Oligo-carrageenan kappa increases C,N and S assimilation,auxin and gibberellin contents,and growth in Pinus radiata trees
Silvia Saucedo•Rodrigo A.Contreras•Alejandra Moenne
Oligo-carrageenan kappa increases C,N and S assimilation,auxin and gibberellin contents,and growth in Pinus radiata trees
Silvia Saucedo1,2•Rodrigo A.Contreras1•Alejandra Moenne1
Oligo-carrageenans(OCs)obtained from pure carrageenans extracted from marine red algae stimulate growth by enhancing photosynthesis and basalmetabolism in tobacco plants and Eucalyptus trees.In addition,OCs stimulate secondary metabolism,increasing the level of metabolites involved in defense against pathogens.In this work,we analyzed the effect of OC kappa on the increase in height,in activities of basal metabolism enzymes involved in carbon,nitrogen and sulphur assimilation,ribulose 1,5 biphosphate carboxylase/oxygenase(rubisco), glutamate dehydrogenase(GDH)and O-acetylserine thiollyase(OASTL),and in the level of growth-promoting hormones,the auxin indole acetic acid(IAA)and the gibberellin GA3,in pine(Pinus radiata)trees treated with OC kappa at concentrations of 1 and 5 mg mL-1and cultivated for9 months withoutadditionaltreatment.Pines treated with OC kappa at 1 mg mL-1showed a similar increase in height but displayed a higher increased in total chlorophyll,activities of rubisco,GDH and OASTL and level of IAA and GA3than those treated with OC kappa at 5 mg mL-1.Thus,OC kappa stimulates growth and basal metabolism and increases the level of growth-promoting hormones in pine trees,mainly at 1 mg mL-1.
Auxin·Basal metabolism·C,N and S assimilation·Gibberellin·Oligo-carrageenan kappa· Pinus radiata
Introduction
It is now clearly established that plant and marine algae oligosaccharides can stimulate or inhibitplant growth and development(Albersheim et al.1992;Gonza´lez et al. 2013a).In particular,oligo-carrageenans(OCs,for models see Vera et al.2011)prepared from marine algae pure carrageenans stimulate growth in tobacco plant,mainly OC kappa and iota(Castro et al.2012).In addition,OCs stimulate growth in Eucalyptus globulus trees,mainly OC kappa(Gonza´lez et al.2013b).It was determined that the stimulation of growth is due to an increase in net photosynthesis,carbon(C)and nitrogen(N)assimilation,and activities ofenzymes involved in basalmetabolism(Castro etal.2012;Gonza´lez etal.2013b,2014a).Moreover,OCs increase contents of polyphenolic compounds in tobacco plants leading to protection against viral,bacterial and fungal infections(Vera et al.2012).Furthermore,OCs increase the contentof essentialoils(volatile terpenes)and polyphenolic compounds with antipathogenic activities in E.globulus trees suggesting that defense against insects and pathogens may be increased(Gonza´lez et al.2013b, 2014b).
Plantgrowth requires the coordinated assimilation of C which is absorbed from air as well as N and S which are absorbed from soil(Kopriva et al.2002).For C assimilation,CO2is reduced to C and incorporated into three-carbon sugars by the enzyme ribulose 1,5 biphosphate carboxylase/oxygenase(rubisco)(Kopriva etal.2002).For nitrogen assimilation,nitrate is reduced to nitrite and then to ammonium and assimilated into the aminoacids glutamine and glutamate by the enzymes glutamine synthetase (GlnS)and glutamate dehydrogenase(GDH),respectively (Kraiser et al.2011).For S assimilation,sulphate is reduced to sulphite and then to sulphide and incorporatedinto the aminoacid cysteine by the enzyme O-acetylserine thiol-lyase(OASTL).Thus,assimilation of C,N and S are reducing processes that occur in a coordinated manner to synthesize biological molecules and macromolecules.
Plant growth and development also require the coordinated effects of growth-promoting hormones,mainly the auxin indole acetic acid(IAA)and the gibberellins GA1and/or GA3.It is now known that auxin and gibberellins display a reciprocal positive interaction and that their effects overlap regarding cell division and expansion,and tissue differentiation(Weiss and Ori 2007).In particular, Arabidopsis thaliana and Pisum sativum plants treated with IAA showed enhanced levels of gibberellins due to an increase in expression of several genes encoding enzymes involved in gibberellin synthesis,mainly gibberellins 20-and 3-oxidases(O’Neill and Ross 2002;Frigeiro et al. 2006).In addition,gibberellins GA3induce an increase in IAA leveland in polartransportof IAA,and IAA and GA3increase expression ofcommon genes mostofthem related to plant growth in Populus trees(Bjo¨rklund et al.2007).
In this work,we analyzed the effects of OC kappa on growth in height,C,N and S assimilation,and auxin and gibberellins levels in Pinus radiata trees.
Materials and methods
Preparation of OC kappa
Twenty grams of pure(free of proteins and secondary metabolites)commercial kappa2 carrageenan(Gelymar S.A.,Santiago,Chile)were solubilized in 2 L of water at 60°C.Concentrated HCl(36.2 N)was added to reach a finalconcentration of0.1 N,the solution was incubated for 45 min at60°C and then NaOH 1 M was added to obtain pH 7.A sample of10μL ofthe depolymerized carrageenan corresponding to OC kappa was analyzed by electrophoresis in an agarose gel(1.5%w/v)using 100 V for 1 h and dextran sulphate of 8 and 10 kDa as standards (Sigma,St.Louis,MO,USA).The gel was stained with 15%w/v Alcian blue dye in 30%v/v acetic acid/waterfor 1 h atroom temperature and washed with 50%v/v acetic acid/waterfor1 h.OC kappa was visualized as a relatively discrete band of around 10 kDa.
Pine culture,treatments,measure of height and sampling
Pine trees(initial height=20 cm)were cultivated outdoors in plastic bags with compost(soilsupplemented with leaves of native Chilean trees).Trees were sprayed on needles with 5 mL of water(control group,n=10)and with an aqueous solution of OC kappa ata concentration of 1 mg mL-1(treatment group 1,n=10),or at a concentration of 5 mg mL-1(treatment group 2,n=10),once per week,four times in total,and cultivated without additional treatment for 9 months.Height of pine trees was measured with a measuring tape.Samples of needles located in the middle partof three pines trees were pooled to constitute an independent sample and this procedure was repeated twice in orderto obtain three independentsamples and to determine enzyme activities at 3,6 and 9 months after treatment.Plant hormones were detected in samples of pine needles obtained 9 months after treatment.
Determination of chlorophyll levels
Pine needles(5 g of fresh tissue)of control and treated trees were frozen in liquid nitrogen and homogenized in a mortar with a pestle.12 mL of hexane–acetone(3:1)were added and the mixture was incubated overnight at room temperature and filtered on Miracloth paper(Calbiochem, Darmstadt,Gemany).The absorbance of chlorophyll a and b was detected at 663 and 646 nm,respectively,using Hewlett-Packard/Agilent spectrophotometer model 8453 (Santa Clara,CA,USA).The levels of chlorophylls a and b were calculated as described in Lichtentalerand Welburn (1983)using the formulae:Chlorophyll a(μg mL-1)=12.5 A663–2.79 A646and Chlorophyll b(μg mL-1)=20.5 A646–5.1 A663.
Preparation of protein extracts
Pine needles(5 g of fresh tissue)of control and treated pines were frozen in liquid nitrogen and pulverized in a mortar with a pestle.Twenty mL of 0.1 M phosphate buffer pH 7.0 containing 5 mMβ-mercaptoethanol was added and the homogenization was pursued untilthawing. The homogenate was filtered through Miracloth(Calbiochem,San Diego,CA).The filtrate was centrifuged at 27,000×g for 30 min and the supernatant was recovered. Proteins were precipitated by addition of 0.6 g of ammonium sulfate per milliliter of extract and the mixture was centrifuged at27,000 g for 30 min.The protein pelletwas solubilized in 2 mL of 0.1 M phosphate buffer(pH 7.0) containing 2 mMβ-mercaptoethanol and 10%glycerol. Protein concentration was determined according to Bradford(1976).Extracts normally contained 1.2 to 1.5 mg mL-1of proteins and were stored at-80°C.
Determination of enzyme activities involved in C,N and S assimilation
Rubisco activity was detected as described in Lilley and Walker(1974)using 1 mL reaction mixture containing 20 mM Tris–HCl(pH 8.0),0.3 mM ribulose 1,5-biphosphate,10 mM NaHCO3,20 mM MgCl2,1 mM ATP,10 U phosphoglycerate kinase,10 U glyceraldehyde 3-phosphate dehydrogenase,0.2 mM NADH and 3μg of protein extract.The decrease in absorbance at 340 nm due to consumption of NADH was detected for 3 min.Rubisco activity was calculated using the extinction coefficient of NADH(ε=6.2 mM-1cm-1).
GDH activity was detected as described by Turano etal. (1996)in 1 mL of reaction mixture containing 100 mM phosphate buffer(pH 7.4),50 mM ammonium sulphate, 2 mM 2-oxoglutarate,0.3 mM NADH and 1μg of protein extract.The decrease in absorbance was detected at 340 nm due to the consumption of NADH for 1 min.The activity of GDH was calculated using the extinction coefficient of NADH(ε=6.2 mM-1cm-1).
OASTL activity was determined as described in Lunn et al.(1990)using 1 mL of reaction mixture containing 100 mM Tris–HCl pH 7.5,5 mM O-acetylserine,5 mM Na2S,5 mM DTT and 30μg protein extract.The reaction was incubated at 37°C for 30 min and the reaction was stopped by adding 500μL ninhydrin reagent(320 mg of ninhydrin dissolved in 3 mL methanoladded to mixture of 32 mL of concentrated acetic acid and 8 mL of concentrated HCl).The mixture was placed in boiling water for 10 min,rapidly cooled in ice and 550μL of 95%(v/v) ethanol were added.The absorbance of the mixture was determined at 560 nm and the activity of OASTL was determined using the extinction coefficient the spirane formed by cysteine and ninhydrin(ε=25 mM-1cm-1).
Extraction and analysis of growth-promoting hormones
Plant hormones were extracted and analyzed as described in Pan etal.(2010)with some modifications.Pine needles (1 g offresh tissue)were obtained from trees cultivated for 9 months after treatment,in the middle partof controland treated trees.Leaves were homogenized with liquid nitrogen in a mortar and 4 mL of 30%(v/v)isopropanol-15 mM HCl were added.The mixture was shaken at 4°C for 30 min to extract plant hormones.Two mL of dichloromethane were added,the mixture was shaken at 4°C for 30 min and centrifuged at14.000 rpm for 15 min and the alcoholic solution containing hormones was recovered.The alcoholic supernatantwas concentrated using a flux of nitrogen(g)to reach a finalvolume of 180μL.
Plant hormones were detected and quantified using an HPLC–ESI–MS/MS system(Agilent 1200 series,MS/MS 5420).The mobile phase was prepared with 0.1%of formic acid(A)and 0.1%of formic acid in methanol(B).A sample of 20μL was separated using a C18 reverse phase column(5 m particle size,150 mm 4.6 mm,Agilent)with a flow rate of 0.3 mL·min-1at room temperature.The elution was done using linear steps of 0–2 min 30%of B, 2–20 min increasing to 100%of B,20–22 min with 100%of B and 22–25 min with 30%of B.The MS/MS detection was performed using a multiple reaction mode (MRM),-4500 V,25 psiand 10 L min-1of nitrogen flow. Twenty uL of a mixture of deuterated standards for IAA and GA3(Olchemim,Olomouc,Czech Republic)at a concentration of 50 ng mL-1were added to each sample. For the detection in the negative mode,the mass-to-charge ratio(m/z)of IAA and GA3were[174.0 129.6;retention time(RT)=15.5]and(345.1 142.7;RT=17.30),respectively.For the detection of internal standard in a negative mode the m/z ratios for d5-IAA and d2-GA3were (347.1 142.7)and(347.1 142.7),respectively.
Statisticalanalysis
Data were analyzed using two-way analysis of variance (ANOVA)followed by Tukey’s multiple comparison tests (T).Mean values were obtained from ten plants and differences between mean values were considered to be significant at a probability of 5%(p<0.05).
Results and discussion
OC kappa was prepared from pure carrageenan kappa and it was visualized as a relatively discrete band of around 10 kDa.In order to analyze whether OC kappa stimulated growth in pine trees,the heightof controland treated pines was measured at 3,6 and 9 months of culture after treatment.The increase in heightcorresponds to the difference between the finalheightand the initialheightof each tree. Control pines showed an increase in height of 32 cm at 9 months after treatment(Fig.1a).Pines treated with OC kappa at a concentration of 1 and 5 mg mL-1showed increases in height of 38 and 39 cm,respectively(Fig.1a), which corresponded to 19 and 22%increases,respectively,compare to control trees.In addition,control trees displayed total chlorophyll(Chl a+Chl b)level of 102μg g-1of fresh tissue at 9 months after treatment (Fig.1b).Pine trees treated with OC kappa at 1 and 5 mg mL-1showed total chlorophyll level of 161 and 124μg g-1of FT,respectively,at 9 months after treatment,which corresponded to increases of 58 and 22%, respectively(Fig.1b).Thus,OC kappa increased growth and total chlorophyll content in pine trees.

Fig.1 Increase in height(a)and in total chlorophylllevelin control pines trees(open circles)and pines treated with OC kappa at a concentration of 1mg mL-1(black circles)and 5 mg mL-1(grey circles).The increase in height is expressed in centimeters and total chlorophyll in micrograms per gram of fresh tissue(FT).Symbols represent mean values of three independent experiments±SD. Different letters represent significant differences(p<0.05)
The increase in height in response to treatmentwith OC kappa observed in pine trees was previously observed in tobacco plants(Castro etal.2012)and in E.globulus trees (Gonza´lez et al.2014a).In this sense,pines are slowgrowing conifers and they appeared earlier in evolution (approx.360 million year ago)than latifoliate plants (aprox.250 million years ago)such as tobacco and E. globulus(Clarke etal.2011).The latter results indicate that conifers and latifoliates are able to sense OC kappa and respond with an increase in height.This suggests that a potentialreceptor for OC kappa mightexistin conifers and latifoliates(Gonza´lez et al.2014a)that might have appeared early in evolution of land plants.In addition,the leveloftotalchlorophyllincrease in pine trees at9 months after treatment(58%)as it did in Eucalyptus trees(44%) cultivated in the field for 3 years(Gonza´lez et al.2013b).
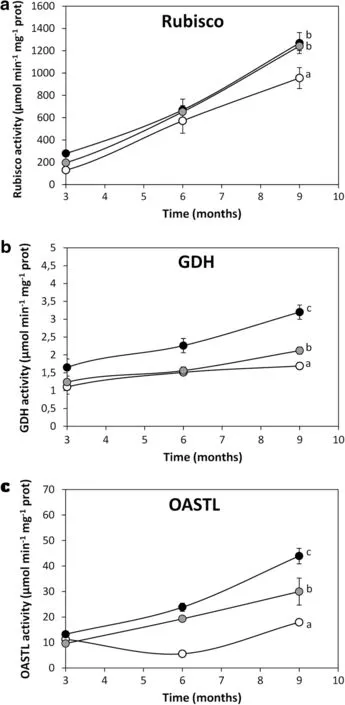
Fig.2 Activities of basal metabolism enzymes involved in C,N and S assimilation ribulose 1,5 biphosphate carboxylase/oxygenase(rubisco,a),glutamate dehydrogenase(GDH,b)and O-acetylserine thiol-lyase(OASTL,c)in controlpine trees(open circles)and pines treated with OC kappa at a concentration of 1 mg mL-1(black circles)and 5 mg mL-1(grey circles).Activities ofrubisco,GSH and OASTL are expressed in micromoles per minute per gram of protein. Symbols represent mean values of three independent experiments ±SD.Different letters represent significant differences(p<0.05)
In order to analyze whether increase in heightis due to an increase in C,N and S assimilation or activities of rubisco, levels of GDH and APR enzymes involved in C,N and S assimilation were quantified in the needles of control and treated pine trees.Controlpine trees showed rubisco,GDH and OASTLactivitiesof956,1.7 and 16.4μmolmin-1mg-1of protein,respectively,at 9 months after treatment (Fig.2a–c).Pines treated with OC kappa at1 mg mL-1displayed rubisco,GDHand OASTLactivitiesof1268(Fig.2a), 3.2(Fig.2b)and 44μmol min-1mg-1of protein(Fig.2c), respectively,at 9 months after treatment,corresponding toincreasesof33,88 and 169%,respectively.In addition,pines treated with OC kappa at5 mg mL-1rubisco showed GDH and OASTL activities of 1241(Fig.2a),2.1(Fig.2b)and 30μmol min-1mg-1of protein(Fig.2c),respectively,at 9 months aftertreatment,which corresponded to increases of 30,24 and 83%,respectively.Thus,OC kappa increased basal metabolism,in particular,C,N and S assimilation in pines trees,mainly ata concentration of1 mL-1.Similarly, OC kappa induced increase in C,N and S assimilation in Eucalyptus trees(Gonza´lez etal.2014a).In particular,Eucalyptus trees treated with OC kappa showed increases in NADPH,ascorbate and glutathione synthesis,which changed the redox status to a more reducing condition.The increase in NADPH increased thioredoxin reductase(TRR)/thioredoxin (TRX)activities which,in turn,increased C,N and S assimilation,basal metabolism and growth(Gonza´lez et al. 2014a).Therefore,it is possible that a similar increase in NADPH,ASCand GSHsynthesismightalso haveoccurred in pines trees treated with OC kappa,leading to an increase in basalmetabolism and growth butthe latterassumption remain to be determined.Itis interesting to note thatthe increase in C assimilation better correlates with the increase in height of pinestreated with OCkappa,suggesting thatCassimilation is the limiting step in pines.Thiscontrastswith resultsobtained for Eucalyptus where the increase S assimilation wasthought to be limiting(Gonza´lez etal.2014a).
We also quantified levels of the growth-promoting hormones auxin IAA and gibberellins GA3and GA1. Control pines showed 2 nmol g-1of FT of IAA and 1.2 nmoles g-1of FT of GA3(Fig.3).GA1was notdetected in controlor treated pine trees.Pines treated with OC kappa at a concentration of 1 mg mL-1 showed 1500%increases in levels ofIAA(Fig.3a)and in 17%increases in levels of GA3(Fig.3b)at 9 months after treatment.In addition, pines treated with OC kappa at a concentration of 5 mg mL-1displayed an increase in IAA contentof 60% (Fig.1a)and in GA3level of 17%(Fig.3b).Thus,OC kappa increased auxin and gibberellin levels,mainly at a concentration of 1 mg mL-1,which may account,at least in part,for the increase in basal metabolism and height in pine trees.In this sense,itis importantto mention thatthe increase in height in pine trees(around 20%)correlate with the increase in gibberellin content(17%)at9 months after treatment which contrasts with the increase in height in Eucalyptus trees(around 30%)and the increase in gibberellins content(600%)at 4 months after treatment (Gonza´lez et al.2014c).In addition,pine trees showed a higher increase in auxin level(1500%)compare with the increase in GA3level(17%,this work)which contrasts with the higher increase in GA3(600%)than auxin IAA level(350%)in Eucalyptus globulus trees(Gonza´lez etal. 2014c).Thus,auxin and gibberellin showed complex interplay regarding the increase in height in trees which depends on the plant species treated with OC kappa.
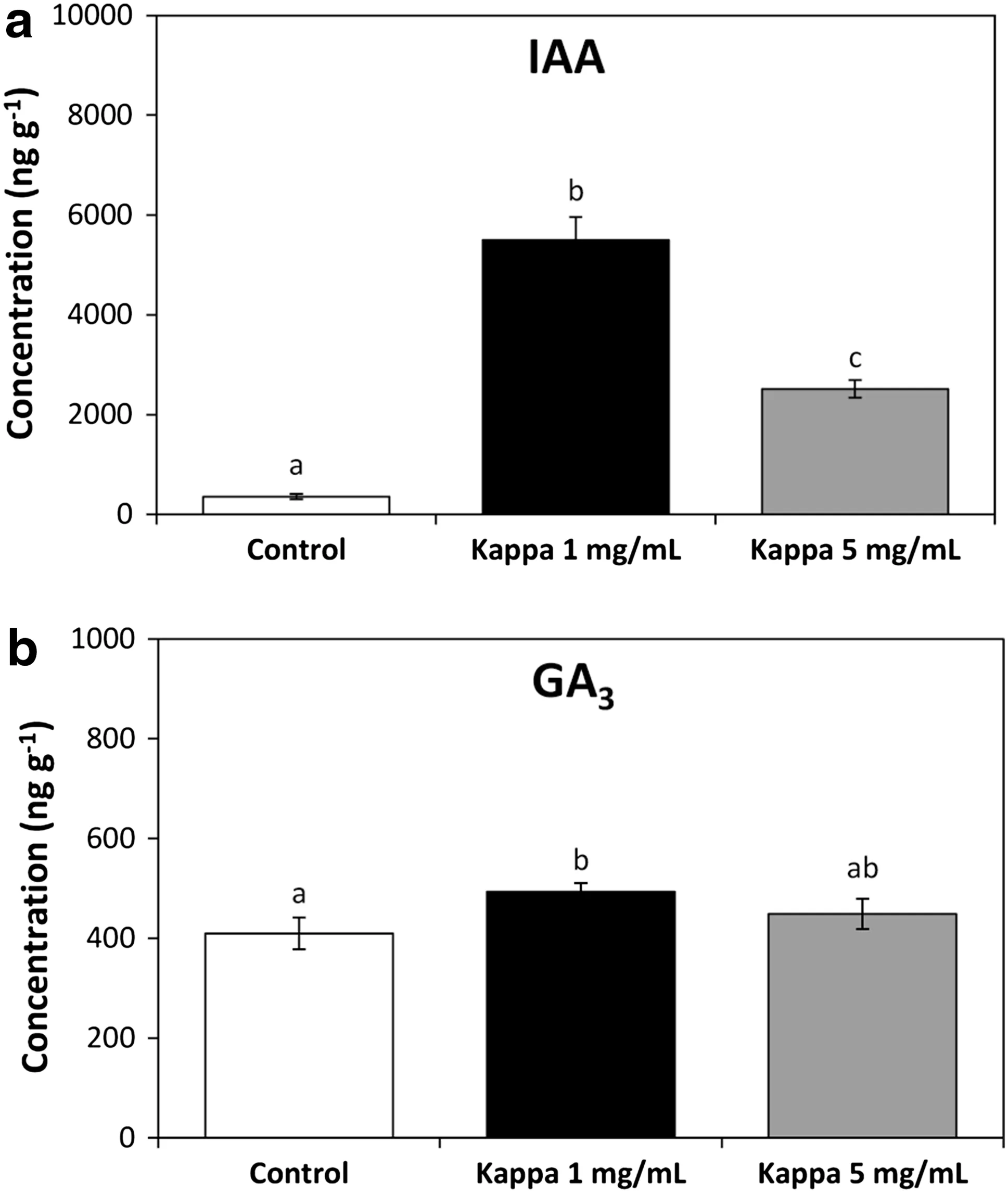
Fig.3 Levels of growth-promoting hormones indole acetic acid (IAA,a)and gibberellin A3(GA3,b)in controlpines(white bar)and pines treated with OC kappa ata concentration of 1 mg mL-1(black bar)and 5 mg mL-1(grey bar)at9 months after treatment.The level of IAA and GA3are expressed in nanomoles per gram of fresh tissue (FT).Bars represent mean values of three independent experiments ±SD.Differentletters represent significantdifferences(p<0.05)
To date,little information has been published on the effect of auxin and gibberellins on C,N or S metabolism. However,it has been shown that an Arabidopsis mutant over-expressing regulatory genes involved in auxin synthesis displayed down-regulation of genes encoding sulfate transporters and sulfate reducing enzymes involved in S assimilation,indicating that auxin level may regulate S assimilation in Arabidopsis(Falkenberg et al.2008).In addition,it is still not clear whether growth-promoting hormones regulate C,N and S assimilation and/or vice versa.
Conclusions
OC kappa stimulates C,N and S assimilation and increased growth-promoting hormone content,and growth in pine trees,mainly at a concentration of 1 mg·mL-1.Thus,OC kappa may constitute a useful biotechnological tool to increase growth in pine forests.
AcknowledgmentsThis work was financed by Sirius Natura S.A. and VRIDEI-USACH.Silvia Saucedo was financed by SENESCYTEcuador,Convocatoria 2011.
Albersheim P,DarvillA,Augur C,Cheong JJ,Eberherd S,Hahn MG, Marfa´V,Mohnen D,O’Neill MA,Spiro MD,York WS(1992) Oligosaccharins-oligosaccharide regulatory molecules.Acc Chem Res 25:7783
Bjo¨rklund S,Antti H,Uddestrand I,Moritz T,Sundberg B(2007) Cross talk between gibberellin and auxin in development of Populus wood:gibberellins stimulate polar auxin transport and has a common transcriptome with auxin.Plant J 52:499511
Bradford MM(1976)A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding.Anal Biochem 72:248254
Castro J,Vera J,Gonza´lez A,Moenne A(2012)Oligo-carrageenans stimulate growth by enhancing photosynthesis,basal metabolism,and cell cycle in tobacco plants(var.Burley).J Plant Growth Regul31:173185
Clarke JT,Warnock RC,Donoghe PCJ(2011)Establishing a timescale for plant evolution.New Phytol 192:266301
Falkenberg B,Witt I,Zano´r MI,Steinhauser D,Mueller-Roeber B, Hesse H,Hoefgen R(2008)Transcription factors relevent to auxin signaling coordinate broad-spectrum metabolic shifts including sulphur metabolism.J Exp Bot 59:28312846
Frigeiro L,Alabadi D,Pe´rez-Go´mez J,Gracia-Cacel L,Phillips AL, Hedden P,Blazquez MA(2006)Transcriptional regulation of gibberellin metabolism genes by auxin signaling in Arabidopsis. Plant Physiol 142:553563
Gonza´lez A,Castro J,Vera J,Moenne A(2013a)Seaweed oligosaccharides stimulate plant growth by enhancing carbon and nitrogen assimilation,basal metabolism and cell division. J Plant Growth Regul 32:443448
Gonza´lez A,Contreras RA,Moenne A(2013b)Oligo-carrageenans enhance growth and contents of cellulose,essential oils and polyphenolic compounds in Eucalyptus globulus trees.Molecules 18:87408751
Gonza´lez A,Contreras RA,Zun˜iga G,Moenne A(2014a)Oligocarrageenan kappa-induced reducing redox status and activation of TRR/TRX system increase the level of indole-3-acetic acid, gibberellin A3and trans-zeatin in Eucalyptus globulus trees. Molecules 19:1269012698
Gonza´lez A,Gutierrez-Cutin˜o M,Moenne A(2014b)Oligo-carrageenan kappa-induced reducing redox status and increase in TRR/TRX activities promote activation and reprogramming of terpenoid metabolism in Eucalyptus trees.Molecules 19:7356–7367
Gonza´lez A,Moenne F,Go´mez M,Sa´ez CA,Contreras RA,Moenne A(2014c)Oligo-carrageenan kappa increases NADPH,ascorbate and glutathione syntheses and TRR/TRX activities enhancing photosynthesis,basalmetabolism,and growth in Eucalyptus trees.Front Plant Sci 5:512
Kopriva S,Suter M,Von Ballmoos O,Hesse H,Kra¨henbu¨hl U, Rennenberg H,Brunold C(2002)Interaction of sulfate metabolism with carbon/nitrogen metabolism in Lemna minor. Plant Physiol 130:14061413
Kraiser T,De Gras AG,Gutie´rrez AG,Gonza´lez B,Gutie´rrez RA (2011)Holistic view of nitrogen acquisition in plants.J Exp Bot 62:14551466
Li ZY,Xu ZS,Chen Y,He GY,Yang GY,Chen M,Li LC,Ma YZ (2013)A novel role for Arabidopsis CBL1 in affecting plant response to glucose and gibberellins during germination and seedling development.PLoS One 8:e56412
Lichtenthaler H,Wellburn AR(1983)Determinations of total carotenoids and chlorophylls a and b in leaf extracts in different solvents.Biochem Soc Trans 11:591–592(Your reference is not cited in the paper.)
Lilley RM,Walker DA(1974)An improved spectrophotometric assay for ribulosebiphosphate carboxylase.Biochim Biophys Acta 358:226229
Lunn J,Droux M,Martin J,Douce R(1990)Localization of ATP sulfurylase and O-acetylserine(thiol)lyase in spinach leaves. Plant Physiol 94:13451352
O’Neill DP,Ross JJ(2002)Auxin regulation of the gibberellin pathway in Arabidopsis.Plant Physiol 130:9741978
Pan X,Welti R,Wang X(2010)Quantitative analysis of major plant hormones in crude plant extracts by high-performance liquid chromatography-mass spectrometry.Nat Protoc 5:986992
Turano FJ,Dashner R,Upadhyaya A,Caldwell CR(1996)Purification of mitochondrialglutamate dehydrogenase from dark-grown soybean seedlings.Plant Physiol 112:13571364
Vera J,Castro J,Gonza´lez A,Moenne A(2011)Seaweed polysaccharides and derived oligosaccharides stimulate defense responses and protection against pathogens in plants.Mar Drugs 9:25142525
Vera J,Castro J,Contreras RA,Gonza´lez A,Moenne A(2012)Oligocarrageenans induced a long-term and broad-range protection against pathogens and the reversion of infections in tobacco plants(var.Xanthi).Physiol Mol Plant Pathol 79:3139
Weiss D,Ori N(2007)Mechanisms ofcross talk between gibberellins and other hormones.Plant Physiol 144:12401246
13 January 2014/Accepted:8 June 2014/Published online:8 May 2015
©Northeast Forestry University and Springer-Verlag Berlin Heidelberg 2015
✉Alejandra Moenne alejandra.moenne@usach.cl
1University of Santiago of Chile,Santiago,Chile
2Facultad de Ciencias Agrarias,Universidad Te´cnica Estatal de Quevedo,63,Quevedo,Ecuador
 Journal of Forestry Research2015年3期
Journal of Forestry Research2015年3期
- Journal of Forestry Research的其它文章
- Management of pests and diseases of tropical sericultural plants by using plant-derived products:a review
- Gamma generalized linear model to investigate the effects of climate variables on the area burned by forest fire in northeast China
- Diversity,abundance,and structure of tree communities in the Uluguru forests in the Morogoro region,Tanzania
- Brazilian savanna re-establishment in a monoculture forest: diversity and environmental relations of native regenerating understory in Pinus caribaea Morelet.stands
- Carbon storage and sequestration rate assessment and allometric model development in young teak plantations of tropical moist deciduous forest,India
- Use of infrared thermal imaging to diagnose health of Ammopiptanthus mongolicus in northwestern China
