Establishment of high frequency shoot regeneration system in Himalayan poplar(Populus ciliata Wall.ex Royle)from petiole explants using Thidiazuron cytokinin as plant growth regulator
G.Aggarwal•A.Gaur•D.K.Srivastava
Establishment of high frequency shoot regeneration system in Himalayan poplar(Populus ciliata Wall.ex Royle)from petiole explants using Thidiazuron cytokinin as plant growth regulator
G.Aggarwal1,2•A.Gaur1•D.K.Srivastava1
Populus species are important resources for industry and in scientific study on biological and agriculturalsystems.Our objective was to enhance the frequency of plantregeneration in Himalayan poplar(Populus ciliata wall.ex Royle).The effect of TDZ alone and in combination with adenine and NAA was studied on the regeneration potential of petiole explants.The explants were excised from Himalayan poplar plants grown in glasshouses.After surface sterilization the explants were cultured on shoot induction medium.High percentage shoot regeneration(86%)was recorded on MS medium supplemented with 0.004 mg L-1TDZ and 79.7 mg L-1adenine.The regenerated shoots for elongation and multiplication were transferred to MS+0.5 mg L-1BAP+0.2 mg L-1IAA+0.3 mg L-1GA3.Root regeneration from shoots developed in vitro was observed on MS medium supplemented with 0.10 mg L-1IBA.Himalayan poplar plantlets could be produced within 2 months after acclimatization in a sterile mixture of sand and soil.We developed a high efficiency plantregeneration protocol from petiole explants of P.ciliata.
In vitro regeneration·Petiole explants· Growth regulator·Thidiazuron·Populus ciliata
Introduction
Plant biotechnology holds promise for circumventing obstacles to the genetic improvement of woody perennials, namely,unwieldy size,long life cycles and lack of basic genetic and inheritance information.An efficient in vitro regeneration system with cell and tissue culture is a prerequisite for applying biotechnology to plantimprovement (Confalonieriet al.2003).The genus Populus L.(poplars, cottonwoods and aspens)comprises about30 species(Park et al.2004)that are widely distributed in the temperate climates of the northern hemisphere.Populus ciliata Wall. ex Royle(Himalayan poplar)is one of the few forest species considered ideal for intercultivation with agricultural crops.It is a large deciduous tree with sexually differentiated male and female plants that occur in temperate and sub-temperate regions of the Himalayas at elevations of 1200-3500 m.Due to its exceptional qualities,such as high capacity for vegetative propagation and fast growth rate,it has been extensively used in the pulp and paper industries,for reforestation of lowlands,and in phytoremediation of contaminated soil(Balatinecz et al.2001; Rishi et al.2001).A growing interest has been shown in further improvement of the economically important traits in Populus,especially with modern methods of genetic engineering.However,P.ciliata is severely affected by many biotic and abiotic stresses leading to considerable loss ofyield.Also,high lignin contentin the species makes the operational costs of delignification in paper manufacturing quite expensive,highlighting the need for in vitro genetic manipulation(Rishi et al.2001).
However,before taking this approach,itis importantto know whether the somatic cells of this species are able to regenerate in such a way so as to give rise to whole plantlet and the conditions required for such plant regeneration. Efforts devoted to the use of explants of mature trees of proven worth for propagation through tissue culture have been reported(Cheema 1989;Jafarietal.1995;Shen etal. 1998;Daietal.2003;Thakur and Srivatava 2006;Thakur et al.2008,2012),but they are limited to very few genotypes and very few reports focused on shoot regeneration from leaf and petiole explants of Himalayan poplar(Thakur and Srivatava 2006;Thakur et al.2008).Therefore,in the present study we established a highly efficient shoot regeneration system from petiole explants of P.ciliata. This is the key step for genetic transformation of this tree species.We also investigated the effect of TDZ concentrations alone and in combination with adenine and NAA. We are currently using this regeneration system for the transformation of Himalayan poplar by genetically engineered Agrobacterium tumefaciens strain.
Materials and methods
Plant material and nutrient medium
Stem cuttings of Populus ciliate Wallex.Royle for use as petiole explants were procured from the fields of Tree Improvement and Genetic Resources,Shilly Solan and then planted in the glass house of department of Biotechnology, Dr.Y.S.Parmar University of Horticulture and Forestry, Nauni,Solan.Petiole explantswere thoroughly washed with teepolforhalfan hourunderrunning tap water,then explants were surface sterilized with 0.2%Bavistin for2–3 min and 0.1%HgCl2for 1–2 min.Explants were then thoroughly washed with sterilized distilled waterto remove the tracesof HgCl2.MS medium(Murashige and Skoog 1962)salts (Macro and Micro),vitamins supplemented with 100 mg L-1mesoinositol,3%sucrose and 0.8%agar–agar (used as gelling agent)was used as basalmedium.Different concentrations of TDZ alone and in combination with adenine and NAA were used in the MS medium for plant regeneration studies.The pHofthe medium wasadjusted to 5.8 before adding agar–agar.The medium was autoclaved at 121°C and 15 pounds per square inch pressure for 20 min. Allthe aseptic manipulationswere carried outundervertical laminarairflow chamber.
Plant regeneration from petiole explants of Himalayan poplar
To optimize the culture medium for high frequency shootregeneration leaf and petiole explants were excised from the stem cuttings,which were grown and maintained in the glass house.Theseexplantswerecutinto approximately 0.5–1.0 cm size and cultured on MS medium supplemented with different concentrationsofTDZaloneand in combinationswith adenine and NAA(Tables 1,2 and 3,Fig.1).Forevery combination, six flasks with five explants were inoculated.Allthe cultures were keptin the culture room under16 h.photoperiod with the light intensity of 40μmol m-2s-1provided by cool white fluorescentlamps,at70–80%humidity and(26±2)°C.The regenerated shootswere separated and individualshootswere transferred to the MS medium containing various concentrationsofauxin and IBAforrootinduction to produce complete plantlets(Table 4).
Hardening of regenerated plantlets
After proper in vitro development the plantlets were removed from the tubes and flask without damage to their rootsystems.The roots were washed gently under running tap water to remove adhering medium.Plantlets were held under running tap water for a few minutes so thatthey did not wilt after transfer to pots.Pots contained a mixture of sterilized sand and soil(1:1).The plantlets were watered and covered with jam jars to maintain high relative humidity.After 9–10 days,when the plantlets showed new leaves,they were gradually uncovered until after 3–4 weeks the plantlets were without cover.
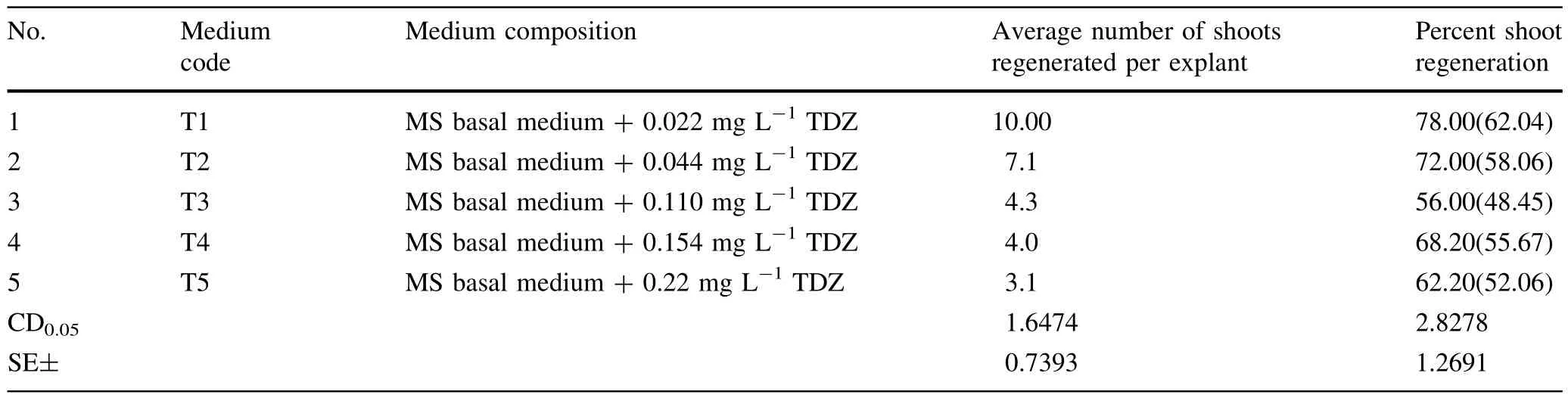
Table 1 Effect of various concentrations of TDZ(in MS basalmedium)on shoot regeneration from petiole explants
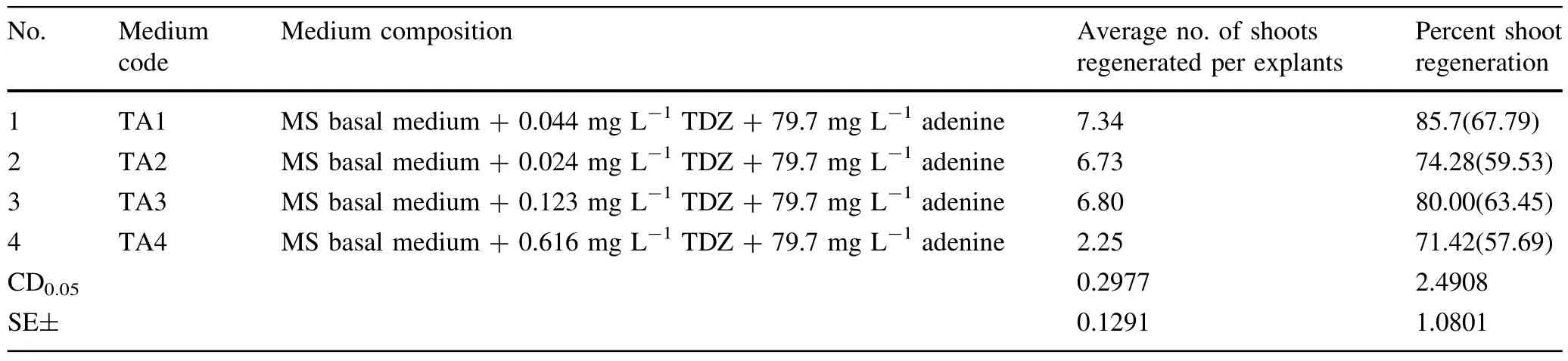
Table 2 Effect of various concentrations and combinations of TDZ and adenine(in MS basal medium)on shoot regeneration from petiole explants
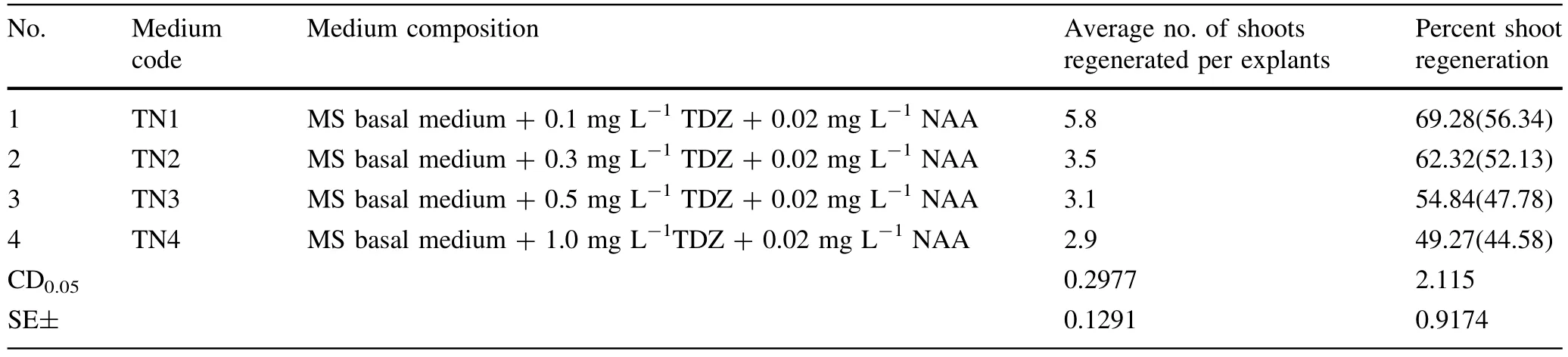
Table 3 Effectof various concentrations and combinations of TDZ and NAA(in MS basalmedium)on shootregeneration from petiole explants

Fig.1 a–e Plant regeneration studies in petiole explants of Himalayan poplar(Populus ciliata).a Petiole explants cultured on shoot regeneration medium at 0 day;b Petiole explants showing shoot proliferation after 28 days of culturing;c Rootregeneration in in vitro developed shoots after 28 days of shoot inoculation; d In vitro regenerated plantlets of Himalayan poplar showing welldeveloped rootsystem; e Young,healthy plantlets successfully acclimatized on the planting substrate after 4 weeks of hardening
Statisticalanalysis
Each treatment consisted of at least 30 explants and each experiment was repeated thrice.The data recorded for different parameters was subjected to Completely Randomized Design(Gomez and Gomez 1984).The statistical analysisbased on mean valuespertreatmentwasmade using analysis of variance for Completely Randomized Design.
Results
Shoot regeneration from petiole explants
The petiole explantsofHimalayan poplarwere inoculated on MS medium supplemented with differentconcentrations of TDZ alone and in combinations with adenine and NAA for the shootregeneration experiment.Petiole explantsturned to light green from green in the first week of culturing.No change in the color of the medium was observed.Petiole explants were swollen and expanded after 10 days of culturing.Directshootregeneration was observed after18 days of culturing from the tissue around the cutedges of explant on differentmedia.Allthe media were able to induce direct organogenesisfrom petiole explants(Tables 1,2 and 3)For multiplication and elongation ofshootsthey were transferred to a different medium(MS+0.5 mg L-1BAP+0.2 mg L-1IAA+0.3 mg L-1GA3).
Of the 13 different concentrations and combinations of TDZ(Tables 1,2 and 3)tried,MS medium supplemented with 0.004 mg L-1TDZ+79.7 mg L-1adenine yielded maximum percent shoot regeneration(85.70%)and maximum average number of shoots(7.34)per explant (Table 2,Fig.1).
Root regeneration in adventitious shoots and hardening of plantlets
Shoots for multiplication and elongation were transferred to a different medium(MS+0.5 mg L-1BAP+0.2 mg L-1IAA+0.3 mg L-1GA3).Elongated shoots(about 2–3 cm in height)obtained from petiole derived cultures were excised and cultured on MS medium supplemented with different concentrations of IBA for root induction (Table 4).Complete root formation was observed 24 days after transfer to the root regeneration medium(Fig.1). Maximum percent(76%)root regeneration was observed with MS medium supplemented with 0.10 mg L-1IBA. After complete development of roots,the plantlets were taken out from the culture tubes and transferred to plastic pots containing a mixture of sterilized sand and soil(1:1). The plantlets were watered and covered with glass jars to maintain high humidity.Himalayan poplar plantlets were able to regenerate within 2–3 months(Fig.1).
Discussion
Biotechnological approaches to in vitro propagation have helped plant breeders to overcome the limitation of conventional techniques for tree improvement(Confalonieri et al.2003).With increasing demand for pulp and paper, reforestation of lowlands and phytoremediation of contaiminated soil,fast growing poplar species are commercially important(Schnoor2000;Balatinecz etal.2001;Rishi etal.2001;Peterneletal.2009).The limitations associated with popularforcommercialapplicationsincludehigh lignin content,which effects the cost of production of paper and vulnerability to many pests.Forgenetic improvementof the species the major prerequisite is to develop an efficientand reliable plantregeneration systemwithoutcompromising the genetic stability ofthe species/genotypes.
Plantregeneration studies of Populus species have been carried outto achieve an efficientand reliable regeneration system(Charenon and Taris 1960;Mathes 1964;Winton 1968;Venverloo 1973;Walter et al.1988;Costache etal. 1995;Gangoo and Khurana 2002;Thakur and Srivatava 2006;Thakur etal.2008).We found thatconcentrations of plant growth regulators and age of the explants proved to be important factor affecting the frequency of shoot regeneration.The young and tender petiole explants were found to be more effective for efficientshootregeneration in P.ciliata,confirming the previously reported results for P.ciliata(Thakurand Srivatava 2006;Thakuretal.2008).
We used TDZ in the MS medium forshootregeneration studies.TDZ is a synthetic phenylurea cytokinin-like compound thatis a highly effective regulator of shoot morphogenesis(Huttenman and Preece 1993;Murthy etal.1998).It is also effective in shootregeneration in many recalcitrant species(Pelah etal.2002;Schween and Schwenkel 2002; Liu etal.2003;Mithila etal.2003).The effect of various concentrations of TDZ alone and in combination with adenine and NAA were studied for enhancing the shootregeneration frequency from the leaf and petiole explants in P. ciliata.Highestshootregeneration frequency was obtained on MS medium supplemented with TDZin combination with adenine.Many published protocols for poplar regeneration, whether they include the transformation step or not,are based on TDZ(De Block 1990;Leple etal.1992;Noeletal. 2002;Ma etal.2004;Cseke etal.2007;Tsvetkov etal.2007;Yevtushenko and Misra2010).Allresearchersreportthatthe TDZ-based media enhanced the frequency of shoot regeneration in Populus species.We also found thatthe concentration of TDZ affects shootregeneration.The concentration ofTDZup to a certain threshold favoured shootregeneration. Further increase in concentration of TDZ led to declining shootregeneration frequency,which supports the results of Ledbetter and Preece(2004).Some reports indicate that higher concentration(5–10μM)and continous exposure to TDZmay cause lossofregeneration in in vitro culture(Malik and Saxena 1992).Hence,a precaution is necessary while using TDZ in in vitro cultures.In Himalayan poplar TDZ in combination with adenine(in MS medium)and an exposure for2 weeks afterregeneration was found to be optimum for maximum in vitro shootregeneration from petiole explants.
TDZ was found to be superior to other cytokinins in promoting shoot regeneration in Populus species.Thakur and Srivatava(2006),Thakur et al.(2008)used various cytokinins and auxins for shoot regeneration in P.ciliata. Their maximum regeneration percentage was 80%for petiole explants of Himalayan poplar.In TDZ-supplemented media 86%shoot regeneration was obtained from petiole explants of Himalayan poplar.In the presentstudy, direct shoot regeneration was observed from petiole explants of P.ciliata.Similarly,Thakuretal.(2008)obtained direct shoot regeneration from the petiole explants of P. ciliata.Direct shoot regeneration was also reported by Gosukonda et al.(1998)for P.deltoides.In contrast,various workers reported plant regeneration from callus cultures derived from leaf discs of Populus species and hybrids(Mehra and Cheema 1980;Son and Hall 1990; Park and Son 1988;Dong et al.1996;Shen et al.1998; Phan et al.2004;Kang etal.2006).
We developed a protocol for high frequency plant regeneration from leaf explants in Himalayan poplar.This protocol allows the regeneration and acclimatization of male plants of Himalayan poplar within 2–3 months.This protocol can be used to carry out transformation studies to transfer various biotic and abiotic resistant genes.In this regard,the present investigation provides a platform for in vitro genetic manipulation and mass multiplication of improved genotypes of Himalayan poplar.
Balatinecz JJ,Kretschmann DE,Leclercq A(2001)Acheivements in the utilization of poplar wood-guideposts for the future.For Chron 77:265–269
Charenon J,Taris B(1960)Remarques sur la structure des tissus neoformes et l’apparition d’organes specializes chez quatre cultivars de Populus et chez Salix alba cultives in vitro.CR Acad Sci 251:2070–2071
Cheema GS(1989)Somatic embryogenesis and plant regeneration from cell suspension and tissue cultures of mature Himalayan poplar(Populus ciliata).Plant Cell Rep 8:124–127
Confalonieri M,Balestrazzi J,BisoffiS,Carbonera D(2003)In vitro culture and genetic engineering of Populus spp.synergy for forest tree improvement.Plant Cell Tissue Organ Cult 72:109–138
Costache IM,Lowe KC,Davey MR,Power JB(1995)Improved micropropagation of Populus spp.by Pluronic F-68.Plant Growth Regul 17:233–239
Cseke LJ,Cseke SB,Podila GK(2007)High efficiency poplar transformation.Plant Cell Rep 26:1529–1538
Dai WH,Cheng ZM,Sargent W(2003)Plant regeneration and Agrobacterium-mediated transformation of two elite aspen hybrid clones from in vitro leaf tissue.In vitro Cell Dev Biology-Plant 39:6–11
De Block M(1990)Factors influencing the tissue culture and Agrobacterium tumefaciens-mediated transformation of hybrid aspen and poplar clones.Plant Physiol 93:1110–1116
Dong SX,Ning ZX,Yuan SJ,Liu XQ,Zhang S(1996)Effects of growth regulators on leaf callus induction and plantlet regeneration in Populus tomentosa.Ningxia J Agric For Sci Technol 6:18–20
Gangoo SK,Khurana DK(2002)In vitro multiplication of poplars: effects of growth regulators and genotypes.Appl Biol Res 4:11–16
Gomez KA,Gomez AA(1984)Statistical procedures for agricultural research.John Wiley and Sons,New York
Gosukonda RM,Beyl CA,Zipf A,Sharma GC(1998)In vitro callus initiation and multiple shoot production of Populus deltoides. Plant Growth Regul Soc Am 26:79–93
Huttenman CA,Preece JE(1993)Thidiazuron:a potentcytokinin for woody plant tissue culture.Plant Cell,Tissue Organ Cult 33:105–119
Jafari MA,Kiss J,Gergacz J,Heszky LE(1995)High efficiency callus induction and plant regeneration in petiole culture of four poplar genotypes.Acta Biol Hung 46:51–59
Kang W,Zheng J,Lıu KY,Peng JX,Hong HZ(2006)Study on plant regeneration of excised leaf from Populus deltoids(I-63×I-69).J Wuhan Bot Res 24:83–86
Ledbetter DI,Preece JE(2004)Thidiazuron stimulates adventitious shoot production from Hydrangea quercifolia Bartr.Leaf explants.Sci Hortic 101:121–126
Leple JC,Brasileiro ACM,MichelMF,Delmotte F,Jouanin L(1992) Transgenic poplars:expression of chimaeric genes using four different constructs.Plant Cell Rep 11:137–141
Liu CZ,Murch SJ,Demerdash MEL,Saxena PK(2003)Regeneration of the Egyptian medicinalplant Artemisia judaica L.Plant Cell Rep 21:525–530
Ma C,Strauss SH,Meilan R(2004)Agrobacterium-mediated transformation of the genome sequenced poplar clone,Nisqually-1(Populus trichocarpa).Plant Mol Biol Rep 22:1–9
Malik KA,Saxena PK(1992)Thidiazuron induces high frequency shoots regeneration in intact seedlings of pea(Pisum sativum), Chickpea(Cicer arietinum)and lentil(Lens culinaris).Aust J Plant Physiol 19:731–740
Mathes MC(1964)Culture of isolated triploid aspen tissue.For Sci 10:35–38
Mehra PN,Cheema GS(1980)Clonal multiplication in vitro of Himalayan poplar(Populus ciliata).Phytomorphology 30:336–343
Mithila J,Hall JC,Victor JMR,Saxena PK(2003)Thidiazuron induces shoot organogenesis at low concentration and somatic embryogenesis athigh concentration on leaf and petiole explants of African violet(Saintpaulia ionantha WEndl.).Plant Cell Rep 21:408–414
Murashige T,Skoog F(1962)Revised medium for rapid growth and bioassays with tobacco tissue cultures.Physiol Planta 15:211–218
Murthy BNS,Murch SJ,Saxena PK(1998)Thidiazuron:a potent regulator ofin vitro plantmorphogenesis.In Vitro Cell Dev Biol 34:267–275
Noel N,Leple JC,Pilate G(2002)Optimization of in vitro micropropagation and regeneration for Populus X interamericana and Populus X euramericana hybrids(P.deltoides, P.trichocarpa,and P.nigra).Plant Cell Rep 20:1150–1155
Park YG,Son SH(1988)In vitro organogenesis and somatic embryogenesis from punctured leaf of Populus nigra×P. maximowiczii.Plant Cell,Tissue Organ Cult 15:95–105
Park S,Oh S,Han KH(2004)Large scale computational analysis of poplar ESTs reveals the repertoire and unique features of expressed genes in the poplar genome.Mol Breed 14:429–440
Pelah D,Kaushik RA,Mizrahi Y,Sitrit Y(2002)Organogenesis in the vine cactus Selenicereus megalanthus using thidiazuron. Plant cell Tissue and Organ Cult 71:81–84
Peternel S,Gabrovsek K,Gogala N,Regvar M(2009)In vitro propagation of European aspen(Populus tremula L.)from axillary buds via organogenesis.Sci Hortic 121:109–112
Phan CT,Jorgensen J,Jouve L,Hausman JF,Polle A,Teichmann T (2004)Micropropagation of Populus euphratica Olivier.Belg J Bot 137:175–180
Rishi AS,Nelson ND,Goyal A(2001)Genetic modification for improvement of Populus.Physiol Mol Biol Plants 7:7–21
Schnoor JL(2000)Phytostabalization of metal using hybrid poplar tree.In:Raskin I,Ensley BD(eds)Pytoremediation of toxic metals:using plantto clean up the environment.John Wiley and Sons Inc,New York,pp 133–150
Schween G,Schwenkel HG(2002)In vitro regeneration in Primula Sp.Via organogenesis.Plant Cell Rep 20:1006–1010
Shen HL,Wantanabe S,Ide Y(1998)Establishment of callus culture system of Populus euphratica,Populus alba cv.Pyramidalis and Populus maximowicziiX Populus plantierensis.BullTokyo Univ For 99:19–26
Son SH,HallRB(1990)Plantregeneration capacity ofcallus derived from leaf,stem and root segments of Populus alba X Populus grandidentata michx.Plant Cell Rep 9:344–347
Thakur AK,Srivatava DK(2006)High efficiency plant regeneration from leaf explants of male Himalayan poplar(Populus ciliata wall.).In vitro Cell Dev Biol Plant 42:144–147
Thakur AK,Sharma S,Srivastava DK(2008)Direct organogenesis and plant regeneration from petiole explant of male Himalayan poplar(Populus ciliata wall.).Phytomorphology 58:49–55
Thakur AK,Saraswat A,Srivastava DK(2012)In vitro plant regeneration through directorganogenesis&in Populus deltoids clone G48 from petiole explants.J Plant Biochem Biotechnol 21:23–29
Tsvetkov I,Hausman JF,Jouve L(2007)Thidiazuron induced regeneration in rootsegmentof white poplar(P.alba L.).Bulg J Agric Sci 13:623–626
Venverloo CJ(1973)Formation of adventitious organs in cytokinin induced formation of leaves and shoots in callus cultures of Populus nigra L.Acta Bot Neerl 22:390–398
Walter MW,Grima-pettenati J,Grand C,Boudet AM,Lamb C(1988) Cinnamyl alcohol dehydrogenase,a molecular marker specific for lignin synthesis:cDNA cloning and mRNA induction by fungal elicitor.Proceedings of the National Academy of Sciences,vol 85,pp 5546–5550
Winton LL(1968)Plantlets from aspen tissue culture.Science 160:1234–1235
Yevtushenko PD,Misra S(2010)Efficient Agrobacterium-mediated transformation of commercial hybrid poplar Populus nigra X Populus maximowiczii.Plant Cell Rep 29:211–221
31 January 2013/Accepted:13 June 2013/Published online:19 May 2015
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
The online version is available at http://www.springerlink.com
Corresponding editor:Zhu Hong
✉G.Aggarwal agaurav7@gmail.com
1Department of Biotechnology,Dr.Yashwant Singh Parmar University of Horticulture and Forestry,Nauni, Solan 173230,HP,India
2Department of Food Technology,Desh Bhagat University, Mandi Gobindgarh 147301,Pb,India
 Journal of Forestry Research2015年3期
Journal of Forestry Research2015年3期
- Journal of Forestry Research的其它文章
- Management of pests and diseases of tropical sericultural plants by using plant-derived products:a review
- Gamma generalized linear model to investigate the effects of climate variables on the area burned by forest fire in northeast China
- Diversity,abundance,and structure of tree communities in the Uluguru forests in the Morogoro region,Tanzania
- Brazilian savanna re-establishment in a monoculture forest: diversity and environmental relations of native regenerating understory in Pinus caribaea Morelet.stands
- Carbon storage and sequestration rate assessment and allometric model development in young teak plantations of tropical moist deciduous forest,India
- Use of infrared thermal imaging to diagnose health of Ammopiptanthus mongolicus in northwestern China
