Purif i cation and structural analysis of the toxin AP-I from the pathogen of Bambusa pervariabilis×Dendrocalamopsis grandis blight
•,2••
ORIGINAL PAPER
Purif i cation and structural analysis of the toxin AP-I from the pathogen of Bambusa pervariabilis×Dendrocalamopsis grandis blight
Shujiang Li1•Tianhui Zhu1,2•Tianmin Qiao1•Shan Han1
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
Bambusapervariabilis×Dendrocalamopsis grandisblight is caused by a toxin from the fungusArthrinium phaeospermum(corda)M.B.Ellis.We used shaking culture in a modif i ed Fries culture medium and methanol extraction to isolate the toxin.The optimal developing solvent mixture(methanol:ethyl acetate:H2O at 7:1.5:3)was selected using thin layer chromatography and used as the eluent for toxin purif i cation by silica gel column chromatography.Two toxic fractions were identifi ed in the bioassay.A f l axen oil substance,AP-I,showed higher toxicity than a toxic white powder,AP-II.The more toxic AP-I was determined to be dibutyl phthalate (C16H22O4,molecular weight of 278)by mass spectrometry,nuclear magnetic resonance,and infrared spectrophotometry.Dibutyl phthalate might contribute to the pathogenesis of bamboo blight.
Arthrinium phaeospermum⋅Bambusa pervariabilis×Dendrocalamopsis grandis⋅Infrared spectrophotometry⋅Mass spectrometry⋅Nuclear magnetic resonance⋅Toxin
Introduction
Bambusa pervariabilis×Dendrocalamopsis grandis,a major species native to China,is an important component of the ecological barrier to soil erosion in the Changjiang River basin.This hybrid bamboo is prevalent in warm and humid bamboo-growing areas all over China,and has signif i cantly contributed to the building of this ecological barrier.Arthrinium phaeospermum(corda)M.B.Ellis, which is a novel pathogenic fungus that causes hybrid bamboo wilting and death,was recently discovered in regions of forest reclamation(from farmland)in the Middle and Upper Reaches of the Changjiang River(Zhu et al. 2009).This fungus can also infect other bamboo species, includingPhyllostachys heterocycla(Xia et al.1995)andPhyllostachys prominens(Ma et al.2003).The occurrence and spread of this bamboo blight has been closely monitored(Zhu et al.2009),but very few studies have been conducted on the pathogenic mechanism ofA. phaeospermum.
Phytotoxins are major pathogenic factors in the infection of host plants.Plant pathogens produce many toxic compounds that can overcome disease resistance of many host species or multiple resistance mechanisms of one host (Dong 1995;Zhang 1996).A total of 21 species of pathogenic fungi from nine genera are known to produce host-specif i c toxins(HSTs),whereas at least 60 species are known to produce nonhost-specif i city toxins(NHSTs) (Andrie et al.2008;Amnuaykanjanasin and Daub 2009; Meca et al.2009;Kim et al.2010).With the development of new extraction and purif i cation technologies,manyfungal toxins have been isolated and characterised(Bok et al.1999).Previous studies focused mainly on polysaccharides(including glycoproteins)(Abang et al.2009), hemiterpenes(Rattan 2010),alkaloids(Fumiharu et al. 2006;Lorenz et al.2009;Uhlig et al.2010)and reductones (Raoudha et al.2006;Xu et al.2010),but the toxicity ofA. phaeospermumcompounds has only been reported in human studies(Vijayakumar et al.1996;Bloor 2008).We reported on the protein AP-toxin produced byA. phaeospermum,which played an important role in mediating the phytotoxic activities ofA.phaeospermum(Li et al.2013).But whether other type of phytotoxin produced from this fungus contribute to bamboo wilting disease,is unknown.
Dibutyl phthalate(DBP)is a common phthalate acid ester(PAE)used in many different industrial processes.As of this writing,most toxicology studies on DBP have focused on its carcinogenic and estrogenic effects(Pedersen and Larsen 1996)on aquatic animals(Patyna and Cooper 2000)and hydrophytes(Staples et al.1985), bioaccumulation in the food chain(Peijnenburg and Struijs 2006),degradation methods(Yuan et al.2002),acute toxicity to mature marine f i sh species(Lin et al.2003)and reproductive toxicity to generative cells of mature organisms(Adams et al.1995).Natural DBP has also been isolated fromMimusops elengi(Ruikar et al.2010),Streptomyces(Lee 2000;Roy et al.2006)andPenicillium bilaii(Savard et al.1994),and can mediate the toxicity and antimicrobial activity of these species.Whether DBP can be produced in toxic quantities byA.phaeospermumhas not been established.
Our primary objective was to isolate and purify the nonprotein toxic secondary metabolite fromA.phaeospermumby column chromatography,thin layer chromatography (TLC)and high-performance liquid chromatography (HPLC).Once the toxin was conf i rmed,our second objective was to determine the molecular structure by mass spectrometry(MS),nuclear magnetic resonance(NMR) and infrared spectroscopy(IR).
Materials and methods
Fungal cultures and plant samples
The pathogenic fungusA.phaeospermumwas provided by the Key Laboratory of Forest Protection of Sichuan Province and isolated from diseasedB.pervariabilis×D. grandis.The fungus was revived from storage at-20°C by pipetting a thawed suspension onto potato dextrose agar (PDA)plates and grown at 25°C for 5 days.Hybrid bamboo(1-year-old)samples were collected from the bamboo-growing areas of reclaimed farmland(elevation 393–1431 m,mean annual temperature of 16°C and annual precipitation of 1400–1700 mm)in Sichuan,China.
Preparation of crude toxin
Mycelial discs(5 mm)were grown in bottles containing 100 mL of modif i ed Fries nutrient solution for liquid culture(Pestka et al.1985)(containing 20 g/L dextrose,5 g/L tartaric acid ammonium,1 g/L NH4NO3,1 g/L KH2PO4, 0.5 g/L MgSO4⋅7H20,0.1 g/L NaCl,0.1 g/L CaC12⋅2H2O, and 1 g/L yeast extract).Sterile distilled water was cultured as a blank control and worked through all the steps below to eliminate the effects of the test apparatus and basic isolation procedure.After 15 days of growth in a rotary shaker(140 rpm)in the dark at 26°C,the culture was f i ltered over double gauze and the supernatant was centrifuged at 6000 rpm for 10 min.The clarif i ed supernatant was then mixed with an equal volume of methanol. The mixture was centrifuged at 6000 rpm for 10 min and condensed to 5 mL by vacuum evaporation at 40°C.This process was repeated three times to obtain three batches of crude toxin sample.Extracts were stored at-20°C.
Determination of the developing solvent
According to the permittivity and polarity of different solvents,more than 100 different solvent systems were tested by varying the volume ratio.The crude toxin and blank control were spotted on silica gel plates (5 cm×10 cm)with an equal volume of solvent and the distance travelled by the solvent system was determined. After the developing solvents were volatilised,the coloration spots were examined with iodine vapour.The optimal developing solvent was determined by the maximum Rf value(distance travelled by the compound/distance travelled by the solvent front).
Purif i cation of the toxin and assay of activity
The crude toxin and blank control were respectively loaded onto silica gel for column chromatography(100–200 mesh) with the selected eluent and the f l ow rate was maintained constant at 2 mL/min.Under these conditions,the volume of each pure compound collected was less than 1 mL after drying by distillation.Samples were arranged in order of elution time until the silica gel column turned white and the eluent contained no material after drying.The compounds were dissolved in H2O and combined with the same samples from the next trial of silica gel column chromatography.The combined products from three purif i cation trials were stored at-20°C.
The impregnation method(Ho et al.1996)was used to test the toxicity of the purif i ed products against hybridbamboo.The clean shoots were cultured in solution containing 1 mL of 100 μg/mL of each purif i ed compound in centrifuge tubes.The symptoms(wilting and brown discoloration)of the branches and leaves were surveyed after culturing at 22°C for 24 h(12 h light and 12 h dark).Each treatment was repeated three times.
Assay of the purity of the toxin
TLC and HPLC were used to test the purity.In TLC,after developing on the TLC,the purif i ed compound was stained with phosphomolybdic acid hydrate and iodine vapour.For HPLC,the purif i ed compound was analysed using an 1101LC system(Agilent,USA)with automatic sampler (G1316A)and variable wavelength detector(G1314A)set to absorbance at 276 nm.The HPLC column was a VPODS C18 5 μm reversed-phase capillary chromatography column(4.6 mm×150 mm).The eluent was a mixture of A and B phases(A phase:65%acetonitrile;B phase:35% H2O)at a f l ow rate of 1.0 mL/min with a linearity of 10 μL at 30°C.The DBP standard was obtained from Beijing Beihua Hengxin Technology Co.Ltd.(China).
Identif i cation of the toxin structure
For mass spectrometry(MS)analysis,the selected sample (AP-1)was scanned using a MS-QP2010 Plus MS(Shimadzu,Japan)with electron impact ionisation(EI).The voltage of the ionisation chamber was 70 eV and the temperature was 250°C.Both1H and13C nuclear magnetic resonance(NMR),with distortionless enhancement by polarisation transfer(DEPT)and heteronuclear multiple quantum coherence(HMQC)NMR(Unity Inova, 400 MHz;Varian,USA),were obtained for AP-1 in the solvent CDCl3.The internal standard was(CH3)4Si.For infrared(IR)spectroscopic analysis,the IR absorption spectrum was analysed using a 170 SX IR spectrophotometer(Nicolet,USA)with disc-formed samples of KBr.
Bioassay
Puncture assay(Cuq et al.1993)was used to test the symptoms induced byA.phaeospermum(5×106CFU/ mL),the purif i ed toxin(100 μg/mL),or standard dibutyl phthalate(100 μg/mL)to bamboo joints in the f i eld. Control plants were treated with an equal volume(1 mL) of the following:1)sterile distilled water and 2)sterile distilled water cultured under the same condition as toxin. Each treatment protocol was repeated on 50 plants.After 5, 10,15,and 20 days,the numbers and areas of brown rhombic spots on the stems were recorded.
The spot area was calculated using the following formula:S(cm2)=(a×b)/2(S:rhombic spot area;a,b(cm):two diagonals).All data were subjected to one-way ANOVA to determine the signif i cance of individual differences atp<0.05.Signif i cant differences between means were computed using the LSD post hoc test.All statistical analyses were conducted using the SPSS commercial statistical package(SPSS,Version 13.0 for Windows,SPSS Inc.,Chicago,USA).
Results
Optimal developing solvent
Only four solvent combinations yielded at least f i ve clear spots on the TLC plates.Among these combinations,we determined that methanol:ethyl acetate:H2O at 7:1.5:3 was the optimal ratio as determined by the Rf values (Table 1).
Purif i ed toxins and their activity
From the crude toxin cultured from mycelia,we collected 51 separate volumes that attained a maximum absorption at 276 nm during the entire elution time(4 days).The optical density of compound No.11 was the highest,whereas Nos. 5,9 and 20 were in the mid-range.No substance was found above sample No.38(Fig.1).
Compound No.11 was obtained as a f l axen oily transparent liquid(A1)and showed one spot on TLC(Fig.2). This sample also had the highest bioactivity(Table 2)and turned the stems of hybrid bamboo to brown and caused the leaves to wilt.The sensitivity of leaves was higher than that of branches(Table 2).After treatment with compound A1for 24 h,the leaves exhibited chlorisis,wilting,and putrescence.By contrast,treatment with another toxin (compound A2,a white powder isolated from sample No. 20)did not evoke this response until 72 h after application. After 5 days,wilting was observed(Fig.3),and compound No.11 yielded the most serious wilting.Based on these results,compound No.11 was subjected to structural analysis.
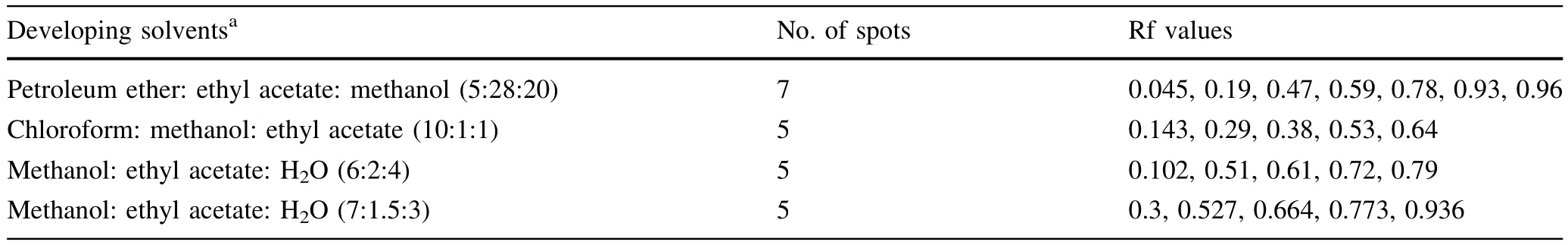
Table 1 Optimisation of the developing solvent

Fig.1 Compounds isolated from A.phaeospermum by ultraviolet spectrophotometry

Fig.2 Pure compound A1 collected by TLC.A1:a f l axen oily transparent liquid developed on the thin layer plate;chromogenic reagent: phosphomolybdic acid hydrate and iodine vapour
HPLC analysis of A1 showed that this compound had an absorption peak at 12.914 min(Fig.4a)and was designated as AP-I according to the naming conventions for plant pathogenic toxins.Compared with the HPLC results of standard substances(Fig.4b),AP-I was presumed to be dibutyl phthalate.
Structure of the toxin AP-I
MS(Fig.5)revealed the peaks of the cracking ions of carboxylate carbonyl carbon at m/z 43,57,and 71,and phthalate base at m/z 149,which was considered for the characterisation of phthalate ester with lateral chains larger than two carbons.Considering an[M+H]+ion peak at 279,the molecular weight of AP-I was inferred to be 278.
NMR analysis(Table 3)indicated the presence of two methyl groups,six methylene units(two O-bearing methylenes),four sp2aromatic carbons,two sp2quaternarycarbons and two carbonyl carbons.The1H NMR spectrum showed a characteristic AA’BB’system at δ7.71(2H,dd,J=9.12,2.11 Hz)and 7.26(2H,dd,J=8.78,2.63 Hz), as well as13C NMR data at δ132.47(s),128.79(d)and 130.86(d),which indicate that the compound had a diortho-substituted aromatic ring.
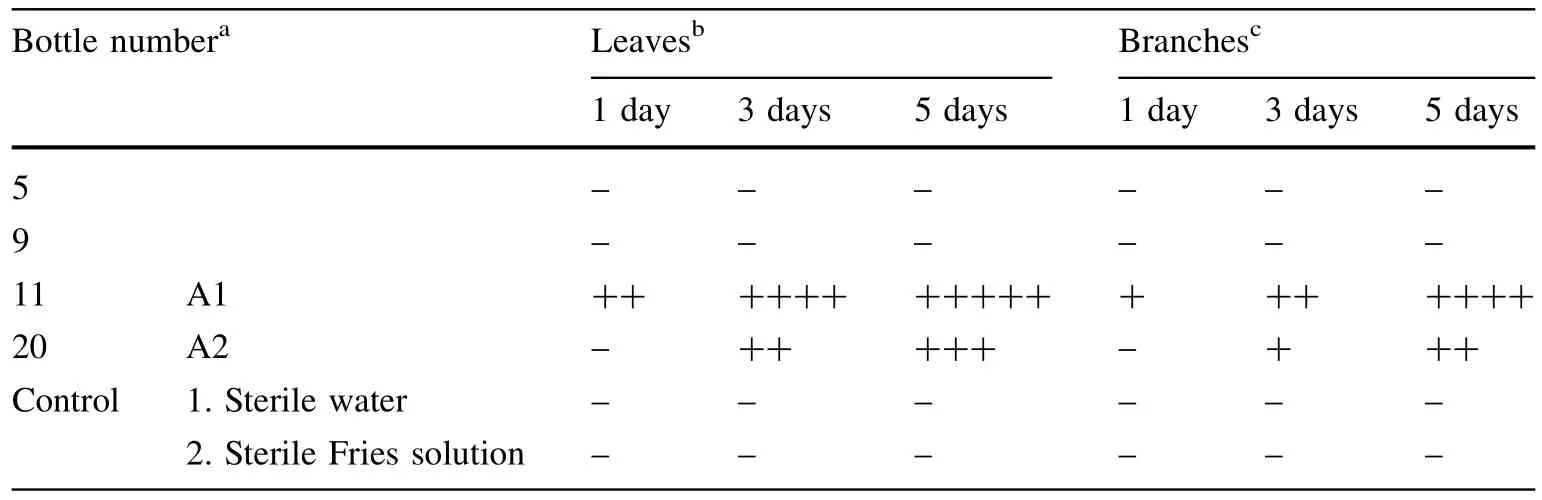
Table 2 Pathogenic activity of compounds isolated by column chromatography
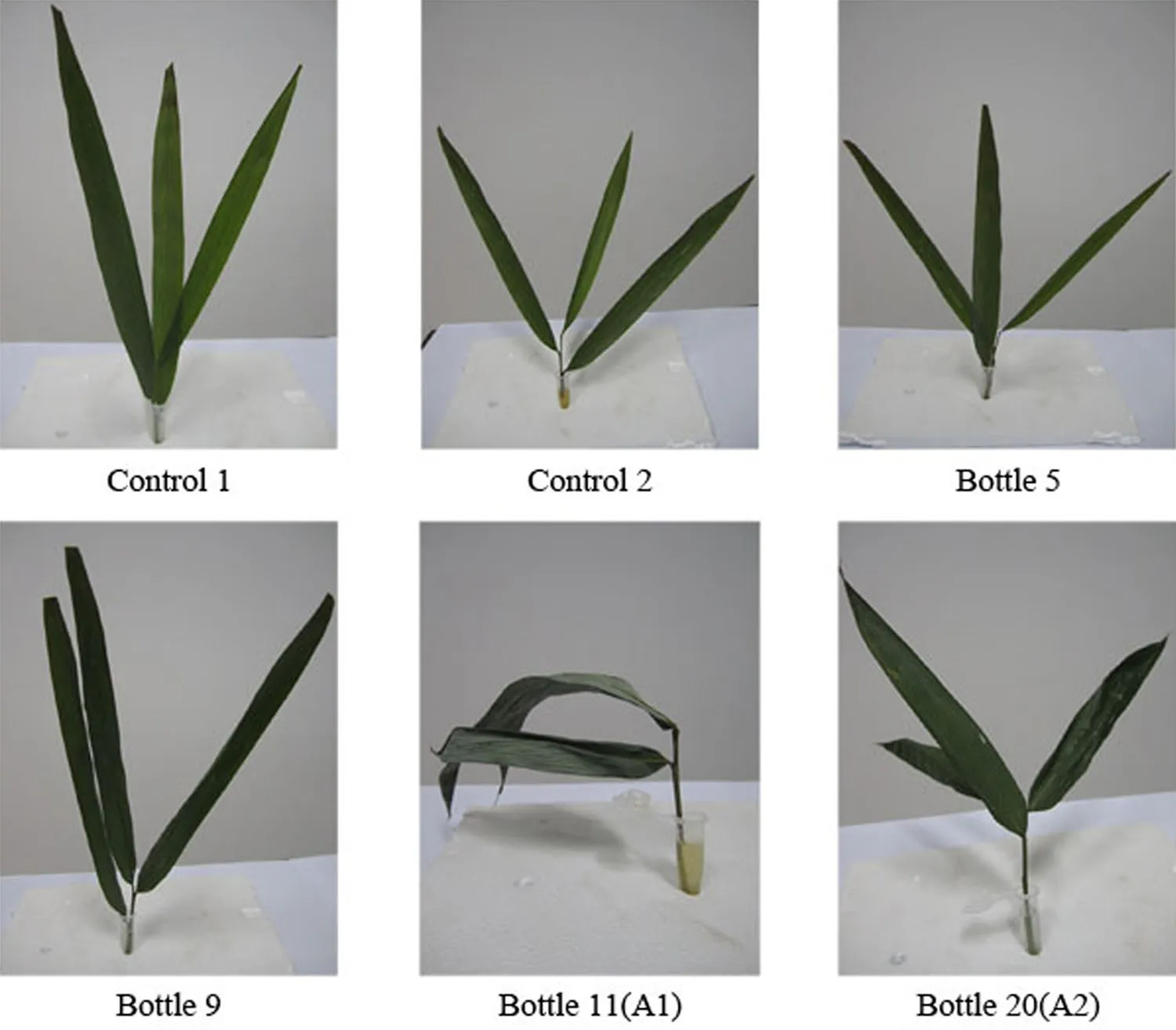
Fig.3 Symptoms of hybrid bamboo infected by toxin. Control 1 sterile water;control 2 sterile Fries solution;bottle 5, 9,11 and 20:the compounds isolated from A.phaeospermum
IR spectrum showed a–C–(CH2)2–CH3group (2860.09–2959.55 cm-1indicatedνCH,1380.59 and 1463.40 cm-1indicated δCH3and δCH2),an aromatic system(3070.53,1600.09,and 742.78 cm-1),and an ester moiety(1731.80 and 1271.03 cm-1),which indicate carbonyl and phenyl functional groups.These data suggestthat compound AP-I was dibutyl phthalate.The structural formula is illustrated in Fig.6.

Fig.4 HPLC on VP-ODS C18 5 μm reversed-phase capillary chromatography column(4.6 mm×150 mm).a HPLC prof i les of compound A1;the solid bar indicates the fraction containing toxicity at 12.914.b HPLC prof i les of DBP standard;the solid bar indicates the fraction containing toxicity at 12.822

Fig.5 MS spectrum compound A1

Table 3 NMR data of compound AP-I
Activity of the toxin AP-I
The symptoms induced by toxin AP-I were compared with those induced byA.phaeospermumand dibutyl phthalate in naturally growing bamboo(50 plants each).Brown rhombic spots appeared on the stems of all 150 plants treated. Moreover,the spot areas of AP-I-treated plants were not signif i cantly different from those produced by DBP throughout the observation period.By contrast,AP-I-induced spots were larger than those produced byA. phaeospermumtreatment before day 10,but similar after day 15(Table 4).This difference in response time may ref l ect the slower action of the live pathogen compared with the purif i ed toxic metabolite.With respect to the shape,area, and colour of these disease spots,the results suggest that the toxin AP-I was the main pathogenic factor ofA. phaeospermum,and it was verif i ed to be dibutyl phthalate.

Fig.6 Structure of compound A1.The numbers are the order of carbon atoms
Discussion
Many studies have reported on the pathogenic toxins produced by fungi(Ueno et al.1973;Brain and Harold 1994; Shizawa et al.1995;Ostry and Anderson 2009).However, the toxins from pathogenicA.phaeospermumhave not been identif i ed.Bloor(2008)demonstrated thatA. phaeospermumcan produce arthrinic acid while infecting humans,which indicated that the toxic substance is a polyhydroxy acid(PHA).However,we found that this fungus produced a novel ester phytotoxic compound, dibutyl phthalate.
TLC and HPLC analyses showed two active toxic compounds,namely,AP-I(a f l axen oil)and AP-II(a whitepowder),with AP-II being the less toxic compound.Thus, compound AP-I was considered to be the pathogenic factor that caused the typical lesions on hybrid bamboos.Moreover,a previous study conf i rmed that dibutyl phthalate can be produced byMicromonosporasp.on a halophyte(Shi et al.2005),Streptomyces albidof l avus(Roy et al.2006),Cryphonectria parasitica(Han and Zhu 2009)andEnterobacter sp.(Fang et al.2010).Further tests by MS,NMR, and IR spectroscopy indicated that compound AP-I was dibutyl phthalate(C16H22O4,molecular weight=278).In addition,the bioassay reconf i rmed that AP-I was the pathogenic factor dibutyl phthalate.

Table 4 Rhombic spot areas of different treatments on hybrid bamboo stems
In the process of purif i cation,bioactive substances produced in low amounts can be easily lost.Many of these rare compounds,or their combinations,can still cause plant damage(Zhu et al.2005).However,once these multiple compounds are separated from each other,their bioactivities become weak or are lost completely and un-detected using a bioassay(Zhang et al.2003;Zhu et al.2003). Similarly,our results show that the activity and action sites of different compounds were distinct,suggesting that plant diseases could be caused by different compounds acting separately or synergistically.Moreover,the purif i ed toxin produced a much more rapid pathological response than the fungal infection,which was probably due to the higher concentration of toxin in the bioassay solution than the concentration of toxin released quickly from the natural pathogen.
In conclusion,highly toxic dibutyl phthalate was isolated fromA.phaeospermum.This study is the f i rst report on the production of non-protein toxin byA.phaeospermum,which also causes bamboo blight.Further preparation and isolation are required to assess the toxic activity of this and other compounds,alone and in combination.Furthermore,the natural concentration and action site should be studied.These results provide the foundation for studies on the pathogenic mechanisms ofA.phaeospermumand may lead to methods for inactivating this toxin and rescuing ecologically valuable bamboo hybrids.
AcknowledgmentsThis research was supported by the National Natural Science Foundation of China(31070578)and the National Natural Science and Technology Resources Sharing Platform of China(2005DKA21207-13).
Abang MM,Abraham WR,Asiedu R,Hoffmann P,Wolf G,Winter S (2009)Secondary metabolite pro fi le and phytotoxic activity of genetically distinct forms ofColletotrichum gloeosporioidesfrom yam(Dioscoreaspp.).Mycol Res 113:130–140
Adams WJ,Biddinger GR,Robillard KA(1995)A summary of the acute toxicity of 14 phthalate esters to representative aquatic organisms.Environ Toxicol Chem 14:1569–1574
Amnuaykanjanasin A,Daub ME(2009)The ABC transporter ATR1 is necessary for ef fl ux of the toxin cercosporin in the fungusCercospora nicotianae.Fungal Genet Biol 46:146–158
Andrie RM,Schoch CL,Hedges R,Spatafora JW,Ciuffetti LM (2008)Homologs ofToxB,a host-selective toxin gene fromPyrenophora tritici-repentis,are present in the genome of sisterspeciesPyrenophora bromiand other members of the Ascomycota.Fungal Genet Biol 45:363–377
Bloor S(2008)Arthrinic acid,a novel antifungal polyhydroxyacid fromArthrinium phaeospermum.J Antibiot 61:515–517
Bok JW,Lermer L,Chilton J(1999)Antitumor sterols from the mycelia ofCordyceps sinensis.Phytochemistry 51:891–898
Brain LB,Harold SG(1994)Characterization of sugarcane response toBipolaris sacchari:inoculations and host-speci fi c HS-toxin. Phytopathology 84:672–676
Cuq F,Henmann-Gorline S,Klaebe A,Rossignol M,Petitprez M (1993)Monocerin inExserohilum turcicumisolates from maize and a study of its phytotoxity.Phytochemistry 34:1265–1270
Dong HS(1995)Induced resistance against diseases in plants principle and practice.Science Press,Beijing,pp 62–66
Fang CR,Yao J,Zheng YG,Jiang CJ,Hu LF,Wu YY,Shen DS (2010)Dibutyl phthalate degradation byEnterobactersp.T5 isolated from municipal solid waste in landf i ll bioreactor.Int Biodeterior Biodegrad 64:442–446
Fumiharu H,Hiroyasu N,Hideo H(2006)Purif i cation and structure determination of glucosides of capsaicin and dihydrocapsaicin from variousCapsicumfruits.J Agric Food Chem 54:5948–5953
Han S,Zhu TH(2009)Isolation,purif i cation and structure of Cp-I Toxin fromCryphonectria parasitica.Mycosystema 28:535–540
Ho SH,Koh L,Ma Y,Huang Y,Sim KY(1996)The oil of garlic,Allium sativumL.(Amaryllidaceae),as a potential grain protectant againstTribolium castaneum(Herbst)andSitophilus zeamaisMotsch.Postharvest Biol Technol 9:41–48
Kim TG,Kim MY,Yang MS(2010)Cholera toxin B subunit-domain III of dengue virus envelope glycoprotein E fusion protein production in transgenic plants.Protein Expr Purif 74:236–241
Lee DS(2000)Dibutyl phthalate,a glucosidase inhibitor fromStreptomyces melanosporofaciens.J Biosci Bioeng 89:271–273
Li SJ,Zhu TH,Zhu HMY,Liang M,Qiao TM,Han S,Che GN(2013) Purif i cation of protein AP-toxin fromArthrinium phaeospermumcausing blight inBambusa pervariabilis×Dendrocalamopsis grandisand its metabolic effects on four bamboo varieties. Phytopathology 103:135–145
Lin Z,Ikonomou MG,Jing H(2003)Determination of phthalate ester congeners and mixtures by LC/ESI-MS in sediments and biota of an urbanized marine inlet.Environ Sci Technol 37:2100–2108
Lorenz N,Haarmann T,Pazˇoutova´S,Jung M,Tudzynski P(2009) The ergot alkaloid gene cluster:functional analyses and evolutionary aspects.Phytochemistry 70:1822–1832
Ma GL,Hu GL,Yu CZ,Wu JL,Xu BC(2003)Phyllostachys prominensplum shoot wilt pathogenic fungoid and it s biological characteristics.J Zhejiang For Coll 20:44–48
Meca G,Sospedra I,Soriano JM,Ritieni A,Valero MA,Man˜es J (2009)Isolation,purif i cation and antibacterial effects of fusaproliferin produced byFusarium subglutinansin submerged culture. Food Chem Toxicol 47:2539–2543
Ostry ME,Anderson NA(2009)Genetics and ecology of theEntoleuca mammata–Populuspathosystem:b Implications for aspen improvement and management.For Ecol Manag 257:390–400
Patyna P,Cooper KR(2000)Multigeneration reproductive effects of three phthalate esters in Japanese medaka(Oryzias latipes).Mar Environ Res 50:194
Pedersen BF,Larsen R(1996)Identif i cation of agricultural crops in Denmark by satellite Imagery.In:Proceedings,NJF seminar, report of the Finnish Geodetic Institute.Finnish Agricultural Research Centre,Jokoinen,vol 96,pp 4–8
Peijnenburg W,Struijs J(2006)Occurrence of phthalate esters in the environment of the Netherlands.Ecotoxicol Environ Safe 63:204–215
Pestka JJ,Bahrawy AE,Hart LP(1985)Deoxynivalenol and 15-monoacetyl deoxynivalenol production byFusarium graminearumR6576 in liquid media.Mycopathologia 91:23–28
Raoudha BAM,Samiha S,Lilia FBF(2006)Purif i cation and structure determination of four bioactive molecules from a newly isolatedStreptomycessp.TN97 strain.Process Biochem 41:1506–1513
Rattan RS(2010)Mechanism of action of insecticidal secondary metabolites of plant origin.Crop Prot 29:913–920
Roy RN,Laskar S,Sen SK(2006)Dibutyl phthalate,the bioactive compound produced byStreptomyces albidof l avus321.2.Microbiol Res 161:121–126
Ruikar AD,Gadkari TV,Phalgune UD,Puranik VG,Deshpande NR (2010)Dibutyl phthalate,a secondary metabolite fromMimusops elengi.Chem Nat Compd 46:955–956
Savard ME,Miller JD,Blais LA,Seifert KA,Samson RA(1994) Secondary metabolites ofPenicillium bilaiistrain PB-50. Mycopathologia 127:19–27
Shi Y,Tian L,Pei YH(2005)The chemical constituents from the mycelia of marine fungusRhizopussp.Chin J Med Chem 15:221–223
Shizawa H,Takahashi M,Takaatsu T(1995)Trachyspic acid,a new metabolite produced byTalaromyces trachyspermusthat inhibits tumourcell heparanase.J Antibiot 48:357–363
Staples CA,Wenrner AF,Hoogheem TJ(1985)Assessment of priority poiiutant concentrations in the United States using STORET database.Environ Toxicol Chem 4:131–142
Ueno Y,Sato N,Ishii K(1973)Biological and chemical detection of trichothecene mycotoxins ofFusariumspecies.Apply Environ Microbiol 25:699–704
Uhlig S,Petersen D,Role`n E,Jacobsen WE,Vra¨lstad T(2010) Ergosedmine,a new peptide ergot alkaloid(ergopeptine)from the ergot fungus,Claviceps purpureaparasitizingCalamagrostis arundinacea.Phytochem Lett 167:1–7
Vijayakumar EK,Roy K,Chatterjee S(1996)Arthrichitin.A new cell wall active metabolite fromArthrinium phaeospermum.J Org Chem 61:6591–6593
Xia LM,Zhang SX,Huang JH(1995)Studies onArthrinium phaeospermumcausing moso bamboo foot rot.J Nanjing For Univ 16:23–28
Xu LS,Jia JG,Lv J,Liang XF,Han DJ,Huang LL,Kang ZS(2010) Characterization of the expression prof i le of a wheat acireductone-dioxygenase-like gene in response to stripe rust pathogen infection and abiotic stresses.Plant Physiol Biochem 48:461–468
Yuan SY,Liu C,Liao CS(2002)Occurrence and microbial degradation of phthalate esters in Taiwan river sediments. Chemosphere 49:1295–1299
Zhang YS(1996)Plant pathology and pathophysiology.Jiangsu Technology Press,Nanjing,p 56
Zhang LH,Liu YH,Dong JG(2003)Isolation and purif i cation of specif i c toxin factions produced byExserohdum tareicum.Acta Phytopathol Sin 33:67–71
Zhu TH,Luo MJ,Ye HZ(2003)Isolation and purif i cation of Pftoxin fromPestalotia funerea.Acta Phytopathol Sin 33: 541–545
Zhu TH,Ye HZ,Luo MJ(2005)The chemical composition of Pftoxin fromPestalotia funerea.Struct Pathog Mater I Mycosystema 24:112–115
Zhu TH,Huang ZC,Gao QZ,Li FL,Luo LJ,Li XD(2009)Pathogen and occurrence regularity ofBambusa ervariabilis×Dendrocalamopsis daiiblight.For Pest Dis 28:10–12
19 March 2013/Accepted:8 January 2014/Published online:5 August 2015
Project founding:This research was supported by the National Natural Science Foundation of China(31070578)and the National Natural Science and Technology Resources Sharing Platform of China(2005DKA21207-13).
The online version is available at http://www.springerlink.com
Corresponding editor:Chai Ruihai
✉Tianhui Zhu
zhuth1227@126.com
1College of Forestry,Sichuan Agricultural University, Chengdu 611130,Sichuan,People’s Republic of China2Key Laboratory of Forest Protection of Sichuan Province, Sichuan Agricultural University,Chengdu 611130,Sichuan, People’s Republic of China
 Journal of Forestry Research2015年4期
Journal of Forestry Research2015年4期
- Journal of Forestry Research的其它文章
- Culm characteristics and volume-weight relationship of a forest bamboo(Melocanna baccifera(Roxb.)Kurz)from northeast India
- Effects of characteristic inhomogeneity of bamboo culm nodes on mechanical properties of bamboo f i ber reinforced composite
- Simulating the heartwood formation process of Erythrophleum fordii in South China
- Wood liquefaction with phenol by microwave heating and FTIR evaluation
- The inf l uences of biotic and abiotic factors on the occurrence and severity of poplar canker disease in Qingfeng County,China and the management implications
- Neofusicoccum parvum causing canker of seedlings of Juglans regia in China
