Synthesis and Separation Performance of Y-type Zeolite Membranes by Pre-Seeding Using Electrophoresis Deposition Method
Cheng Zhilin; Han Shuai
(College of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002)
Synthesis and Separation Performance of Y-type Zeolite Membranes by Pre-Seeding Using Electrophoresis Deposition Method
Cheng Zhilin; Han Shuai
(College of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou 225002)
Y-type zeolite membranes were synthesized by a two-step approach in which a particle seed layer was prepared by electrophoresis deposition (EPD) at first, followed by densification through secondary growth. The pre-seeding adopted the directing agent for Y-type zeolite synthesis serving as seeds. The effects of aging time of the directing agent, electrophoresis voltage and electrophoresis deposition time on seed layers quality as well as the quality of zeolite membranes were investigated. The results indicated that the zeolite seeds derived from the directing agent could be evenly deposited on substrate under certain EPD conditions. The XRD patterns of the seeded substrates after the secondary growth showed that the pure as-synthesized Y-type zeolite membranes had successfully grown on the substrates. The SEM images indicated that the substrate was covered by the highly intergrown zeolite crystals when the seeding solution employed the directing agent with an aging time of 2 days. The separation performance of zeolite membrane was evaluated using a CO2/N2mixture (with a mole ratio of 1:1) at different temperatures. Furthermore, the pervaporation measurements were carried out for the dehydration of isopropanol aqueous solutions with different mass fractions. The as-synthesized Y-type zeolite membranes exhibited a relatively high selectivity of water from isopropanol and sustainable permeation flux.
zeolite membrane; crystal growth; electrophoresis deposition; separation
1 Introduction
The FAU-type zeolite membrane, one of the most important zeolite membranes, has potential applications in solvent dehydration, separation of gases and organic liquids, and membrane catalysis. Zhang, et al.[1]reported that the separation performance on self-supporting faujasite zeolite membrane in an alcohol/water system demonstrated that the permeate flux and the separation factor could achieve values as high as 1.41 kg/(h·m2) and 16.8, respectively, at a feed temperature of 50 ℃ and an alcohol concentration of 70 wt%. Zhang, et al.[2]reported that the fluoride-derived NaY zeolite membranes prepared in fluoride media had fluxes of 2.6 and separation factors of 1000 upon treating 90% n-butanol/ethanol aqueous solution at 348 K. Sato, et al.[3]realized the synthesis of Y-type zeolite membranes using the asymmetric alumina supports. These membranes exhibited high fluxes of 9.1—10.1 kg/(h·m2) and separation factors in the range of 190—170 for the same standard water/ethanol system. Wang, et al. reported that NaY zeolite membranes with high performance prepared by a variable-temperature synthesis method characteristic of separation factors exceeding 5000 with fluxes of over 3 kg/(h·m2) were obtained in the separation of methanol/methyl methacrylate (10%/90%) and methanol/methyl acrylate (10%/90%) mixtures at 60 ℃. K. Kusakabe, et al.[5]reported that the separation factors of CO2/N2mixture on Y-type zeolite membranes attained 30. Zhu, et al.[6]reported that the FAU-type zeolite membranes synthesized by the microwave-assisted in situ crystallization method had a 526 separation selectivity for the water/ isopropanol mixture. Since the secondary growth method appeared, many valued zeolite membrane types have been successfully synthesized. While this method generally can give good results[7-9], the quality and thickness of the eventual mem-brane depend strongly on the quality of the seed layer, in particular its uniformity and thickness. The seed layer must cover the (porous) support completely, and should consist of a dense-packing of individual dense particles with similar morphology and dimensions. Therefore, improvements in seed layer deposition techniques are important for the development in chemical syntheses of zeolites[10]. Many methods have been used to coat the zeolite seeds onto the porous support, such as rub-coating[11], spin-coating[12], dip-coating[13], vacuum coating[14], electrophoretic deposition method[15-18], vapor phase transformation[19-20], etc. Electrophoretic deposition (EPD) is a colloidal process for forming zeolite bodies from suspensions with zeolite particles. This process has advantages such as the uniformity of deposition even for complex and large forms, thickness control of the deposit and simplicity of operation. Thus EPD seems to be a favorable method for forming zeolite seed layers, as compared with other methods including the rubbing, dip coating and laser ablation. In the present work, a novel synthesis of Y-type zeolite membrane via pre-seeding of the directing agent of Y-type zeolite synthesis employing EPD for the first time was developed. The seeding conditions under EPD were investigated. Furthermore, the properties of the resulting zeolite membrane were examined on the performance for separation of CO2/N2gas mixture and water/ isopropanol liquid mixtures.
2 Experimental
2.1 Preparation of seeded substrate
A porous stainless disc (20 mm×2 mm) with a porosity of 75% and an average pore size of 0.2 μm was polished with sandpaper, treated in an aqueous solution of sodium hydroxide (10 mol/L) for 12 h, dried at 120 ℃ for 24 h and then transferred into a dessiccator for further use. The seeding solution was obtained from the directing agent of Y-type zeolite synthesis with a molar composition of 16Na2O:1Al2O3:15SiO2:310H2O which was diluted by deionized water and then was subject to adjustment of the pH value of the seed solution to 9.5. An electrophoresis deposition method was used for seeding on the above substrate. The porous stainless plate was used as the electrodes immersed into the above-mentioned diluted directing agent after cleaning and degreasing treatments, when the distance between the electrodes was set at 1 cm. The seed deposition was performed under an electric field of 3 V for 15 seconds under slow stirring. At the end of run, the seeded substrate was washed by deionized water and dried in an oven at 100 ℃ for 24 h. In addition, another seeded substrate was prepared by using the dip-coating method.
2.2 Preparation of zeolite membrane
The Y-type zeolite membranes were synthesized by using the in-situ synthesis and secondary growth method, respectively. A reactive mixture solution of secondary growth was prepared to comply with a molar composition of 3.2Na2O:10SiO2:1Al2O3:300H2O with addition of the above-mentioned directing agent. The unseeded substrate and the above prepared seeded substrates were placed vertically in a Teflon vessel. The teflon vessels were placed into the oven and were maintained at 90 ℃ for 48 h. After the synthesis, the substrates were cooled down, washed for several times with deionized water until a neutral reaction was identified, and then were dried at 120 ℃ for 3 h.
2.3 Material characterization and permeability evaluation
The formation of zeolite membranes was confirmed by X-ray diffraction (XRD) using a Bruker-AXS D8 Advance powder diffractometer. The surface morphology was examined by a scanning electron microscope (SEM) (S4800 II, Hitachi) at an acceleration voltage of 15 kV. The gas separation measurements were carried out using the sweep gas method as described in our previous work[20]. Before measurements, the membranes were put in the permeating cell for drying at 120 ℃ at a temperature increase rate of 1 ℃/min for the overnight drying. The permeance of the gases was tested at room temperature under a pressure difference of 0.10 MPa. The permselectivity of A/B is defined as the permeance ratio of gas A to gas B. The volume ratio of CO2/N2mixture feed is 50/50. The permselectivity of gas mixture was analyzed by an online gas chromatograph. The performance of zeolite membranes was evaluated by the pervaporation (PV) towardsthe water/isopropanol mixture at 70 ℃. The apparatus and experimental details of PV measurements are consistent with that described elsewhere[1]. During pervaporation, the disc-shaped FAU zeolite membrane was sealed in a membrane fixture. A vacuum was applied to the downstream side and the permeation pressure was maintained at below 0.1 kPa. The compositions of the feed and the permeate were analyzed by a gas chromatograph equipped with a TCD detector. The pervaporation performance of membrane was evaluated by the permeation flux J (mass flux) in kg/(h·m2) and the separation factor (α). The total mass flux (J) was de fined as

where m is the mass of the permeate collected during PV (kg); A is the effective membrane surface area (m2); and t is the permeate collection time interval (h). The separation factor α(i/j) of component i over component j was calculated from the equation:

where yiand yjare the mass fractions of components i and j in permeate side and, xiand xjare the mass fractions of components i and j in the feed side.
3 Results and Discussion
3.1 Investigation of seeding conditions
Figure 1 displays the SEM images of the seed layer deposited on the anode substrate as a function of the aging time of directing agent. The porous morphology of substrate is shown in Figure 1-a. As it is well known, the directing agent functioning as the adding seed plays an important role in the synthesis of industry Y-type zeolite[21]. As the quality and thickness of the eventual synthesized zeolite membrane depend strongly on the quality of the seed layer, in particular its uniformity and thickness, the dispersed state of seed solution and seed layer deposition techniques are important for obtaining a synthesized zeolite membrane with good quality. Compared to the microsized seed dispersion, the nanosized seeds can achieve better stability in seeding solution thanks to the ready availability of negative potential on their surface under alkaline condition[22-23]. Just as shown in Figure 1, the porous stainless substrates were completely covered by the coatings derived from the directing agent seed with different aging times through EPD. Under an aging timeof 2 days, the coating on substrate consists of the regular particles with a size of ca. 200 nm possessing a distinct boundary. Regretfully, the feature peaks of FAU zeolite were not observed on the XRD patterns of all seeded substrates (not shown). This should be ascribed to the fact that the directing agent of NaY zeolite synthesis is comprised of the crystal nucleus aggregates of FAU zeolite belonging to the half-baked crystals.

Figure 1 SEM images of the seeding membranes deposited on the anode at 3 V as function of the aging time of directing agent (electrophoresis deposition time for 15 s)
Figure 2 displays the SEM images of the seed layers obtained at different electrophoresis deposition voltages. Obviously, the voltage for the directing agent seeds deposited on substrate has a remarkable influence. Without applying voltage, no seeds are found on the surface of substrate. Nevertheless, upon applying the different voltages on the substrate, the regular particle-like coatings appear on the substrate. Moreover, the coating becomes more even and compact with an increasing voltage applied thereby. There is reason to believe that the crystal nucleus aggregates derived from the directing agent with negative potential on surface were drawn to the substrate and thereby formed an even seed layer under electrophoresis.

Figure 2 SEM images of the seeding membranes deposited on the anode using the directing agent of aging time for 2 d as function of voltage (electrophoresis deposition time for 15 s)
Figure 3 displays the SEM images of the seed layers obtained at different electrophoresis deposition times. With an increasing electrophoresis deposition time, the seed layers on substrate were gradually transformed from a sporadic coating to the compact particle-like layers. These results mentioned above suggested that the formation of seed layer using the directing agent could be achieved by the electrophoresis deposition method. This may provide a facile and controllable method to prepare the seed layers with good quality for the Y-type zeolite membrane synthesis. Compared to the microsized or nanosized zeolite seeds, the preparation of seeds in this work is quite promising for practical application in large scale preparation process.
3.2 Synthesis and separation performance of assynthesized Y-type zeolite membrane
The XRD patterns of as-synthesized Y-type zeolite membranes formed on seeded substrates via secondary growth under different EPD conditions are shown in Figure 4. There are no feature peaks of Y-type zeolite on XRD patterns of the seeded substrate (Figure 4 a). The reason isthat the directing agent of Y-type zeolite synthesis consists of the crystal nucleus aggregates, which have not verified any diffraction feature of the zeolite. However, the standard feature peaks of Y-type zeolite appear on the seeded substrates after secondary growth, demonstrating that the Y-type zeolite membranes have grown on these seeded substrates. The peak intensity of Y-type zeolite membrane represents the integrity of zeolite membrane. As shown in Figure 5, the SEM images display the assynthesized Y-type zeolite membranes synthesized by pre-seeding using the dip-coating method and the EPD method, respectively. It can be seen that the as-synthesized Y-type zeolite membrane with pre-seeding using the dip-coating method is a discontinuous membrane which contains a large amount of defect holes, thereby exhibiting a poor quality (Figure 5 a). This indicates that the Y-type zeolite membrane with high quality obtained via the dip-coating method using the directing agent is quite difficult. However, these seeded substrates achieved by EPD method after secondary growth all were completely covered with zeolite membranes with better quality (Figure 5 b, c, d). These zeolite membranes consist of highly intergrown zeolite crystals, thereby exhibiting a better integrity. Among these good quality membranes, the as-synthesized Y-type zeolite membrane was formed via pre-seeding under the condition of an aging time of 2 d, a voltage of 5 V and an electrophoresis deposition time of 15 s, indicating toa defect-free integrity (Figure 5 c). These results demonstrate that the preparation of the seed layer derived from the directing agent via EPD is a desirable approach for the synthesis of a high-quality Y-type zeolite membrane. The thickness of seed layers and the NaY zeolite membrane attains ca. 20 μm and 30 μm, respectively. Figure 6 shows the CO2permeance and the CO2/N2selectivity from the binary mixture as a function of permeating temperature for the above Y-type membrane (Figure 5 c). The membrane shows a CO2permeance of 7.4×10-7mol/Pa·m2·s and a CO2/N2selectivity of 38 at room temperature. With an increasing permeating tem-perature, the CO2permeance obviously increases, whereas the CO2/N2selectivity slightly decreases from 38 at room temperature to 25.4 at 160 ℃. This suggests that the NaY zeolite membrane exhibits a better stability under different permeating temperatures.

Figure 4 XRD patterns of as-synthesized Y-type zeolite membranes via pre-seeding under different EPD conditions●—support;◆—NaYa—Seeded substrate; b—Membrane obtained at an aging time of 3 d, a voltage of 3 V, and an electrophoresis deposition time of 15 s; c—Membrane obtained at an aging time of 2 d, a voltage of 5 V, and an electrophoresis deposition time of 15 s; d—Membrane obtained at an aging time of 2 d, a voltage of 3 V, and an electrophoresis deposition time of 30 s.

Figure 5 SEM images of as-synthesized Y-type zeolite membranes obtained via dip-coating seeding and EPD pre-seeding under different conditionsa—Dip-coating seeding; b—Membrane obtained at an aging time of 3 d, a voltage of 3 V, and an electrophoresis deposition time of 15 s; c—Membrane obtained at an aging time of 2 d, a voltage of 5 V, and an electrophoresis deposition time of 15 s; d—Membrane obtained at an aging time of 2 d, a voltage of 3 V, and an electrophoresis deposition time of 30 s.
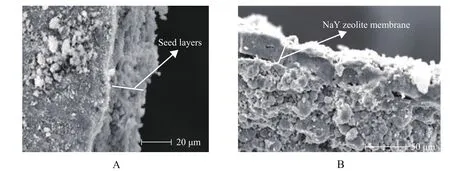
Figure 6 SEM images of cross section of seed layers and NaY zeolite membraneA—Seed layers formed via EPD at an aging time of 2d, a voltage of 5V and an electrophoresis deposition time of 15s; B—NaY zeolite membrane grown on substrate with seed layers
The comparison of permeance and separation performance of a CO2/N2mixture using the FAU membranes prepared in this work and the previous other reports is listed in Table 1. The results indicate that the separation performance of CO2/N2mixture on zeolite membrane in this work is higher than those values, such as 20 reported by Seike, et al.[18], 30 reported by Kusakabe, et al.[5], 19 reported by Hasegawa, et al.[24]and 2.5 obtained in our previous work[25]. Unfortunately, it is still lower than the value referred to in our previous work[20], but the CO2permeance is higher than that of our previous study. A high permeance while keeping a certain appropriate separation performance for zeolite membrane is more desirable in practical application. Most importantly, the CO2permeance and CO2/N2selectivity on zeolite membrane in this work are significantly larger than those reported by Takahiro Seike, et al. who developed the synthesis of FAU type zeolite membrane by pre-seeding using the Y-type zeolite seed with a particle size of 1 μm under EPD[18].
Table 2 lists the PV performance of Y-type zeolite membrane for water/isopropanol mixtures. The membrane achieves a relatively high separation selectivity for water from isopropanol. No isopropanol was detected in the permeating side when isopropanol content in the feed was between 90% and 94%. When the isopropanol content in the feed increased to 98%, its content in the permeating side was detected which could reach up to 6%. Furthermore, with an increasing isopropanol content in the feed, the total flux obviously decreases because the kinetic diameter of isopropanol molecule (0.58 nm) is larger than that of water (0.265 nm). In contrast, the separation factor of the FAU-type zeolite membranes reported by Wang, et al. was equal to 139[4], as compared to 380 reported by Zhu, et al.[26], 420 reported by Sato, et al.[3], and 526 reported by Zhu, et al.[6]However, the as-synthesized Y-type zeolite membranes function even better, and moreover its maximum separation factor can reach up to 767.

Table 1 Comparison of the performance of as-synthesized Y-type zeolite membranes
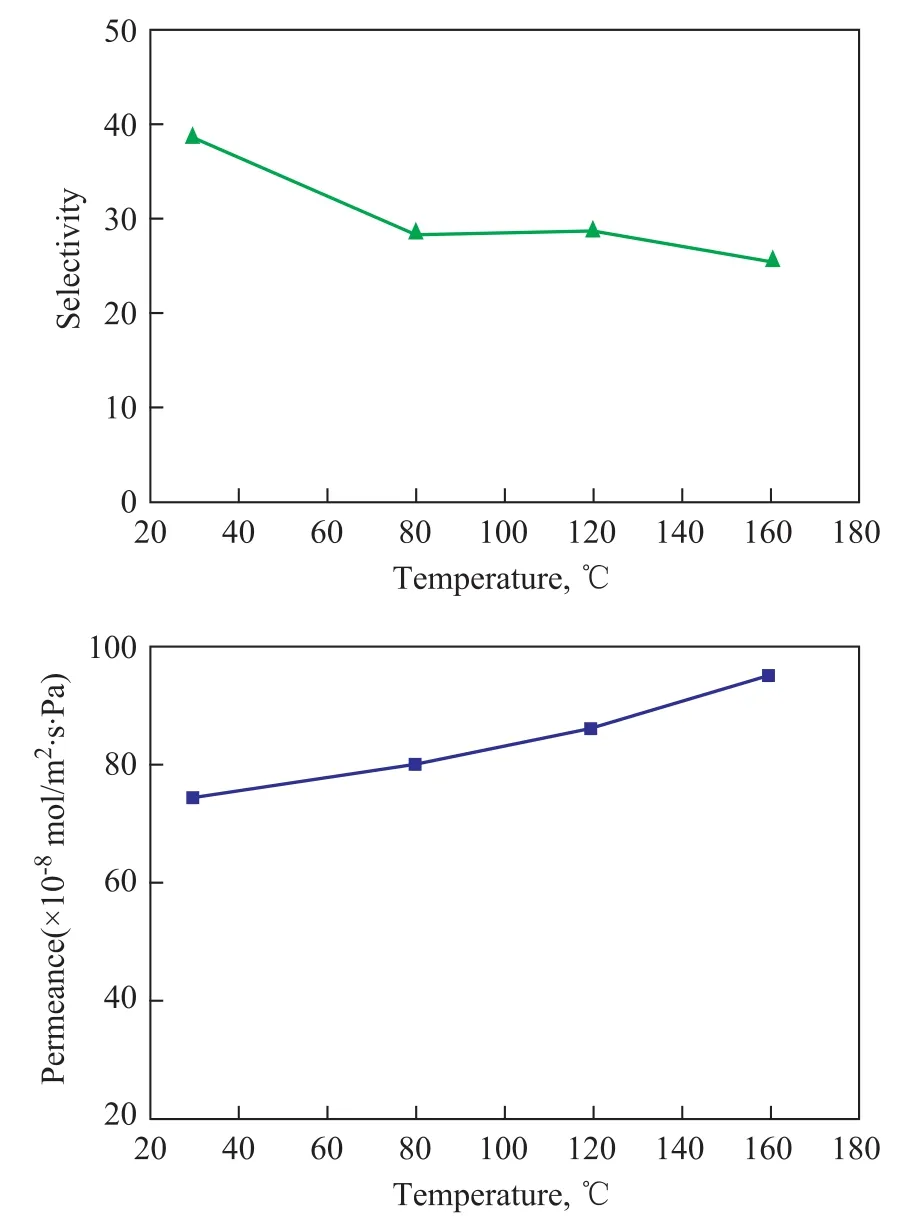
Figure 7 Temperature dependence of the CO2permeation
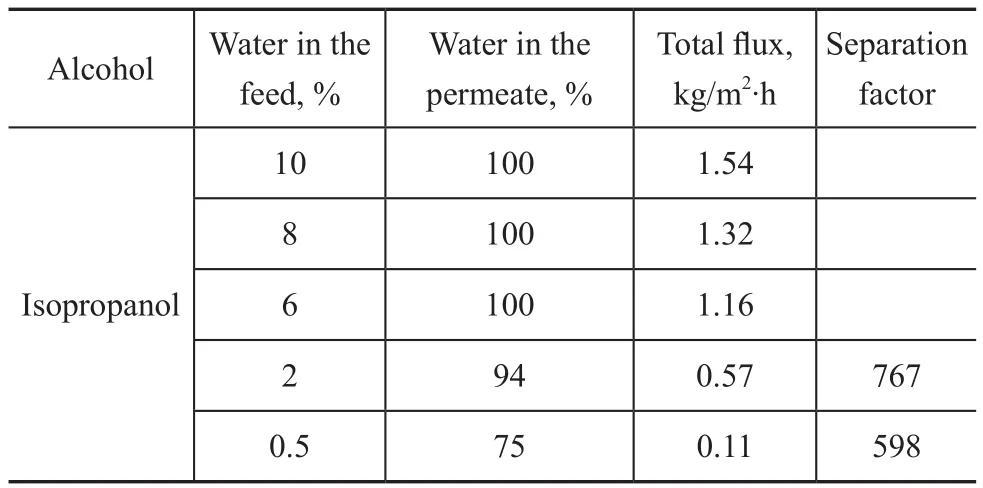
Table 2 PV performance of as-synthesized Y-type zeolite membranes for water/ isopropanol mixtures at 70℃
4 Conclusions
In summary, we have successfully prepared the Y-type zeolite membranes via pre-seeding using the directing agent of Y-type zeolite synthesis via EPD and secondary growth. The seeding conditions using EPD were investigated. The characterization tests demonstrated that the Y-type zeolite membranes prepared by means of this method exhibited a better integrity. The separation performance of CO2/N2mixture on the synthesized Y-type membrane attained 38 at room temperature and kept a good stability at elevated permeating temperatures. Moreover, the zeolite membranes exhibited a good separation factor for water/ isopropanol mixtures, with its maximum value reaching up to 767.
Acknowledgements:This work was supported by the Talent Introduction Fund of Yangzhou University, the Jiangsu Social Development Project—Science and Technology Support Program (BE2014613) and Six Talent Peaks of Jiangsu province (2014-XCL-013). The authors also acknowledge the Project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. The data of this paper were originated from the Test Center of Yangzhou University.
[1] Zhang Jin, He Yan, Wang Yipin, et al. Synthesis of a selfsupporting faujasite zeolite membrane using geopolymer gel for separation of alcohol/water mixture[J]. Mater Lett, 2014, 116(2): 167-170
[2] Zhang Fei, Xu Longnü, Hu Na, et al. Preparation of NaY zeolite membranes in fluoride media and their application in dehydration of bio-alcohols[J]. Sep Purif Tech, 2014, 129: 9-17
[3] Sato K, Sugimoto K, Nakane T. Synthesis of industrial scale Y-type zeolite membranes and ethanol permeating performance in pervaporation and vapor permeation up to 130 ℃and 570 kPa[J]. J Membr Sci, 2008, 310: 161-173.
[4] Wang Z Z, Kumakiria I, Tanaka K, et al. NaY zeolite membranes with high performance prepared by a variabletemperature synthesis[J]. Micropor Mesopor Mater, 2013, 182(12): 250-258.
[5] Kusakabe K, Kuroda T, Uchino K, et al. Gas permeation properties of ion-exchanged faujasite-type zeolite membrane[J]. AIChE J, 2004, 45(6): 1220-1226.
[6] Zhu Guangqi, Li Yanshuo, Zhou Han, et al. FAU-type zeolite membranes synthesized by microwave assisted in situ crystallization[J]. Mater Lett, 2008, 62(28): 4357-4359
[7] Huang Aisheng, Yang Weishen. Hydrothermal synthesis of NaA zeolite membrane together with microwave heating and conventional heating[J]. Mater Lett, 2007, 61(20): 5129-5132
[8] Xu Xiaochun, Yang Weishen, Liu Jie, et al. Synthesis of a high-permeance NaA zeolite membrane by microwave heating[J]. Adv Mater, 2000, 12(3): 195-196
[9] Chen Xiaobo, Yang Weishen, Liu Jie, et al. Synthesis of zeolite NaA membranes with high permeance under microwave radiation on mesoporous-layer-modified macroporous substrates for gas separation[J]. J Membr Sci, 2005, 255(1/2): 201-211
[10] Xomeritakis G, Gouzinis A, Nair S, et al. Growth, microstructure, and permeation properties of supported zeolite (MFI) films and membranes prepared by secondary growth[J]. Chem Eng Sci, 1999, 54(15/16): 3521-3531.
[11] Kusakabe K, Kuroda T, Murata A, et al. Formation of a YType Zeolite Membrane on a Porous α-Alumina Tube for Gas Separation[J]. Ind Eng Chem Res, 1997, 36(3): 649-655
[12] Mintova S, Bein T. Microporous films prepared by spincoating stable colloidal suspensions of zeolites[J]. Adv Mater, 2001, 13(24): 1880-1883.
[13] Bernal M P, Xomeritakis G, Tsapatsis M. Tubular Mfizeolite membranes made by secondary (seeded) growth[J]. Catal Today, 2001, 67(1): 101-107
[14] Huang Aisheng, Lin Yongsheng, Yang Weishen. Synthesis and properties of A-type zeolite membranes by secondary growth method with vacuum seeding[J]. J Membr Sci, 2004, 245(1/2): 41-51.
[15] Mohammadi T, Pak A. Making zeolite A membrane from kaolin by electrophoresis[J]. Micro Meso Mater, 2002, 56(1): 81-88
[16] Uchikoshi T, Matsunaga C, Suzuki T S, et al. Electrophoretic deposition of orientation-controlled zeolite L layer on porous ceramic substrate[J]. J Ceram Soc Jpn, 2013, 121(1412): 370-372.
[17] Negishi H, Imura T, Kitamoto D, et al. Preparation of tubular silicalite membranes by hydrothermal synthesis with electrophoretic deposition as a seeding technique[J]. J Am Ceram Soc, 2006, 89(1): 124-130.
[18] Seike T, Matsuda M, Miyake M. Preparation of FAU typezeolite membranes by electrophoretic deposition and their separation properties[J]. J Mater Chem, 2002, 12: 366-368
[19] Cheng Zhilin, Sun Wei, Han Shuai, et al. Microwave-assisted Synthesis of NaA Zeolite Membrane with High Separating Performance by Seeding Using VPT Method[J]. Chem Lett, 2013, 42(4): 436-437
[20] Cheng Zhilin, Gao Enqing, Wan, Huilin. Novel synthesis of FAU-type zeolite membrane with high performance[J]. Chem Commun, 2004, 15: 1718-1719
[21] Ginter D M, Went G T, Bell A T, et al. A physicochemical study of the ageing of colloidal silica gels used in zeolite Y synthesis[J]. Zeolites, 1992, 12(6): 733-742
[22] Hedlund J, Schoeman B, Sterte J. Ultrathin oriented zeolite LTA films[J]. Chem Commun, 1997, 13(13): 1193-1194
[23] Kuzniatsova T, Kim Y, Shqau K, et al. Zeta potential measurements of zeolite Y: Application in homogeneous deposition of particle coatings[J]. Micropor Mesopor Mater, 2007, 103(1/3): 102-107
[24] Hasegawa Y, Watanabe K, Kusakabe K, et al. The separation of CO2using Y-type zeolite membranes ionexchanged with alkali metal cations[J]. Sep Purif Technol, 2001, 22–23: 319-321
[25] Cheng Zhilin, Lin Haiqiang, Chao Zisheng, et al. NaY zeolite membrane prepared by using pre-absorbed nanosized NaY zeolite synthesized by microwave heating[J]. Chem J Chinese U, 2003, 24(10): 1857-1861
[26] Zhu Guangqi, Li Yanshuo, Chen Hongliang, et al. An in situ approach to synthesize pure phase FAU-type zeolite membranes: Effect of aging and formation mechanism[J]. J Mater Sci, 2008, 43(9): 3279-3288.
Commercialized Technology for Methanol-to-Butene Production with Coproduction of Propylene Passed Appraisal
On September 14, 2015 the 10 kt/a-class commercialized test project relating to the technology for methanolto-butene production with co-production of propylene (hereafter abbreviated as CMTX) has passed the scientific achievement appraisal organized by the China Petroleum and Chemical Industries Federation (CPCIF).
The CMTX technology was jointly developed by the Shaanxi Coal Chemical Industry Research Institute Limited Liability Company, the Shanghai Bike Clean Energy Technology Co., Ltd. and the Shanghai Hetu Engineering Stock Company. The said technology adopts the circulating fluidized bed (CFB) process to manufacture butene and propylene from methanol. The process development work and tests on a 100 t/a pilot unit were completed by the end of 2013. The commercial scale test operation of a 10 kt/a process unit was conducted in July 2015, and the test outcome was checked and calibrated on the site within 72 hours spanning July 24—July 27, 2015 under supervision of a special team organized by CPCIF.
The technical personnel of relevant institutions have creatively developed the fluidized bed process and its special catalyst for manufacturing butene from methanol with coproduction of propylene, as well as the process for lowtemperature regeneration of the catalyst. The CMTX process developed thereby features high space velocity, low water/methanol ratio, high throughput of the process unit, low investment cost, and low energy consumption. The special catalyst which was independently developed by these relevant institutions is characteristic of high selectivity, low coke yield, high hydrothermal stability, reasonable catalyst particle distribution, good fluidization performance, high attrition resistance and good adaptability. The said CMTX process and its catalyst as a whole will have a rosy application prospect.
date: 2015-05-18; Accepted date: 2015-07-15.
Prof. Cheng Zhilin, Telephone: +86-514-87975590-7202, Fax: +86-0514-87975590-7202; E-mail: zlcheng224@126.com.
- 中国炼油与石油化工的其它文章
- Computational Fluid Dynamics Simulation of Liquid-Phase FCC Diesel Hydrotreating in Tubular Reactor
- Hydrothermal Liquefaction of Wheat Straw in Sub-critical Water/Ethanol with Ionic Liquid for Bio-oil Production
- Microbial Characterization of Denitrifying Sulfide Removal Sludge Using High-Throughput Amplicon Sequencing Method
- Promotional Effect of CoO(OH) on Selective Hydrogenation of Maleic Anhydride to γ-Butyrolactone over Supported Ruthenium Catalyst
- Quantitative Analysis Using Fourier Transform Ion Cyclotron Resonance Mass Spectrometry and Correlation between Mass Spectrometry Data and Sulfur Content of Crude Oils
- Design and Control of Self-Heat Recuperative Distillation Process for Separation of Close-Boiling Mixtures: n-Butanol and iso-Butanol

