Promotional Effect of CoO(OH) on Selective Hydrogenation of Maleic Anhydride to γ-Butyrolactone over Supported Ruthenium Catalyst
Zhou Yafen; Wang Qing; Wang Manman; Yang Wenjuan; Zhou Limei; Ma Xiaoyan
(1. Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province, College of Chemistry and Chemical Engineering, China West Normal University, Nanchong 637002; 2. College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059)
Promotional Effect of CoO(OH) on Selective Hydrogenation of Maleic Anhydride to γ-Butyrolactone over Supported Ruthenium Catalyst
Zhou Yafen1; Wang Qing1; Wang Manman1; Yang Wenjuan1; Zhou Limei1; Ma Xiaoyan2
(1. Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province, College of Chemistry and Chemical Engineering, China West Normal University, Nanchong 637002; 2. College of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059)
A decorated ruthenium catalyst was prepared by the coprecipitation method and used for the selective hydrogenation of maleic anhydride (MA) to γ-butyrolactone (GBL). The as-prepared catalyst was characterized by XRD, TGDTG and N2adsorption techniques. The characterization tests revealed that the catalyst carrier was composed of monoclinic zirconia (m-ZrO2) and hydroxyl cobalt oxide (CoO(OH)). The hydrogenation results showed that the content of CoO(OH), the reaction temperature, the hydrogen pressure and the reaction time significantly affected the catalytic selectivity to GBL. The promotional effect of CoO(OH) was remarkable, which led to an obvious increase in GBL selectivity. An 100% MA conversion and 92.0% selectivity to GBL were achieved over the Ru/ZrO2-CoO(OH)(35%) catalyst in water solvent under the conditions involving a reaction temperature of 180 ℃, a hydrogen pressure of 3.0 MPa, and a reaction time of 6 h.
ruthenium catalyst; hydrogenation; maleic anhydride; γ-butyrolactone
1 Introduction
Hydrogenation of maleic anhydride (MA) is of great academic and industrial significance. The products resulted from hydrogenation of MA, such as succinic anhydride (SA), γ-butyrolactone (GBL), 1,4-butanediol (BDO) and tetrahydrofuran (THF) are important intermediates and can be obtained selectively by using different catalysts or regulating and controlling the reaction conditions[1-2]. The reaction pathway for the hydrogenation of MA is illustrated in Figure 1[3-4]. In particular, GBL is currently an alternative to the non eco-friendly chlorinated solvent and also an important intermediate widely used in the fine chemical and pharmaceutical industries. For example, GBL is used for the production of pyrrolidone, N-methylpyrrolidone and polyvinylpyrrolidone, which are employed in the processes for manufacture of pigment, medicine, and polymers. GBL is mainly manufactured by two processes, viz.: the dehydrogenation of BDO[5]and the hydrogenation of maleic acid[6], succinic acid[7]and their dialkyl esters (e.g. dimethyl maleate, diethyl succinate)[8-9]. In the former process, BDO is manufactured by the Reppe process based on raw materials, such as the explosion-prone acetylene and possibly carcinogenic formaldehyde. In the latter process, the severe conditions such as high temperature and high pressure are required in the hydrogenation of maleic acid or succinic acid. Also, the additional costs related to the processes of esterification and alcohol recovery should be taken into account for the hydrogenation of maleic or succinic diesters. In recent years, MA can be produced in a large scale and at a low cost by partial oxidation of n-butane[10]. Therefore, the direct hydrogenation of MA has become the most promising method for the synthesis of GBL.
The key to the hydrogenation of MA to GBL is the preparation of catalysts. During the past decades, both homogeneous and heterogeneous catalysts were used for the hydrogenation of MA to GBL in liquid or gas phase. Although a high γ-butyrolactone yield can be obtained under milder conditions by using homogeneous noble metal complex catalysts[11-12], there are still some problems suchas high cost, difficulty in the preparation of organometallic complex and difficulty in the separation of catalyst and products. These shortcomings can be overcome by using heterogeneous noble metal catalysts[13-16]which have advantages of high activity and good selectivity to target product. The heterogeneous copper-based catalysts are extensively used for the hydrogenation of MA. However, the early Cu-Cr catalysts have brought about Cr pollution to the environment[17]. As regards the recently developed chromium-free copper based catalysts such as Cu-Zn-Ti[1], Cu-Zn-Zr[4], Cu-Zn-Ce[18], and Cu-CeO2-Al2O3[19], there still exist some disadvantages, for example, the sintering and deactivation of catalysts at high reaction temperature. On the other hand, the cobalt-based catalysts are widely used in several hydrogenation reactions. For example, Co/CNTs[20]and CoxP/SiO2[21]are used for the hydrogenation of CO. The Co/SiO2catalyst is active in both the selective hydrogenation of MA and the hydrogenolysis of succinic anhydride[22]. In addition, the remarkable improvement of Co in catalytic performance has been observed in the hydrogenation of dimethyl oxalate[23]. It is also found that the Rh/Co bimetallic catalyst can efficiently promote hydrogenation of unsaturated hydrocarbons with hydrous hydrazine as a hydrogen source[24].
In this paper, we report a study on the hydrogenation of MA to GBL over ZrO2, CoO(OH), and ZrO2-CoO(OH) supported ruthenium catalysts. The catalysts were characterized by means of XRD, TG-DTG and BET techniques. The aim of the present work is to study the promotional effect of CoO(OH) on supported ruthenium catalyst. Moreover, the effects of the reaction conditions were also investigated to take into account the potential industrial relevance of one-step hydrogenation of MA to GBL over the Ru/ZrO2-CoO(OH) catalyst.

Figure 1 Reaction pathway for the hydrogenation of maleic anhydride
2 Experimental
2.1 Catalyst preparation
A series of Ru/ZrO2-CoO(OH) catalysts containing 2.0% of Ru and different contents of CoO(OH) were prepared by the coprecipitation method. Typically, a certain amount of ZrOCl2·8H2O and CoCl2·6H2O was dissolved in 15 mL of distilled water, followed by the introduction of RuCl3aqueous solution. Then a 25% NaOH solution was added to the above mixed solution drop by drop under vigorous stirring until the pH value reached 10. The resulted suspension was stirred continuously for 6 h followed by ageing at room temperature for 12 h. After that, the precipitate was filtered and washed with distilled water until the pH value reached 7. Finally, after drying in vacuum overnight at 60 ℃, the Ru/ZrO2-CoO(OH) catalyst was obtained. All the catalyst samples were used directly without activation.
2.2 Catalyst characterization
The X-ray diffraction (XRD) analysis was performed on a Rigaku D/max-rA diffractometer using a Cu Kαradiation, operating at 40 kV and 110 mA and scanning from 10° to 90° (2θ). The thermogravimetric analysis (TG) and derivative thermogravimetry (DTG) curves were determined using a STA 449 F3 Jupiter analyzer at a heating rate of 10 ℃/min. The BET specific surface area was determined by a Micromeritics ASAP 2020 apparatus and by physical adsorption of N2at -196 ℃.
2.3 Catalytic test
Selective hydrogenation of MA to GBL was carried out in a 60-mL stainless steel autoclave equipped with a magnetic stirrer and an electric temperature controller. In a typical experiment, the autoclave was charged with weighed amounts of catalyst, MA, and solvent. The autoclave was purged with H2for five times, and then pressurized with H2to the designed pressure. Under a stirring rate of 1 000 r/min, the reaction was conducted at the given temperature for a certain time. At the end of reaction, the autoclave was cooled down to room temperature and was slowly depressurized. The MA conversion and product selectivity were determined by a GC-7890 chromatograph(Agilent) equipped with a FID detector and a HP-5 capillary column. The reactant and product were identified by comparison with the standard samples and the GC-MS results.
3 Results and Discussion
3.1 Characterization of catalyst
The XRD patterns of the as-prepared Ru/ZrO2(a), Ru/ZrO2-CoO(OH) (b) and Ru/CoO(OH) (c) catalysts are shown in Figure 2. It can be seen that the pattern of the Ru/ZrO2catalyst showed some broad and dispersive peaks, indicating that the structure of ZrO2was mainly amorphous. In addition, the most obvious peak at 2θ=31.4○corresponded to the maximum characteristic peak of monoclinic zirconia (m-ZrO2) (PDF#37-1484). This suggested that the short-range ordered structure of the amorphous state was similar to the crystal structure of m-ZrO2. The XRD pattern of the Ru/CoO(OH) catalyst exhibited the characteristic peaks of CoO(OH) (PDF#07-0169), appearing at 2θ=20.2○, 38.9○, 50.6○, 65.3○and 69.2○. As regards the Ru/ZrO2-CoO(OH) catalyst, a dispersive characteristic peak of m-ZrO2appeared at 2θ=31.4○. Simultaneously, the narrow, well defined characteristic peaks assigned to CoO(OH) appeared at 20.2○, 38.9○and 50.6○. No diffraction peaks of Ru species were identified in the XRD patterns of all the catalysts, indicating that Ru species were finely dispersed on the catalyst support.

Figure 2 XRD patterns of catalysts: (a) Ru/ZrO2;(b) Ru/ ZrO2-CoO(OH)(35%); and (c) Ru/CoO(OH)
The TG and DTG curves of the as-prepared Ru/ZrO2-CoO(OH)(35%) catalyst are illustrated in Figure 3. The thermal analysis of the catalyst showed two decomposition steps. The first step, from 43 ℃ to 100 ℃, with a mass loss of about 11% corresponding to the peak at around 90 ℃ on the DTG curve, was attributed to the removal of physically adsorbed water. The second step, from 110 ℃ to 320 ℃, with a mass loss of about 12% corresponding to the peak at about 300 ℃ on the DTG curve, was in accordance with the process of CoO(OH) conversion to Co3O4[25].

Figure 3 TG and DTG curves of the prepared Ru/ZrO2-CoO(OH) (35%) catalyst
3.2 Effect of CoO(OH) content
Table 1 shows the catalytic performance of a series of the Ru/ZrO2-CoO(OH) catalysts with different CoO(OH) content for the hydrogenation of MA. For all the catalysts investigated here, 100% conversion of MA was obtained. However, the selectivity to GBL changed obviously with the decorated CoO(OH) content. It can be seen from Table 1 that all the decorated Ru/ZrO2-CoO(OH) catalysts exhibited higher GBL selectivity than the undecorated Ru/ZrO2or Ru/CoO(OH) catalysts, over which the GBL selectivity was only 65.1% and 50.1%, respectively. In addition, the GBL selectivity increased with an increasing CoO(OH) content, reaching a maximum value of 92.0% when the content of CoO(OH) was 35%. A further increase of the CoO(OH) content in the Ru/ZrO2-CoO(OH) catalyst led to a decrease in GBL selectivity. According to the specific surface area data of the investigated catalysts, decoration of CoO(OH) changed the catalyst specific surface area. The catalyst with a CoO(OH) content of 35% exhibited the largest specific surface area to achieve a highest GBL selectivity of 92.0%. So the Ru/ZrO2-CoO(OH)(35%) catalyst was used in the further investigations. The prominent catalytic behavior of the Ru/ZrO2-CoO(OH)(35%) catalyst might be related to its structure.
The above XRD and TG-DTG characterization tests showed that the catalyst contained rich water and surface hydroxyls, which provided the catalyst with strong hydrophilicity to be readily dispersed in aqueous solution. Both the high specific surface area and high dispersion of the catalyst made it capable of being in full contact with the substrate, so that the GBL selectivity was greatly increased. In addition, this finding is of economical and environmental significance since water is a cheap and safe solvent. Therefore, in the subsequent experiments, reaction conditions for the catalytic hydrogenation of MA over the Ru/ZrO2-CoO(OH)(35%) catalyst using water as the solvent were optimized.

Table 1 Effect of CoO(OH) content on the hydrogenation of maleic anhydride
3.3 Optimization of reaction conditions
The effect of reaction temperature on hydrogenation of MA is shown in Figure 4. Under the surveyed range of temperatures, the conversion of MA always reached 100%, while the selectivity to GBL was affected signif icantly by the reaction temperature. It can be seen from Figure 4 that the selectivity to GBL increased with an increasing temperature, reaching a maximum (92.0%) at 180 ℃, and then decreased. At a low temperature, a poor selectivity to GBL was identified because of hydrolyzation of MA to succinic acid. For example, the selectivity to GBL was only 15.4% at 120 ℃. When the temperature was beyond 180 ℃, excessive hydrogenation of MA was found, leading to a decrease of GBL selectivity.
The hydrogenation of MA over the Ru/ZrO2-CoO(OH) (35%) catalyst was carried out at a hydrogen pressure of 1.0—5.0 MPa. As shown in Figure 5, an 100% conversion of MA was obtained under the studied range of H2pressures. However, the selectivity to GBL varied evidently with changes in H2pressure. The selectivity to GBL increased from 59.4% to 92.0% as the H2pressure increased from 1.0 to 3.0 MPa, while the GBL selectivity decreased slightly when the H2pressure was higher than 3.0 MPa as a result of excessive hydrogenation.

Figure 4 Effect of reaction temperature on the hydrogenation of maleic anhydride■—Conversion;●—Selectivity
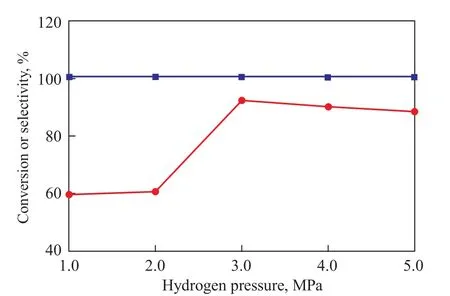
Figure 5 Effect of hydrogen pressure on the hydrogenation of maleic anhydride■—Conversion;●—Selectivity
The effect of reaction time on the performance of the Ru/ZrO2-CoO(OH) (35%) catalyst is shown in Figure 6. It can be seen from Figure 6 that the yield of GBL attained 63.1% when the reaction time was only 2 h, indicating to the high catalytic activity of the catalyst. The selectivity to GBL increased with the extension of reaction time at first. The maximum GBL selectivity was achieved at a reaction time of 6 h (92.0%), and then theselectivity decreased slowly with the extension of reaction time, which was also related to the further hydrogenation reaction.

Figure 6 Effect of reaction time on the hydrogenation of maleic anhydride■—Conversion;●—Selectivity
4 Conclusions
Selective hydrogenation of MA to GBL over ZrO2, CoO(OH), and ZrO2-CoO(OH) supported ruthenium catalysts was investigated. It was found that the decoration of CoO(OH) could greatly improve the catalytic selectivity to GBL, with the conversion of MA maitained at 100%. When the content of CoO(OH) was 35%, the Ru/ZrO2-CoO(OH) catalyst exhibited a highest specific surface area and a maximum selectivity to GBL. Under the optimized conditions involving a reaction temperature of 180 ℃, a hydrogen pressure of 3.0 MPa, and a reaction time of 6 h in the presence of water functioning as the solvent, the conversion of MA and the selectivity to GBL over the Ru/ZrO2-CoO(OH)(35%) catalyst reached 100% and 92.0%, respectively.
Acknowledgements:The authors are grateful to the financial support from the Natural Science Foundation of China (No. 21303139), the Key Fund Project of Educational Department of Sichuan Province (No. 14ZA0126), and the Open Project of Chemical Synthesis and Pollution Control Key Laboratory of Sichuan Province (No. CSPC2013-1).
[1] Hu T J, Yin H B, Zhang R C, et al. Gas phase hydrogenation of maleic anhydride to γ-butyrolactone over Cu-Zn-Ti catalysts[J]. Catal Commun, 2007, 8(2): 193-199
[2] Zhang R C, Yin H B, Zhang D Z, et al. Gas phase hydrogenation of maleic anhydride to tetrahydrofuran over Cu/ZnO/TiO2catalysts in the presence of n-butanol[J]. Chem Eng J, 2008, 140(1/3): 488-496
[3] Guo S F, Tian W P, Shi L. Hydrogenation of maleic anhydride to succinic anhydride over nickel/clay catalysts[J]. Transition Met Chem, 2012, 37(8): 757-763
[4] Zhang D Z, Yin H B, Ge C, et al. Selective hydrogenation of maleic anhydride to γ-butyrolactone and tetrahydrofuran over Cu-Zn-Zr catalyst in the presence of ethanol[J]. J Ind Eng Chem, 2009, 15(4): 537-543
[5] Zhang B, Zhu Y L, Ding G Q, et al. Modification of the supported Cu/SiO2catalyst by alkaline earth metals in the selective conversion of 1,4-butanediol to γ-butyrolactone[J]. Appl Catal A: Gen, 2012, 443/444: 191-201
[6] Chaudhari R V, Rode C V, Deshpande R M, et al. Kinetics of hydrogenation of maleic acid in a batch slurry reactor using a bimetallic Ru–Re/C catalyst[J]. Chem Eng Sci, 2003, 58(3): 627-632
[7] Deshpande R M, Buwa V V, Rode C V, et al. Tailoring of activity and selectivity using bimetallic catalyst in hydrogenation of succinic acid[J]. Catal Commun, 2002, 3(7): 269-274
[8] Ohlinger C, Kraushaar-Czarnetzki B. Improved processing stability in the hydrogenation of dimethyl maleate to γ-butyrolactone, 1,4-butanediol and tetrahydrofuran[J]. Chem Eng Sci, 2003, 58(8): 1453-1461
[9] Vaidya S H, Rode C V, Chaudhari R V. Bimetallic Pt-Sn/ γ-alumina catalyst for highly selective liquid phase hydrogenation of diethyl succinate to γ-butyrolactone[J]. Catal Commun, 2007, 8(3): 340-344
[10] Fernández J R, Vega A, Díez F V. Partial oxidation of nbutane to maleic anhydride over VPO in a simulated circulating fluidized bed reactor[J]. Appl Catal A: Gen, 2010, 376(1/2): 76-82
[11] Hara Y and Takahashi K. A novel production of γ-butyrolactone catalyzed by homogeneous ruthenium complexes[J]. Catal Surv Jpn, 2002, 6(1): 73-78
[12] Wang Q, Cheng H Y, Liu R X, et al. Selective hydrogenation of maleic anhydride to γ-butyrolactone in supercritical carbon dioxide[J]. Catal Commun, 2009, 10(5): 592-595
[13] Pillai U R and Sahle-Demessie E. Selective hydrogenation of maleic anhydride to γ-butyrolactone over Pd/Al2O3catalyst using supercritical CO2as solvent[J]. Chem Commun, 2002, 422-423
[14] Jung S M, Godard E, Jung S Y, et al. Liquid-phase hydrogenation of maleic anhydride over Pd/SiO2: Effect of tinon catalytic activity and deactivation[J]. J Mol Catal A: Chem, 2003, 198(1/2): 297-302
[15] Jeong H, Kim T H, Kim K I, et al. The hydrogenation of maleic anhydride to γ-butyrolactone using mixed metal oxide catalysts in a batch-type reactor[J]. Fuel Process Technol, 2006, 87(6): 497-503
[16] Wang Q, Cheng H Y, Liu R X, et al. Influence of metal particle size on the hydrogenation of maleic anhydride over Pd/C catalysts in scCO2[J]. Catal Today, 2009, 148(3/4): 368-372
[17] Messori M and Vaccari A. Reaction pathway in vapour phase hydrogenation of maleic anhydride and its esters to γ-butyrolactone[J]. J Catal, 1994, 150(1): 177-185
[18] Zhang D Z, Yin H B, Zhang R C, et al. Gas phase hydrogenation of maleic anhydride to γ-butyrolactone by Cu-Zn-Ce catalyst in the presence of n-butanol[J]. Catal Lett, 2008, 122(1/2): 176-182
[19] Yu Y, Guo Y L, Zhan W C, et al. Gas-phase hydrogenation of maleic anhydride to γ-butyrolactone at atmospheric pressure over Cu-CeO2-Al2O3catalyst[J]. J Mol Catal A: Chem, 2011, 337(1/2): 77-81
[20] Lv J, Ma X B, Bai S L, et al. Hydrogenation of carbon monoxide over cobalt nanoparticles supported on carbon nanotubes[J]. Int J Hydrogen Energ, 2011, 36(4): 8365-8372
[21] Song X G, Ding Y J, Chen W M, et al. Synthesis and characterization of silica-supported cobalt phosphide catalysts for CO hydrogenation[J]. Energ Fuel, 2012, 26(11): 6559-6566
[22] Meyer C I, Regenhardt S A, Marchi A J, et al. Gas phase hydrogenation of maleic anhydride at low pressure over silica-supported cobalt and nickel catalysts[J]. Appl Catal A: Gen, 2012, 417/418: 59-65
[23] Wen C, Cui Y Y, Yin A Y, et al. Remarkable improvement of catalytic performance for a new cobalt-decorated Cu/ HMS catalyst in the hydrogenation of dimethyloxalate[J]. Chem Cat Chem, 2013, 5(1): 138-141
[24] Lin J, Chen J, Su W P. Rhodium-cobalt bimetallic nanoparticles: a catalyst for selective hydrogenation of unsaturated carbon-carbon bonds with hydrous hydrazine[J]. Adv Synth Catal, 2013, 355(1): 41-46
[25] Singh Gaur R P, Hydrogen reduction of heterogenite to make sub-micron cobalt metal powder for hard metal applications[J]. Int J Refract Met H, 2012, 35: 300-305
Application of Coalescence Separation Technology in Manufacture of Nitrobenzene
The commercial application project for reducing the sodium content in raw nitrobenzene which was performed by the Research Institute of the SINOPEC Nanjing Chemical Company (NCC) has passed the appraisal organized by the Science and Technology Division of the Sinopec Corp. The appraisal team has recognized that the overall technology of this project has reached the internationally advanced level.
This project has for the first time adopted the coalescence separation technique in washing and separation of nitrobenzene, while concurrently developing a new process for reducing sodium content to less than 6 wppm in raw nitrobenzene. The said process has been tested in commercial scale on the 180 kt/a nitrobenzene unit at NCC, with the process unit running uninterruptedly and smoothly. The results of application of this achievement have revealed that the average sodium content in nitrobenzene has decreased from 18.42 μg/g to 5.42 μg/g, which was equal to a 70.6% reduction in sodium content, while the average phenate content was decreased from 2.32 μg/g to 1.17 μg/g, which was a 49.6% reduction in phenate, resulting in an increased security of the processing system. NCC has applied for 2 Chinese invention patents on this technology, among which one invention patent has been awarded to NCC by the China Patent Office.
date: 2015-10-14; Accepted date: 2015-10-31.
Professor Zhou Yafen, Telephone: +86-817-2568081; E-mail: cwnuzyf@163.com.
- 中国炼油与石油化工的其它文章
- Computational Fluid Dynamics Simulation of Liquid-Phase FCC Diesel Hydrotreating in Tubular Reactor
- Quantitative Analysis Using Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and Correlation between Mass Spectrometry Data and Sulfur Content of Crude Oils
- Hydrothermal Liquefaction of Wheat Straw in Sub-critical Water/Ethanol with Ionic Liquid for Bio-oil Production
- Microbial Characterization of Denitrifying Sulfide Removal Sludge Using High-Throughput Amplicon Sequencing Method
- Synthesis and Separation Performance of Y-type Zeolite Membranes by Pre-Seeding Using Electrophoresis Deposition Method
- Design and Control of Self-Heat Recuperative Distillation Process for Separation of Close-Boiling Mixtures: n-Butanol and iso-Butanol

