Differential expression of zinc accumulation during two growing seasons of Acacia victoriae
Ali Mahdavi•Khadijeh Khermandar
Differential expression of zinc accumulation during two growing seasons of Acacia victoriae
Ali Mahdavi1•Khadijeh Khermandar2
It is important to understand seasonal heavy metal accumulation in different parts of plants in order to develop the best phytoremediation practices for contaminated soils.Forthis purpose we exposed,1 yearold A. victoriae seedlings to ZnSO4in 4 differentconcentrations: 0,50,250 and 500 mg Zn L-1for 45 days over two growing seasons.Subsequently,bioaccumulation of Zn in differentplanttissues(roots,shoots and leafs)was assessed by Atomic Absorption Spectroscopy(AAS)for two periods.In addition,various growth attributes(dry biomass, shoot and root lengths,plant appearance)and functional traits(leaf area,chlorophylla,b and total)were measured. The accumulation of Zn was influenced by the Zn concentration in the growth medium and the number of growing seasons.The amounts of Zn concentrated in the roottissues mightindicate A.victoriae as a good option for phytostabilization of soils contaminated by Zn.We recommend that if A.victoriae is used for phytoextraction purposes,then it should be harvested at the end of the first growing season(fall)because at this time the concentrations of Zn in the above-ground parts willbe maximal.
Heavy metal·Phytoremediation· Phytoextraction·Phytostabilization
Introduction
Heavy metal contamination issues are becoming increasingly common(Fernandes and Henriques 1991).Soilis the vital medium in the natural environment and heavy metal contamination is one of the most serious environmental problems in soil(Fernandes and Henriques 1991).The idea of using plants that hyperaccumulate metals to selectively remove and recycle excessive soilmetals was introduced in 1983,gained public exposure in 1990,and has increasingly been examined as a potential practical and more cost-effective technology than soilreplacement,solidification and washing strategies presently used(Chaney et al.1997). Phytoremediation is best applied at sites with shallow contamination of organic,nutrient,or metalpollutants.Itis well-suited for use at very large field sites where other methods of remediation are not cost-effective or practicable(Brooks 1998).To date,about 400 hyperaccumulators have been identified(Qishlaqietal.2009).The remediation potential of hyperaccumulators relies upon their growth rates(i.e.,biomass production)and metal accumulation rate(g metal per kg of plant tissue).In fact,most hyperaccumulators produce little biomass.This could be compensated by high biomass plants even if they are usually not metal-specific and accumulate low to average heavy metal concentrations(Roosens et al.2003).Thus,phytoremediation could be a promising technique forremoving soil pollutants where hyperaccumulators or accumulators are used to take up large quantities ofpollutantmetals(Salt et al.1995;Roosens et al.2003).Both essential and unessential metals ruin the balance of the ecosystem,withincreasing economic loss and human health damage (Qishlaqi et al.2009).The most important toxic elements belong to a group of metals,including Copper(Cu),Zinc (Zn)and Lead(Pb)and more rarely Cadmium(Cd), Chromium(Cr),Cobalt(Co),Nickel(Ni)and Mercury (Hg).A few metals,including Cu,Mn and Zn,are required by plants in trace amounts.It is only when metals are presented in bioavailable forms at excessive levels where they have the potential to become toxic to plants(Reichman 2002).
Zinc is an essential nutrientfor plants.This element is a co-factor requirement for the structure and function of numerous proteins(Grotz and Guerinot 2006),energy production and structural integrity of membranes(Hansch and Mendel2009).Zn deficiency is a common problem in plants grown in high pH,calcareous soils(as also for Fe deficiency)(Cakmak et al.1996).Zn can be toxic,and affected plants can show symptoms similar to those resulting from other heavy metaltoxicities,such as those of Cd or Pb(Foy et al.1978).High concentrations of Zn inhibitmany plantmetabolic functions resulting in retarded growth and senescence.Zn toxicity in plants limits the growth of both roots and shoots and produces young leaf chlorosis.Even though Zn is not redox active,high levels of this metalare toxic because itcan displace other metals (e.g.Fe,Mn and Cu)in the cell(Pilon et al.2009;Yadav 2010).Because of this,Zn homeostasis is also strongly regulated in plant cells.Human activities releasing Zn to the environmentinclude fossilfuelcombustion and the use of sewage sludge,manure and lime.In contaminated and acid soils some crops and species have a high Zn uptake capacity(Broadley et al.2007).The range of hyperaccumulators of Zn is>1.0%of the dry weights of leafs and stems irrespective of the metal concentration in the soil (Raskin et al.1994).Several studies showed that Zn concentrations in the leafs of Thlaspi calaminare and Viola calaminaria can reach 3.5 and 1.0%,respectively(Reeves and Brooks 1983;Lasat 2000).Ernst in(1986)reported Cardaminopsis halleri(Brassicaceae)as a hyperaccumulator of Zn.
This study is focused on the phytoremediation potential of Zn heavy metalcontaminated soils by A.victoriae.Our assumptions forthis study were thatthe accumulatorplants take up heavy metals according to seasonal growth patterns.Therefore,matching the phytoextraction practices to those patterns can maximize the remediation efficiency of contaminated soils,and increase metal uptake and accumulation.However there is not much knowledge about the pattern of heavy metal uptake and accumulation into the tissues during the growing seasons.This understanding is important to schedule phytoremediation practices to coincide with periods of high metal accumulation in aboveground tissues thereby maximizing the phytoextraction efficiency.In addition,the objective of the study is to investigate the effects of high concentrations of Zn in the soil medium on growth and photosynthetic characteristics of A. victoriae.The results of this study show the potentialuse of A.victoriae trees for phytoremediation purposes in Zn contaminated soils.
Materials and methods
Experiments were performed under natural conditions using 24 seedlings(12 seedlings for each growing season)of one year old A.victoriae that were replanted into 30 cm blackened plastic pots,filled with 2.5 kg(dry weight)of loamy-silt soils.A loamy-silt soil was prepared for filling all the pots(including the control treatments)by proportions of 1:1:2 for manure(dry animaldung),sand and soil, respectively(Table 1).The seedlings were exposed to Zn (SO4)solution in 4 differentconcentrations:0(control),50, 250 and 500 mg Zn L-1by supplementing them into irrigation water each time(the seedling were irrigated 20 times)during 45 days in both seasons.For each Zn concentration three replicates were evaluated.The seedlings were irrigated based on 60%of Field Capacity(FC).
Plant growth and biomass measurements
At the time of harvesting(45 days after being exposed to Zn2+treatments in both growing seasons),A.victoriae seedlings were removed from the pots,separated into leaf, shoot and root portions,rinsed with deionized water,dried to constant weight at 70°C for 48 h,and weighed for biomass determination.Plant height from the soil level to the top was measured before uprooting.Growth measurements were used to estimate the lead-tolerance index(TI). The rootlength can be used as an important TI(Piechalak et al.2002).TI was calculated as follows(Maldonado-Magana et al.2011;Mahdavi et al.2014).

where Ti is the tolerance index,R1 is the root elongation with Zn and R2is the rootelongation without Zn.
Determination of pigment content
Chlorophyll content in A.victoriae leaf samples was determined on fresh weight basis.Fresh leafs weighing 100 mg were pummeled and placed in 10 mL 80%acetone in a sealed,dark bottle.The bottle was placed into a laboratory tabletop centrifuge at 3000 rotations/min for 15 min.Then,1 mL of the surface solution was removed and adjusted with 4 mL 80%acetone.After that,theoptical density of the solution was measured by PD-303 UV spectrophotometer at different wavelengths i.e.,645, 663 nm and chlorophyll content was calculated using relevant formulae(Strain and Svec 1966;Mahdavi et al. 2014).
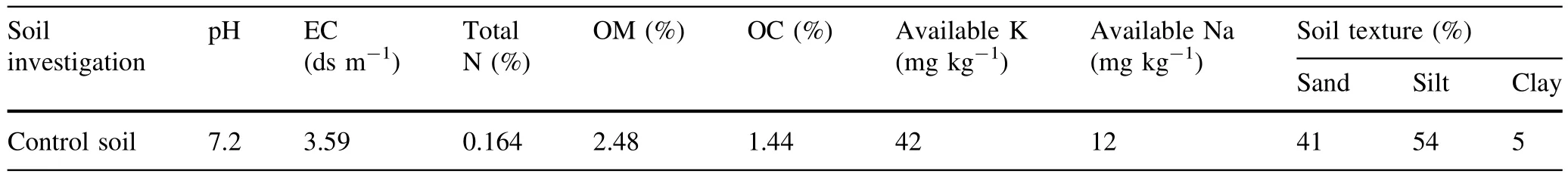
Table 1 Physico-chemical characterizations of control soil
Estimation of Zn accumulation
The dried samples(roots,shoots,and leafs)were digested in a microwave oven at high temperature(up to 235°C) using 8 mL of HNO3(65%),2 mL of H2SO4,1 mL of HClO4(70%)(Moreira etal.2011;Mahdavietal.2014). Zn concentrations were determined by an Atomic Absorption Spectrophotometer(AAS)(CTA-2000 AAS, Chem Tech Analytical)and the values were expressed as mg per Kg dry weight.Zn concentrations were used to estimate the bio-concentration factor(BCF)and the translocation factor(TF).
Bioconcentration factor(BCF)
Bioconcentration factor(BCF)was defined as the ratio of Zn concentrations in planttissues(roots,shoots and leafs) ([Zn]plant)to Zn concentration in the soil medium ([Zn]medium)(Maldonado-Magana et al.2011;Mahdavi et al.2014).That is a dimensionless number representing how much ofa chemicalis in a tissue relevantto how much of that chemical exists in the medium.

where BCFis the Bioconcentration factor,Zpis Zn concentrations in plant tissues(roots,shoots and leafs)and Zsis the Zn concentration in the soil medium.
Translocation factor
The translocation factor(TF)indicates the ability of plant to translocate heavy metals from roots to the harvestable aerial part(Waranusantigul et al.2008;Mahdavi et al. 2014).It was calculated on a dry weight basis by dividing the metal concentration in shoots and leafs by the metal concentration in roots(Waranusantigul et al.2008;Mahdavi et al.2014)

where tFis the translocation factor,ZSLis the metal concentrations in shoots and leafs and ZRis the Zn concentration in roots.
To test the phytotoxicity of Zn,the Grade of Growth Inhibition(GGI)was calculated as follows.

where,C and T represent the dry weight of tissues of control(C)and the metal-treated plants(T)(Leita et al. 1993;Mahdavietal.2014).
Statistical analysis
Combined analysis of variance was used to estimate the average response to given treatments and to test consistency of the responses from summer season to fall season i.e.interaction of the treatment effects with seasons. Combined analysis of variance provided an overview of the magnitude of variance between the experimental seasons, the variation between treatments(Zn concentrations)and especially the treatment×season interaction.We used a randomized block design and the least significant difference test(LSD)for comparing means.The level of statistical significance was set at P<0.01 and<0.05.All the results are expressed as means and letters indicate statistical differences between means.The software used for the combined analysis of variance was SAS for windows program(SAS-9.1-portable).
Results
Analysis of variance
In the combined analysis of variance over seasons,the effects of Zn concentrations were compared to the Zn accumulations in different plant tissues and grwoth characteristics.Some traits such as Zn contents in different tissues,TF rations and BCF had different responses related to treatments in two seasons(Table 2).In addition,alltraits including root length,plant height,leafs,shoots and rootsdry matter and so on varied significantly between treatments at 1 and 5%probability levels(Table 2).
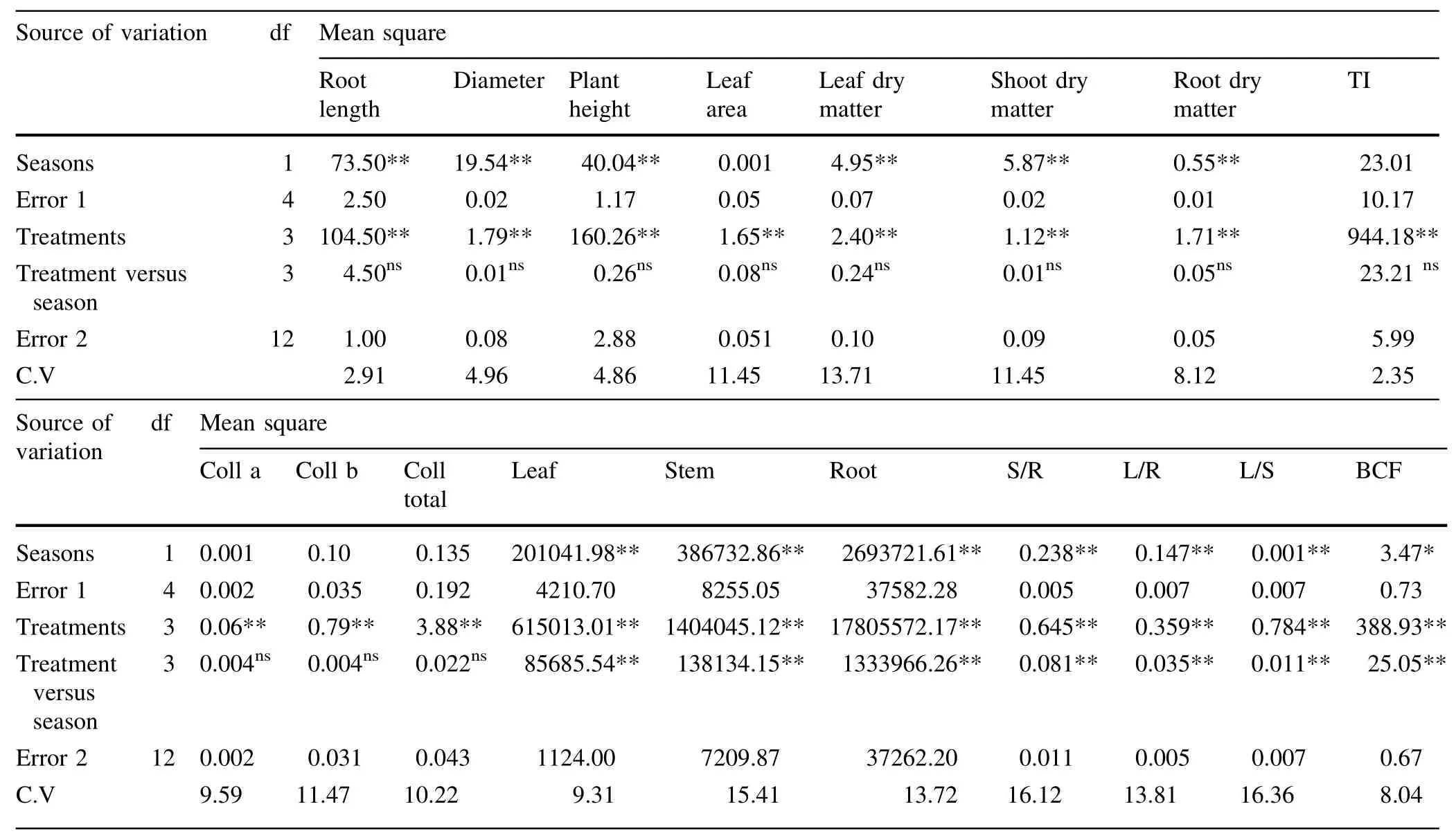
Table 2 Combined analysis of variance from the evaluation of Acacia victoriae traits over two seasons for 4 Zn concentrations
Growth and biomass responses of seedlings
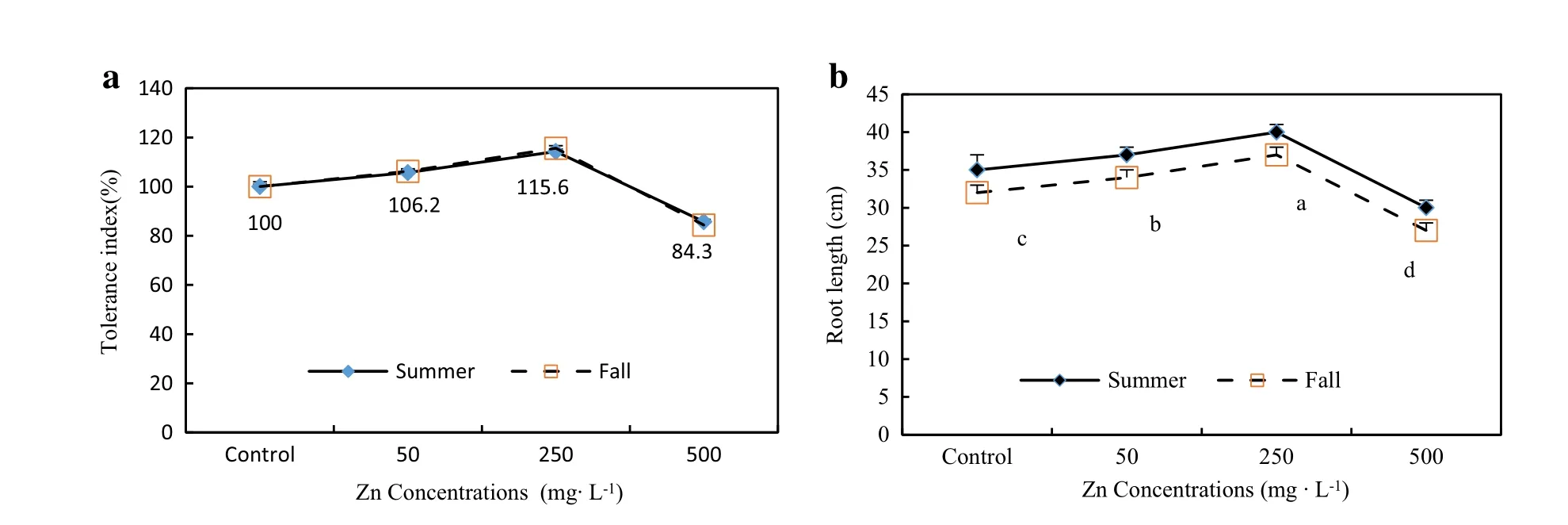
Fig.1 Effectof Zn concentrations on the Zinc-tolerance index(TI)(a)and rootlength(b)of A.victoriae seedlings in two seasons for45 days. (For any given Zn concentration during the same growing season,values followed by the same letter do not differ at p=0.05)
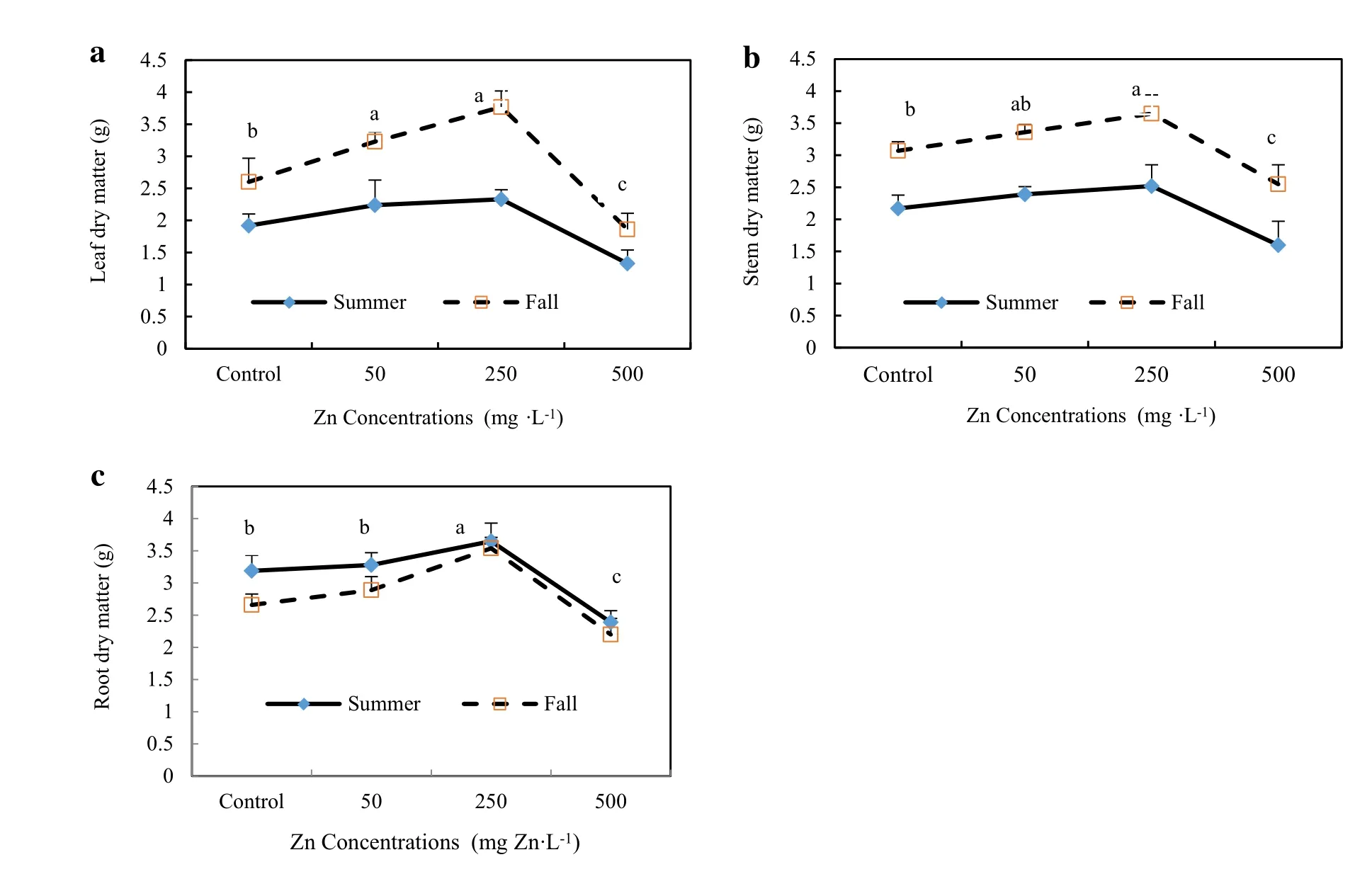
Fig.2 Effectof Zn concentrations on the Leafdry matter(a)Stem dry matter(b)and Rootdry matter(c)of A.Victoria seedlings in two seasons for 45 days.(For any given Zn concentration during the same growing season,values followed by the same letter do not differ at p=0.05)
There was a significantdifferentbetween the treatments in each season butthe trend of effectof Zn concentrations on rootlength,plantheightand dry matter of differenttissues forboth seasons were the same(Figs.1,2).The increase of Zn concentration from 50 to 250 mg L-1significantly increased the root length of seedlings(5 to 20%higher than control seedlings)and TI for both seasons.In all treatments,TI ranged from 120%the highest value(in seedlings exposed to 250 mg Zn L-1)to 85%the lowest value(in seedlings exposed to 500 mg Zn L-1).Therefore, when Zn concentration in the soilmedium was increased to 500 mg L-1,the TI declined significantly(Fig.1).Plant height and dry biomass of different parts of seedlings increased significantly with increasing Zn concentration from 50 to 250 mg L-1in the medium as compared to the controls without Zn(Table 3).The maximum dry biomass (10.86 g plant-1for the fall season and 8.5 g plant-1for the summer season)was recorded for seedlings exposed to 250 mg Zn L-1.In seedlings exposed to 50 and 250 mg Zn L-1,growth was stimulated,reaching total dry biomass between 13 and 30%higherforfalland 8 to 16% higher for summer than the controls(Table 3;Fig.2). However,plant height,root length,leafs,shoots and roots dry mattertreated with the highestconcentration of500 mg Zn L-1declined significantly(Figs.1,2;Table 2).However,the seedlings exposed to a Zn concentration of 500 mg Zn L-1did not show visible symptoms of Zn toxicity such as chlorotic spots or necrotic lesions at the leaf surface.But we recorded reductions in rootlength and plant height(up to 25%reduction as compared with controls),reduction of total chlorophyll(30 and 27%reduction,respectively,as compared with controls for summer and fall),and reduction in growth and dry biomass(to 27%reduction as compared with controls for the summer season)(Fig.2;Table 3).
Discussion
Chlorophyll
In higher plants the contents of chlorophyll a and chlorophyll b are usually in the ratio 3:1(chlorophylla dominates)(Porra 2002).Changes in the ratio of chlorophyll a:b can reflect the negative influence of heavy metals to the photosynthetic apparatus of plants(Porra 2002).A typical phytotoxicity critical concentration is about 500μg Zn g-1(Reichman 2002).The results of chlorophyll pigment analysis showed that in treatments exposed to 250 mg Zn L-1,the Chlorophyll(a,b and total)contents were significantly higher than for the other treatments.The lowestchlorophyll(a,b and total)contents were found in 500 mg Zn L-1treatments and reduced total chlorophyll to about 25%of the control(or increased the ratio of chlorophyll a:b)in both studied growing seasons.A significantincrease was seen in the chlorophyll a:b ratio for Zn alone,suggesting that the chlorophyll b pool is more sensitive to Zn exposure(Macfarlane and Burchett 2001). There was no significantdifference in chlorophyll(a,b and total)contents between two seasons in all treatments (Fig.3).
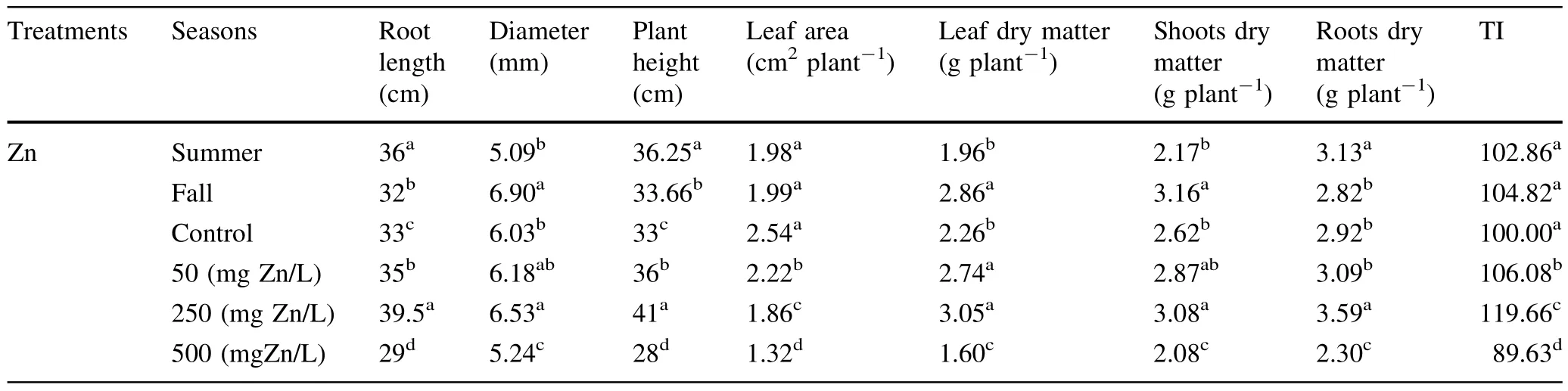
Table 3 Growth parameters of A.victoriae seedlings,grown in pots and exposed to four concentrations of Zn over two seasons for 45 days
Zn content in the tissues
Metaluptake was dependentupon the metalconcentrations in the soilmedium.Significantpositive correlations existed between Zn concentrations in the soil medium and Zn contents in root shoot and leaf tissues(Table 4).Zn accumulation in all treatments was significantly different between the two seasons(Table 2).A.victoriae accumulated large concentrations of Zn during the two growing seasons.Concentrations of Zn in leafs and shoots of A. victoriae seedlings in fall growing season were significantly larger than summer(in alltreatments,Fig.4a,b). While in alltreatments,Zn concentrations in roottissues of A.victoriae seedlings in summer growing season were greater than fallseason(Fig.4c).In alltreatments,Zn was mainly located in the roots and mostZn was transported to aboveground tissues in the shoots.The concentration gradients of Zn in different parts of seedlings ranked as follows:roots>shoots>leafs(Fig.4).The highest Zn accumulations in leafs,shoots and roots of A.victoriae seedlings(755,1141 and 3843 mg kg-1dry matter,respectively)were found in the seedlings exposed to 500 mg Zn L-1(Table 4).BCF values were between 9.63 and 19.60 for treatments at 500 and 50 mg Zn L-1,respectively.These high BCF values show the higheramount of metalaccumulated in the roots than shoots and leafs.In all treatments an average of 42–67%of the Zn accumulated in the entire plant tissues was retained in the roots. These results were associated with values (0.23≤TFs≥0.79)of the TFs from roots in all the treatments(Table 4).
Toxic metals usually cause reduction of plant growth (Chaoui et al.1997).This can be expressed as reduced growth rate,leaf area and root biomass,and can be followed by reduction of total biomass production(Begonia et al.1998).The first symptom of Zn toxicity in most species is a generalchlorosis of younger leafs(Fontes and Cox 1995).Plants exhibiting Zn toxicity have smaller leafs than control plants(Ren et al.1993).The present study revealed that A.victoriae did not reduce growth or biomass,but actually increased growth and biomass when seedlings were treated with 50 and 250 mg Zn L-1.These results can be expressed because of A.victoriae tolerance to relatively high amounts of Zn.Kabata-Pendias(2011) determined the mean content of Zn in plants leafs of a series of plant species and divided this content of Zn into three groups:deficient content(10–20 mg Zn/kg DW), sufficient content(27–150 mg Zn/kg DW)and toxic content(100–400 mg Zn/kg DW).Of course,Zn is an essential nutrient for plants and the threshold of Zn toxicity varies among plant species,time of exposure to Zn stress and composition of the nutrient growth medium(Soares et al.2001).Some studies reported low levels of heavy metals such as cadmium(Arduini et al.2004),lead(Begonia et al.1998;Kadukova et al.2008;Mahdavi et al. 2014)and arsenic and cadmium(Fayiga et al.2004)that had positive effects on plant growth and biomassproduction.Although we recorded no visible symptoms of Zn toxicity in seedlings treated with 500 mg Zn L-1we recorded reduced totaldry biomass by 27%in comparison with control seedlings.These results were compared with the results of Marchiol et al.(2004)who did not observed any obvious symptoms of metal toxicity with the mixture of Cd,Pb,Cr,Ni,Cu and Zn during treatmentbutrecorded reduced growth and biomass production in Brassica napus and Raphanus sativus.Doncheva et al.(2001)reported plant growth inhibition extended in Eucalyptus maculate and E.urophylla by five weeks after addition of 400μM ZnSO4.
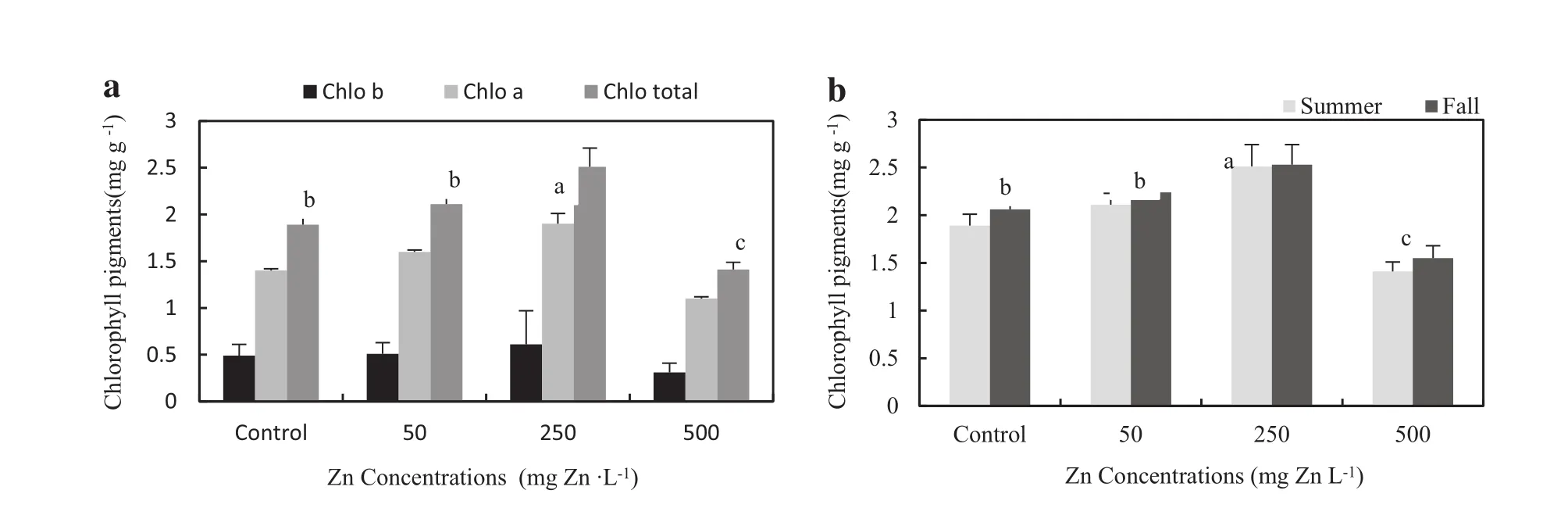
Fig.3 Chlorophyllpigments(a,b and total)(mg/g)in A.victoria leaves(a)and comparison of total Chlorophyllbetween two seasons(b).(For any given Zn concentration during the same growing season,values followed by the same letter do not differ at p=0.05)
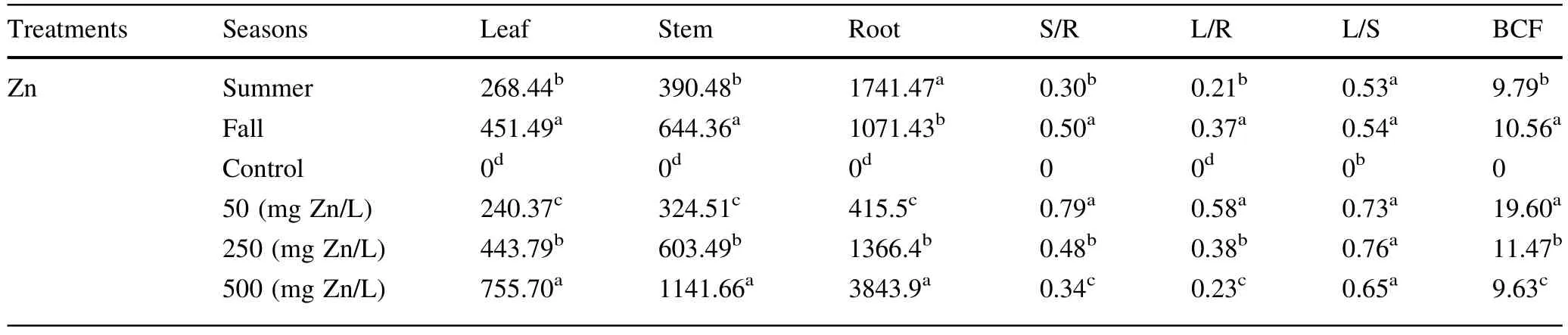
Table 4 Zn contents(mg Zn Kg-1 dry biomass)in roots,shoots and leaves,Zn translocation factors(TFs,leaf/root Zn ratios(L/R),leaf/ shootZn ratios(L/S)and shoot/rootZn ratios(S/R),Bio-concentration factor(BCF),of A.victoriae seedlings grown in pots and exposed to four concentrations of Zn over two seasons for 45 days
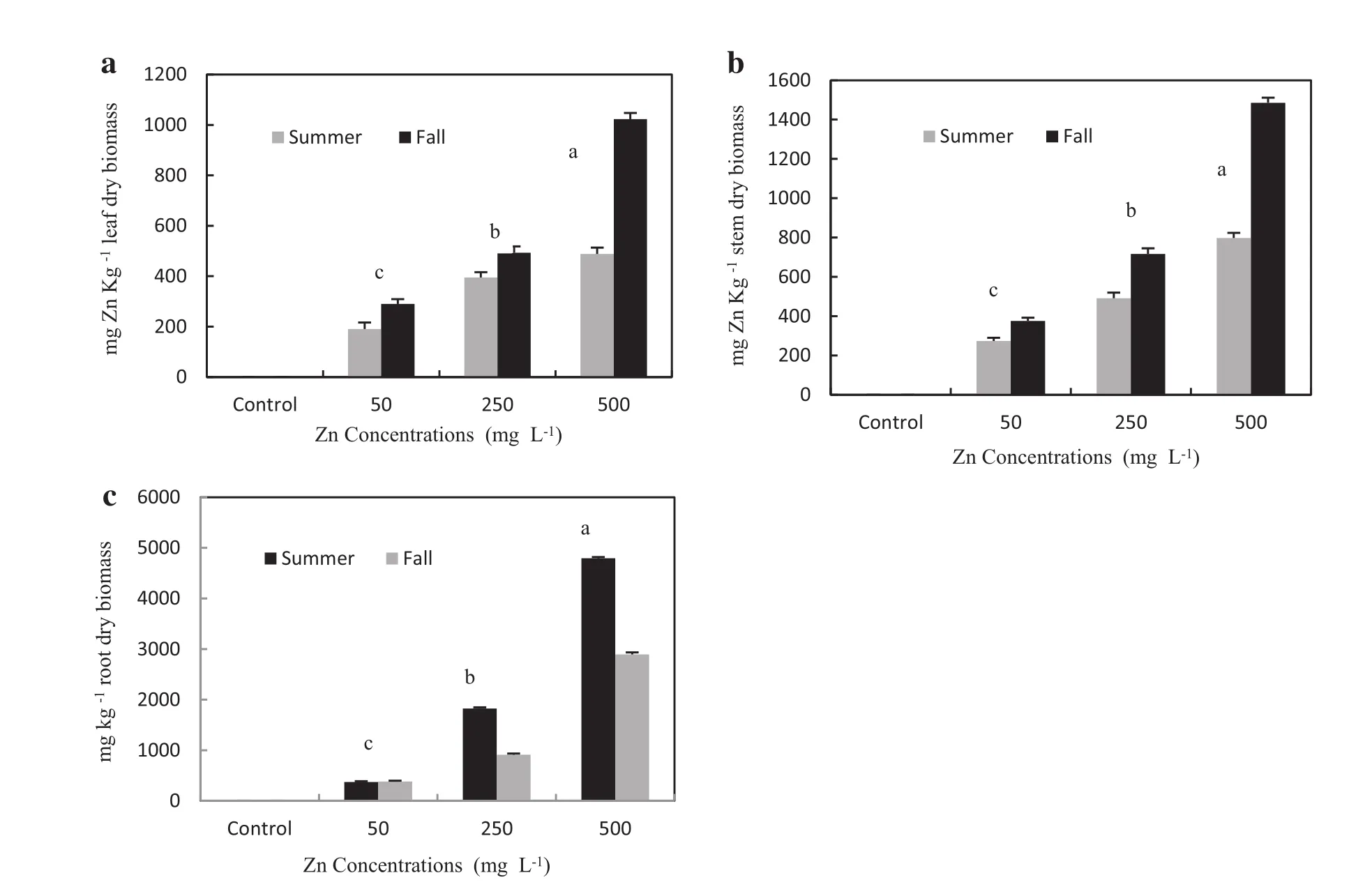
Fig.4 Zn concentrations in Leaves(a),Shoots(b)and Roots(c)of A.victoria seedlings subjectto differentconcentration of Zn treatments in two seasons
Zn toxicity in roots is apparentas a reduction in the growth ofthe main root,fewerand shorterlateralroots and a yellowing of roots(Ren et al.1993).Our root length measurements (Fig.1)showed that shorter root length was found in 500 (mg Zn L-1)treatments.This negative effect on root length wasalso accompanied by reduction in plantheight,decrease in chlorophyll a,b and totalas wellas totaldry biomass(Judith etal.1977;Sagardoy etal.2009)(Figs.1,2,3;Table 3).
The study showed that plant height was significantly greater after treatment with 250 mg Zn L-1compared to controls,whereas in of 500 mg Zn L-1treatment plant height was significantly less than in the control seedlings.The reduction in the plant heightmight have been mainly due to reduced root growth and regulation of less nutrient and water transport to the aerialparts of the plant(Shanker et al.2005).Others showed the same results(Judith et al. 1977;Sagardoy et al.2009).
Concentrations of Zn differed between two seasons.The seedling response to soil Zn varied both in the nature and the amount in two seasons(Fig.4a–c).Our study showed that because of the ability of A.victoriae to accumulate higher concentrations of Zn in roots in summer as compared to fall(Table 4),ithas the potentialto be used for Zn phytostabilisation(Rizzi et al.2004).This study showed that,at 500 mg Zn L-1treatments,Zn concentrations in the leafs and shoots of A.victoriae seedlings exceeded 1000 mg kg-1in fallgrowing season(Table 4 and Fig.4). In addition,the plantwas also able to grow normally atZn concentrations up to 500 mg L-1and the symptoms of toxicity(chlorosis and necrosis on leafs and roots)were not revealed at this treatment.Therefore,the results indicate that although A.victoriae was not a Zn hyperaccumulator, it can be used for phytoextraction purposes and should be harvested at the end of the growing season(fall season) because the concentration ofthe metalin the above-ground tissues was maximal at this time.
Conclusions
Heavy metal toxicity in soils is a significant global problem.Although at low concentrations Zn acts as micronutrient,itbecomes toxic athigh concentrations.In this study we described the pattern of metal(Zn)accumulation in A. victoriae over two growing seasons so that we can effectively manage Zn contaminated areas.The highest concentrations of Zn were found in roots.Therefore,A. victoriae could be considered as a root bioaccumulator species.However significant concentrations of Zn were found in both shoots and leaf,especially in fall.Finally, from the results ofstudy,itcan be concluded thatdue to the ability of A.victoriae to accumulate high concentrations of Zn in its tissues,produce relatively large biomass and maintain high tolerance to drought and saline soils,is a suitable and effective choice to be used as a tool for phytoremediation(phytostabilization or phytoextraction)for industrialsites in arid zones.
Arduini I,Masoni A,Mariotti M,Ercoli L(2004)Low cadmium application increase miscanthus growth and cadmium translocation.Environ Exp Bot 52:89–100
Begonia GB,Davis CD,Begonia MFT,Gray CN(1998)Growth responses of Indian Mustard(Brassica juncea L.Czern.)and its phytoextraction of lead from a contaminated soil.Bull Environ Contam Toxicol 61:38–43
Brooks RR(1998)Plants thathyperaccumulate heavy metals,1stedn. Cambridge University Press,Cambridge,pp 88–102
Cakmak I,Yilmaz A,Kalayci M,Ekiz H,Torun B,Erenoglu B, Braun HJ(1996)Zinc deficiency as a critical problem in wheat production in Central Anatolia.Plant Soil 180(2):165–172
Chaney RL,Malik M,Li YM,Brown SL,Brewer EP,Angle JS, Baker AJ(1997)Phytoremediation of soil metals.Curr Opin Biotechnol 8(3):279–284
Chaoui A,Mazhoudi S,Ghorbal MH,El Ferjani E(1997)Cadmium and zinc induction of lipid peroxidation and effects of antioxidant enzyme activities in beans(Phaseolus vulgaris L.). Plant Sci 127(2):139–147
Doncheva S,Stoynova Z,Velikova V(2001)Influence of succinate on zinc toxicity of pea plants.J Plant Nutr 24(6):789–804
Ernst W(1968)Das Violetum calaminariae westfalicum,eine Schwermetallpflanzengesellschaft bei Blankenrode in West falen.Floristisch Soziologische Arbeitsgemeinschaft 13:263–268
Fayiga AO,Ma LQ,Cao X,Rathinasabapathi B(2004)Effects of heavy metals on growth and arsenic accumulation in the arsenic hyperaccumulator Pteris vittata L.Environ Pollut 132(2):289–296
Fernandes JC,Henriques FS(1991)Biochemical,physiological and structural effects of excess copper on Vigna radiata plants.Bot Rev 57:246–273
Fontes RLF,Cox FR(1995)Effects of sulfur supply on soybean plants exposed to zinc toxicity.J Plant Nutr 18(9):1893–1906
Foy CD,Chaney RL,White MC(1978)The physiology of metal toxicity in plants.Ann Rev Plant Physiol 29:511–566
Grotz N,Guerinot ML(2006)Molecular aspects of Cu,Fe and Zn homeostasis in plants.Biochim Biophys Acta(BBA)Mol Cell Res 1763(7):595–608
Hansch R,Mendel RR(2009)Physiological functions of mineral micronutrients(Cu,Zn,Mn,Fe,Ni,Mo,B,Cl).Curr Opin Plant Biol 12(3):259–266
Kabata-Pendias A(2011)Trace elements in soils and plants(fourth edition).CRC Press,Taylor&Francis Group,pp 275–287
Kadukova J,Manousaki E,Kalogerakis N(2008)Pb and Cd Accumulation and Phyto-excretion by Salt Cedar(Tamarix smyrnensis Bunge).Int J Phytorem 10(1):31–46
Lasat MM(2000)Phytoextraction ofmetals from contaminated soil:a review of plant,soil,metal interaction and assessment of pertinent agronomic issues.J Hazard Subst Res 2(5):1–25
Leita L,De Nobili M,Mondini C,Baca Garcia MT(1993)Response of Leguminosae to cadmium exposure.J Plant Nutr 16(10): 2010–2012
Mahdavi A,Khermandar K,Ahmady-Asbchin S,Tabaraki R(2014) Lead accumulation potentialin Acacia victoriae.Int J Phytorem 16(6):582–592
Maldonado-Magana A,Favela-Torres E,Rivera-Cabrera F,Volke-Sepulveda TL(2011)Lead bioaccumulation in Acacia farnesiana and its effect on lipid peroxidation and glutathione production.Plant Soil 339(1–2):377–389
Marchiol L,Assolari S,Sacco P,Zerbi G(2004)Phytoextraction of heavy metals by canola(Brassica napus)and radish(Raphanus sativus)grown on multicontaminated soil.Environ Pollut 132(1):21–27
Moreira H,Marques APGC,Rangel AOSS,Castro PML(2011) Heavy metal accumulation in plant species indigenous to a contaminated Portuguese site:prospects for Phytoremediation. Water Air Soil Pollut 221(14):377–389
Piechalak A,Tomaszewska B,Baralkiewicz D,Malecka A(2002) Accumulation and detoxification of lead ions in legumes. Phytochemistry 60(2):153–162
Pilon M,Cohu CM,Ravet K,Abdel-Ghany SE,Gaymard F(2009) Essentialtransition metalhomeostasis in plants.Curr Opin Plant Biol 12(3):347–357
Porra RJ(2002)The chequered history of the developmentand use of simultaneous equations for the accurate determination of chlorophylls a and b.Photosynth Res 73(1–3):149–156
Qishlaqi A,Moore F,Forghani G(2009)Characterization of metal pollution in soils under two landuse patterns in the Angouran region,NW Iran,a study based on multivariate data analysis. J Hazard Mater 172(1):374–384
Raskin I,Nanda Kumar PBA,Dushenkov S,Salt DE(1994) Bioconcentration of heavy metals by plants.Curr Opin Biotechnol 5(3):285–290
Reeves RD,Brooks RR(1983)Hyperaccumulation of lead and zinc by two metallophytes from mining areas of Central Europe. Environ Pollut Ser A Ecol Biol 31(4):277–285
Reichman SM(2002)The responses of plants to metal toxicity:a review focusing on copper,manganese and zinc.Occasional Paper No.14,Melbourne:Australian minerals and energy environment foundation press,ISBN 1-876205-13-X,pp 5–26
Ren F,Liu T,Liu H,Hu B(1993)Influence of zinc on the growth, distribution of elements,and metabolism of one-year old American ginseng plants.J Plant Nutr 16:393–405
Rizzi L,Petruzzelli G,Poggio G,Vigna Guidi G(2004)Soilphysical changes and plant availability of Zn and Pb in a treatability test of phytostabilization.Chemosphere 57(9):1039–1046
Roosens N,Verbruggen N,Meerts P,Ximenez-Embun P,Smith JAC (2003)Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from Western Europe.Plant,Cell Environ 26(10):1657–1672
Sagardoy R,Morales F,Lopez-Millan AF,Abadia A,Abadia J(2009) Effects of zinc toxicity on sugar beet(Beta vulgaris L.)plants grown in hydroponics.Plant Biol 11(3):339–350
Salt DE,Prince RC,Pickering IJ,Raskin I(1995)Mechanisms of cadmium mobility and accumulation in Indian mustard.Plant Physiol 109(4):1427–1433
Shanker AK,Cervantes C,Loza-Tavera H,Avudainayagam S(2005) Chromium toxicity in plants.Environ Int 31(5):739–753
Soares CRFS,Grazziotti PH,Siqueira JO,De Carvalho JG,Moreira FMS(2001)Toxidez de zinco no crescimento e nutricao de Eucalyptus maculata e Eucalyptus urophylla em solucao nutritiva.Pesquisa Agropecuaria Brasileira 36(2):333–348
Strain HH,Svec WA(1966)Extraction separation,estimation and isolation of the chlorophylls.In:Varnon LP,Seely GR(eds)The chlorophylls.Academic Press,New York,pp 21–65
Waranusantigul P,Kruatrachue M,Pokethitiyook P,Auesukaree C (2008)Evaluation of Pb phytoremediation potential in Buddleja asiatica and B.paniculata.Water Air Soil Pollut193(1–4):79–90
Yadav SK(2010)Heavy metals toxicity in plants:an overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants.South Afr J Bot 76(2):167–179
27 February 2014/Accepted:27 August 2014/Published online:9 May 2015
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
The online version is available at http://www.springerlink.com
Corresponding editor:Zhu Hong
✉Ali Mahdavi a_amoli646@yahoo.com Khadijeh Khermandar khermandar625@yahoo.com
1Department of Forest Science,Faculty of Agriculture, University of Ilam,P.O.Box 69315-516,Ilam,Iran
2Department Range and Watershed Management,Faculty of Agriculture,University of Ilam,P.O.Box 69315-516,Ilam, Iran
 Journal of Forestry Research2015年3期
Journal of Forestry Research2015年3期
- Journal of Forestry Research的其它文章
- Management of pests and diseases of tropical sericultural plants by using plant-derived products:a review
- Gamma generalized linear model to investigate the effects of climate variables on the area burned by forest fire in northeast China
- Diversity,abundance,and structure of tree communities in the Uluguru forests in the Morogoro region,Tanzania
- Brazilian savanna re-establishment in a monoculture forest: diversity and environmental relations of native regenerating understory in Pinus caribaea Morelet.stands
- Carbon storage and sequestration rate assessment and allometric model development in young teak plantations of tropical moist deciduous forest,India
- Use of infrared thermal imaging to diagnose health of Ammopiptanthus mongolicus in northwestern China
