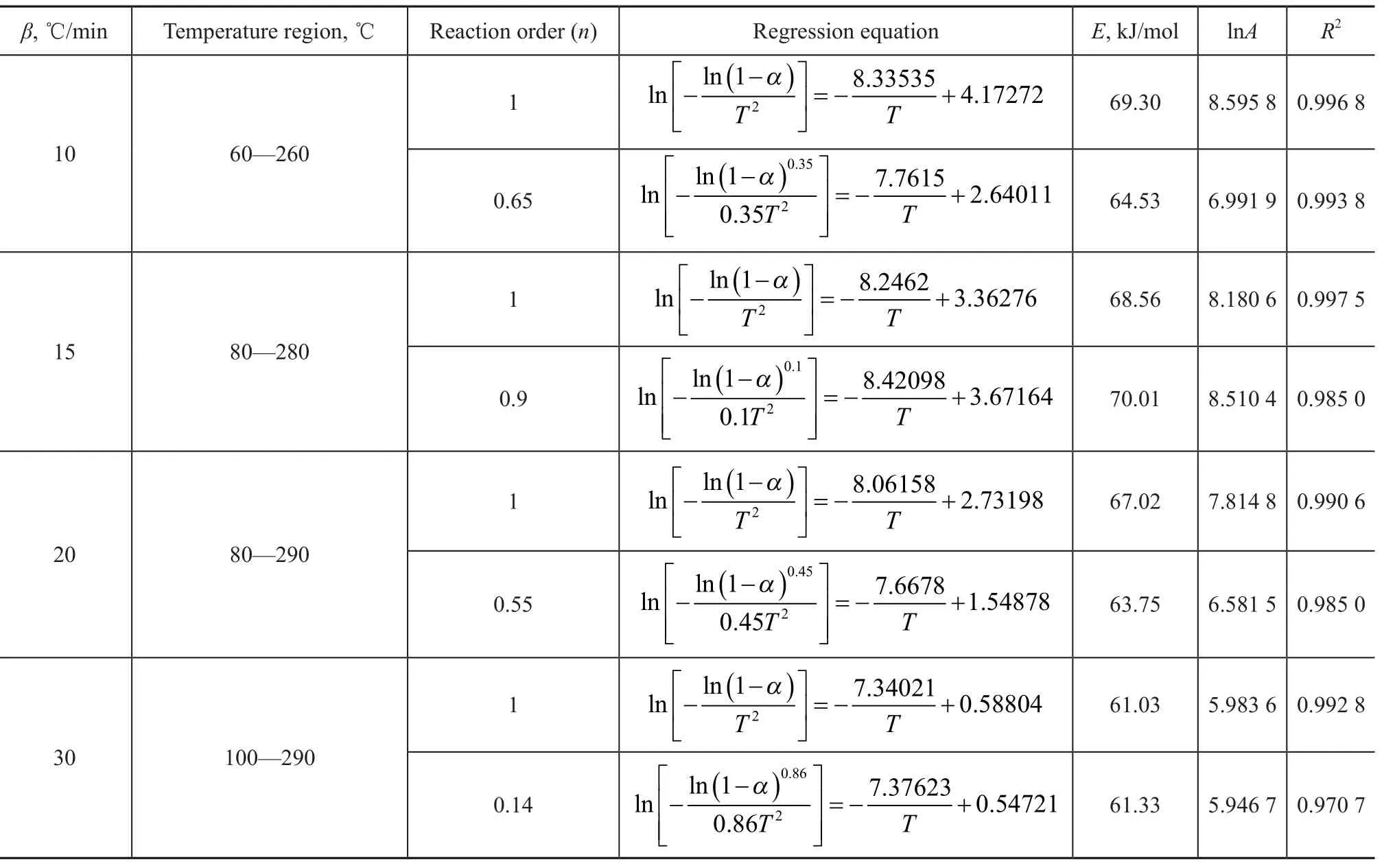
Table 3 Pyrolysis kinetic parameters of MOE measured at different heating rate
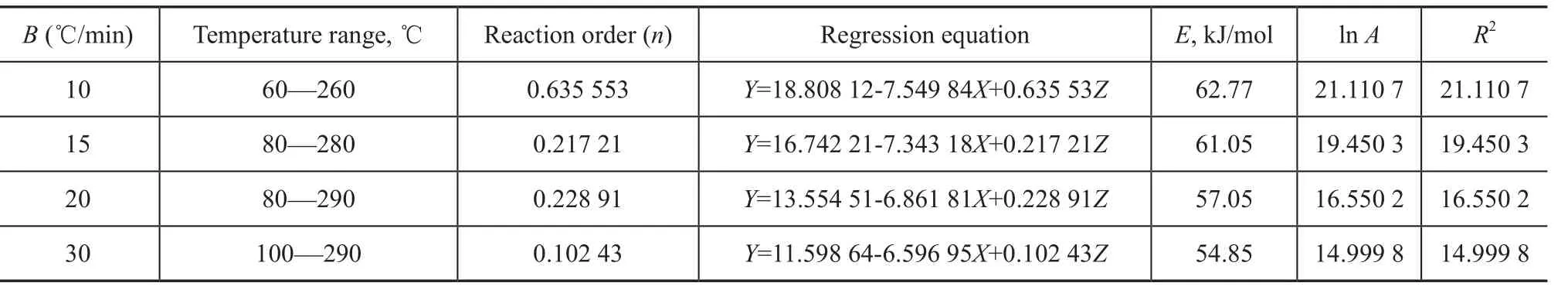
Table 4 Pyrolysis kinetic parameters of MOE by multiple linear regression method
3.2.4 Kinetic compensation effect in pyrolysis of MOE
It has been found that for thermal decomposition reactions, the kinetic parameters such as the pre-exponential factor A and the activation energy E have the following relationship[22-23]:
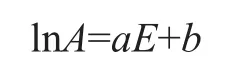
in which a and b are constant coefficients. This relationship is referred to as the kinetic compensation effect, meaning that the pre-exponential factor of a thermal decomposition reaction is not a constant but changes with the variation of activation energy. Therefore, based on the kinetic parameters listed in Table 3 and Table 4, the relationship between lnA and E for pyrolysis of MOE was plotted, as shown in Figure 8. It can be observed clearly from Figure 8 that, with the correlation coefficients ranging from 0.982 7 to 0.995 4, the good linear relationship between lnA and E demonstrated thatthe pre-exponential factor for thermal decomposition of MOE was kinetically well compensated by the activation energy.

Figure 6 Experimental data and the calculation data obtained by the Coats-Redfern integral method

Figure 7 Experimental data and the calculation data obtained by the multiple linear regression method

Table 5 Numerical statistical errors for different methods

Figure 8 Natural logarithm of pre-exponential factor versus activation energy by different calculating methods
4 Conclusions
During the thermal decomposition of methyl oleate under nitrogen atmosphere, the initial decomposition temperatures, the maximum weight loss temperatures and the peak decomposition temperatures increased with an increasing heating rate due to the increase of the activated energy and the delay in heat transfer. Furthermore, CO, CO2and H2O were the typical gaseous decomposition species. In addition, a kinetic model for thermal decomposition of methyl oleate was built up, and the kinetic parameters such as the activation energy, the pre-exponential factor and the reaction order were obtained. The kinetic model was reliable in predicting the pyrolysis characteristics of methyl oleate. Finally, the kinetic compensation effect between the pre-exponential factor and the activation energy for the pyrolysis of methyl oleate was confirmed.
Acknowledgements: The authors gratefully acknowledge the financial support provided by the National Natural Science Foundation of China (Project No. 51375491) and the Natural Science Foundation of Chongqing (No. CSTC, 2014 JCYAA 50021).
[1] Lin L, Cunshan Z, Vittayapadung S, et al. Opportunities and challenges for biodiesel fuel[J]. Applied Energy, 2011, 88 (4): 1020-1031
[2] Demirbas A. Progress and recent trends in biodiesel fuels[J]. Energy Conversion and Management, 2009, 50(1): 14-34
[3] Chen Wuhua, Chen Jian, Jiang Jinxing, et al. Crystal precipitation law of biodiesel at low temperatures[J]. Petroleum Processing and Petrochemicals, 2014, 45(3): 14-17 (in Chinese)
[4] Wang Yunpu, Liu Yuhuan, Ruan Rongsheng, et al. Microwave-assisted decarboxylation of sodium oleate and renewable hydrocarbon fuel production[J]. China Petroleum Processing and Petrochemical Technology, 2013, 15(3): 19-27
[5] Nigam P S, Singh A. Production of liquid biofuels from re-newable resources[J]. Progress in Energy and Combustion Science, 2011, 37(1): 52-68
[6] Candeia R A, Silva M C D, Carvalho Filho J R, et al. Influence of soybean biodiesel content on basic properties of biodiesel-diesel blends[J]. Fuel, 2009, 88(4): 738-743
[7] Leung D Y C, Wu X, Leung M K H. A review on biodiesel production using catalyzed transesterification[J]. Applied Energy, 2010, 87(4): 1083-1095
[8] Moser B R, Vaughn S F. Coriander seed oil methyl esters as biodiesel fuel: unique fatty acid composition and excellent oxidative stability[J]. Biomass Bioenergy, 2010, 34(4): 550-558
[9] Knothe G, Van Gerpen J, Krahl J. The Biodiesel Handbook[M]. Champaign, IL: AOCS Press, 2005
[10] Gili F, Igartua A, Luther R, et al. The impact of biofuels on engine oil’s performance[J]. Lubrication Science, 2011, 23(7): 313-330
[11] Watson S A G, Wong V W. The Effects of Fuel Dilution With Biodiesel and Low Sulfur Diesel on Lubricant Acidity, Oxidation and Corrosion: A Bench Scale Study With CJ-4 and CI-4+ Lubricants[C]//STLE/ASME 2008 International Joint Tribology Conference. American Society of Mechanical Engineers, 2008: 233-235
[12] Wang Z, Xu G, Huang H, et al. Reliability test of diesel engine fueled with biodiesel[J]. Transactions of the Chinese Society of Agricultural Engineering, 2009, 25(11): 169-172
[13] Park Y H, Kim J, Kim S S, et al. Pyrolysis characteristics and kinetics of oak trees using thermogravimetric analyzer and micro-tubing reactor[J]. Bioresource Technology, 2009, 100(1): 400-405
[14] Chen M, Qi X, Wang J, et al. Catalytic pyrolysis characteristic and kinetic of cotton stalk [J]. Journal of Fuel Chemistry and Technology, 2011, 39(8): 585-589 (in Chinese)
[15] Li D, Chen L, Zhang X, et al. Pyrolytic characteristics and kinetic studies of three kinds of red algae[J]. Biomass and Bioenergy, 2011, 35(5): 1765-1772
[16] Liang Y, Cheng B, Si Y, et al. Thermal decomposition kinetics and characteristics of Spartina alterniflora via thermogravimetric analysis[J]. Renewable Energy, 2014, 68: 111-117
[17] Li S, Lyons-Hart J, Banyasz J, et al. Real-time evolved gas analysis by FTIR method: An experimental study of cellulose pyrolysis[J]. Fuel, 2001, 80(12): 1809-1817
[18] S. Li, J. L. Hart, J. Banyasz, et al. TG-FTIR analysis of biomass pyrolysis[J]. Fuel, 2001, 80(12): 1765-1786
[19] Duan J, Cai G, Shuai C. The relationship between IR characteristic peak and microstructure of the glass used as optical fiber[J]. Journal of Central South University of Technology, 2006, 13(3): 238-241 (in Chinese)
[20] Guo X, Wang S, Guo Z, et al. Pyrolysis characteristics of bio-oil fractions separated by molecular distillation[J]. Applied Energy, 2010, 87(9): 2892-2898
[21] Zou S P, Wu Y L, Yang M D, et al. Pyrolysis characteristics and kinetics of the marine microalgae Dunaliella tertiolecta using thermogravimetric analyzer[J]. Bioresource Technology, 2010, 101(1): 359-365
[22] Cai J M, BilS. Kinetic analysis of wheat straw pyrolysis using isoconversional methods[J]. Journal of Thermal Analysis and Calorimetry, 2009, 98(1): 325-330
[23] Li D, Chen L, Zhang X, et al. Pyrolytic characteristics and kinetic studies of three kinds of red algae[J]. Biomass and Bioenergy, 2011, 35(5): 1765-1772
date: 2014-09-21; Accepted date: 2014-12-11.
Prof. Fang Jianhua, Telephone: +86-23-86731410; E-mail: fangjianhua71225@sina.com.
- 中国炼油与石油化工的其它文章
- A Highly Efficient and Selective Water-Soluble Bimetallic Catalyst for Hydrogenation of Chloronitrobenzene to Chloroaniline
- Development of RSDS-III Technology for Ultra-Low-Sulfur Gasoline Production
- Effects of Fe2+, Co2+and Ni2+Ions on Biological Methane Production from Residual Heavy Oil
- Curing Mechanism of Condensed Polynuclear Aromatic Resin and Thermal Stability of Cured Resin
- A Novel Thermally Coupled Reactive Distillation Column for the Hydrolysis of Methyl Acetate
- Synthesis of Waterborne Polyurethane Modified by Nano-SiO2Silicone and Properties of the WPU Coated RDX

