两个由4-酰基吡唑啉酮衍生物构筑的Mn配合物的合成、晶体结构及性质
余万学 张 丽 许贯诚 张艳慧 贾殿赠
(清洁能源材料与技术教育部省部共建重点实验室,新疆先进功能材料自治区重点实验室,新疆大学应用化学研究所,乌鲁木齐830046)
余万学 张 丽*许贯诚 张艳慧 贾殿赠*
(清洁能源材料与技术教育部省部共建重点实验室,新疆先进功能材料自治区重点实验室,新疆大学应用化学研究所,乌鲁木齐830046)
以4-酰基吡唑啉酮衍生物为配体合成了2个Mn配合物[Mn2L2(μ-CH3OH)2(CH3OH)2](1)和{[MnL(μ-CH3OH)]·CH3OH· CHCl3}n(2)(H2L=N-(1-苯基-3-苄基-4-丙烯基-5-吡唑啉酮)-异烟酰肼),利用元素分析、红外光谱、紫外光谱、热重和X-射线单晶衍射分析进行了表征。结果表明反应体系的pH值影响配体的配位方式,所得配合物1为双核结构,而2为2D网状结构。热重分析表明,配合物1的稳定性高于配合物2的。
4-酰基吡唑啉酮衍生物;锰配合物;晶体结构;热稳定性
0 Introduction
The rational design and assembly of metal-organic coordinationpolymershavereceivedremarkable attention due to their fascinating structural diversities and potential applications in the areas including optical,electronic,magnetic fields,gas storage,ionexchange and catalysis[1-4].Investigations show that the designed construction of metal-organic polymers is influenced by many factors such as the nature of the organic building block,the coordination preference of the central metal ion,crystallization conditions,the molar ratio of metal and ligand and preparation methods,etc[5].Among these,proper choice of organicligand is the key factor governing the structure of coordination polymer,as the geometry of ligating atoms within a multi-chelateing ligand,the flexibility of the ligand backbone,and the additional functional groups of the ligands all play important roles in directing the extended structure of theresulting complex[6].Thus,considerable efforts have been devoted tochoose or design new multidentate ligands to construct novel coordination frameworks with tunable properties.
Pyrazolone-5,especially 4-acyl-pyrazolone,form an important class of organic compounds and widely used in biological,analytical applications,catalysis and extraction metallurgy[7].Moreover,4-acyl-pyrazolone derivatives can be easily tailored by incorporating otherfragmentwithvariouscomplexities,which facilitates the preparation of chelating molecules[8]. Due to the various electron-rich donor centers and tautomeric effect of the enol form and keto form of the 4-acyl-pyrazolonederivatives,thecoordination behavior of 4-acyl-pyrazolone derivatives has been extensively explored[7-11].Meanwhile,as the compound containing hydrazide moiety and their metal complex always possess biological activities,especially serving aspotentialinhibitorsformanyenzymes,the compounds bearing 4-hydrazone moieties have also warranted considerable attention in recent years[12-13]. In this paper,two manganese compounds of a new Schiff base derivative of 4-acyl-pyrazolone,N-(1-phenyl-3-benzyl-4-propylene-5-pyrazolone)-isonicotinic hydrazide,have been synthesized and characterized. The structural analyses reveal that one is a dinuclear complex,and the other one represents a 2D network based on the dinuclar unit.When a weak organic base was used or not,the ligand performed in different kind of coordination modes,which play an important roleinthestructureofthefinalcompound. Furthermore,thethermalstabilitiesofthetwo manganese complexes are also investigated.
1 Experimental
1.1 Materials and general physical measurements
All reagents were purchased from commercial sources and used as received.1-phenyl-3-benzyl-4-propionyl-5-pyrazoloneandisoniazidewere synthesized and purified according to the methods in the literature[11].
Elemental analyses(C,H and N)were performed onaFLASHEA1112SeriesNCHS-Oanalyzer. Melting point was measured with a TECH XT-6 melting point apparatus.IR spectra were recorded on BRUKEREQUINOX-55spectrophotometerwithin 400~4 000 cm-1using the samples prepared as pellets with KBr.UV-Vis absorption spectra were measured in solid state at room temperature on a UV-3010 spectrometer equipped with an integrating sphere. Thermal analyses were carried out on NETZSCH STA 449F3 instrument at a temperature range of 30~800℃with a heating rate of 10℃·min-1in the flowing air atmosphere.
1.2 Synthesis of the ligand
The ligand N-(1-phenyl-3-benzyl-4-propylene-5-pyrazolone)-isonicotinic hydrazide(H2L)was prepared by refluxing equimolar 1-phenyl-3-benzyl-4-propionyl-5-pyrazolone and isoniazide in ethanol at 80℃for 4 h,adding a few drops of glacial acetic acid as a catalyst.Upon cooling,the yellow precipitate was deposited and collected by filtrating,washed and dried in air.Yield:75%.m.p.222.4~225.2℃.Anal. Calcd.for C25H23N5O2(%):C,70.57;H,5.45;N,16.46. Found:C,70.51;H,5.49;N,16.52.FT-IR(cm-1):2 967 (b,νNH),1 623(m,νC=O),1 591(m,νC=O).
1.3 Preparation of the complexes and basic analytical data
1.3.1 [Mn2L2(μ-CH3OH)2(CH3OH)2](1)
To a solution of H2L(0.213 g,0.5 mmol)in 100 mL of methanol was added a solution of Mn(OAc)2· 4H2O(0.125 g,0.5 mmol)in 20 mL of methanol at 80℃with continuous stirring for 3 h.The resulting solution was allowed to cool in room temperature,and thebrownprecipitatewascollectedbysuction filtration,washed with methanol and dried in air.The filtrate was left to stand for over one week under ambient conditions to produce brown crystals.Yield: 75%.Anal.Calcd.for C54H58N10O8Mn2(%):C,59.78;H, 5.39;N,12.91.Found:C,59.55;H,5.49;N,13.25. FT-IR(cm-1):1 604,1 596(m,νC=N-N=C),1 346(m,νC-O-),534(m,νM-O),458(m,νM-N).
1.3.2 {[MnL(μ-CH3OH)]·CH3OH·CHCl3}n(2)
4,4′-bipyridine(0.047 g,0.3 mmol)was added to a 100 mL methanol solution of H2L(0.128 g,0.3 mmol) at reflux temperature,followed by the addition of a 20 mL methanol solution of Mn(OAc)2·4H2O(0.075 g, 0.3 mmol)after fifteen minutes.The mixture was stirred for 4 h,the resulting solution was cooled to room temperature and then filtered off.The dark brownsinglecrystalssuitableforanalyseswere obtained by slowly evaporating the mixed solution of filtrate and CHCl3at room temperature for a few days. Yield:52%.Anal.Calcd.for C28H30Cl3N5O4Mn(%):C, 50.81;H,4.57;N,10.58.Found:C,51.33;H,4.46; N,10.34.FT-IR(cm-1):1 607,1 595(m,νC=N-N=C),1 360 (m,νC-O-),537(m,νM-O),473(m,νM-N).
1.4 X-ray crystallography
ThesinglecrystalX-raydiffractiondataof complexes 1 and 2 were performed on a Rigaku R-axis Spider diffractometer with graphite monochromatic Mo Kα radiation(λ=0.071 073 nm)at 153 and 296 K. The crystal structure was solved by direct method using SHELXS-97 program and all non-hydrogen atoms were refined anisotropically on F2by the fullmatrix least-squares technique using the SHELXTL-97 crystallographic software package[14].All non-hydrogen atoms were refined anisotropically.Hydrogen atoms were added geometrically and refined using the riding model.Details of the data collection parameters and crystallographic information for complex 1 and 2 are summarized in Table 1.Selected bond lengths and angles with their estimated standard deviations are listed in Table 2.Summary for hydrogen bond data are listed in Table 3.
CCDC:997907,1;997908,2.
2 Results and discussion
2.1 Synthesis
The ligand N-(1-phenyl-3-benzyl-4-propylene-5-pyrazolone)-isonicotinic hydrazide(H2L),possessing an adequate number of coordination sites,was designed to fabricate manganesecomplex,as shown inScheme 1.The reactions of manganeseacetate with H2L in the different pH value of the reaction system produced[Mn2L2(μ-CH3OH)2(CH3OH)2](1)and{[MnL (μ-CH3OH)]·CH3OH·CHCl3}n(2)in good yield.4,4′-Bipyridine was used as a weak organic base to modulate the pH value of the reaction system.
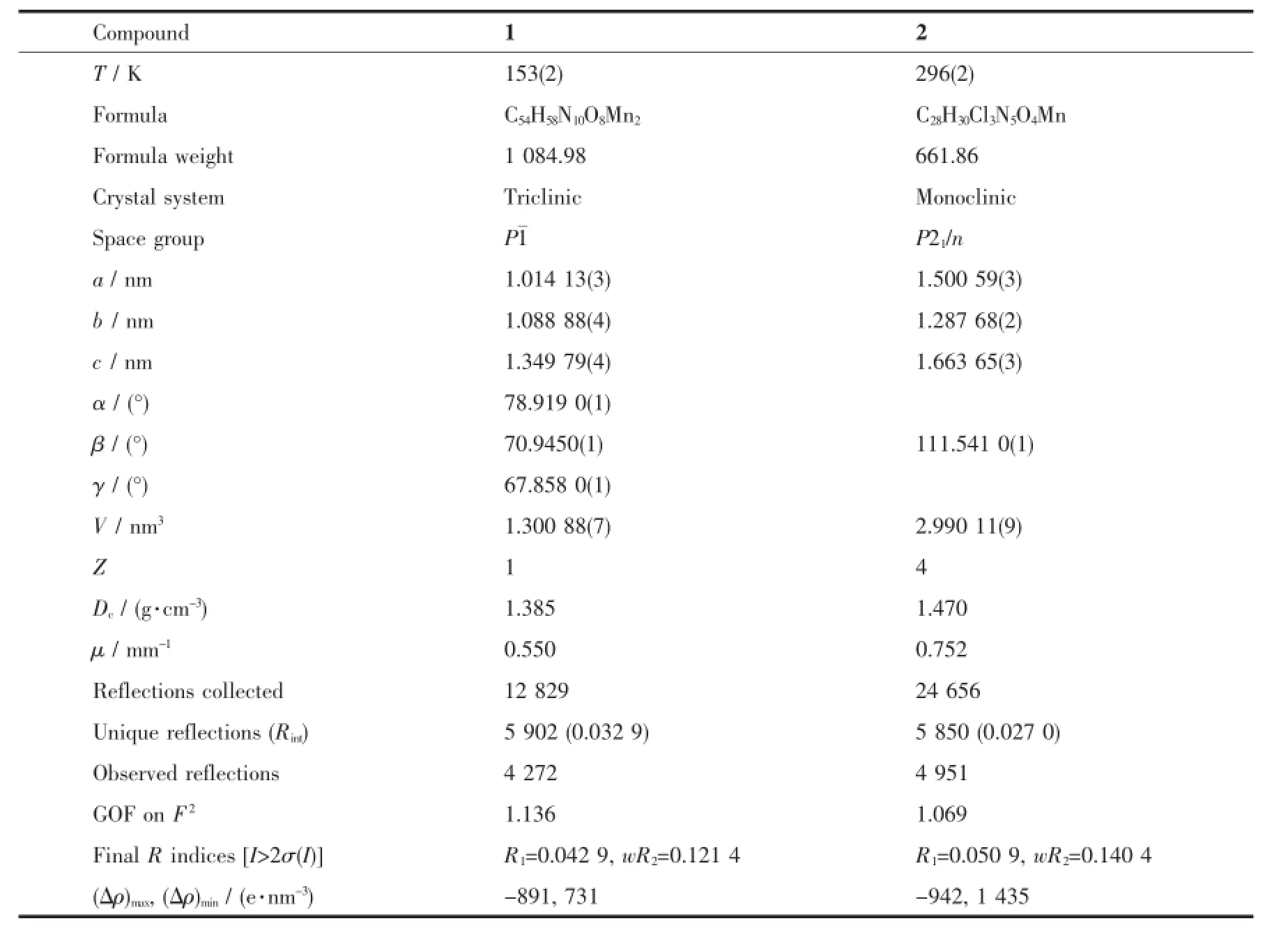
Table1 Crystallographic data and structure refinements for 1 and 2
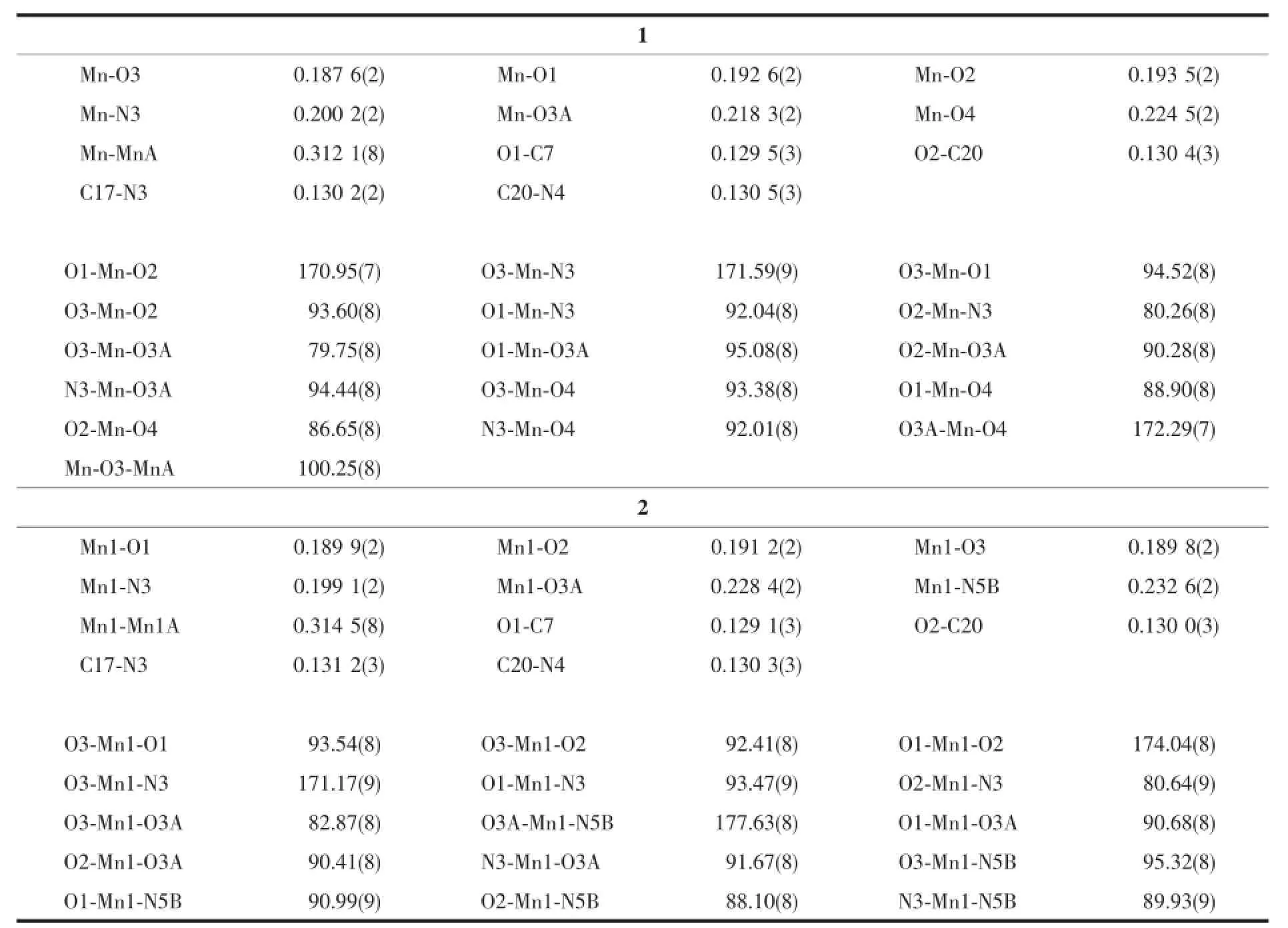
Table2 Selected bond lengths(nm)and angles(°)for 1 and 2
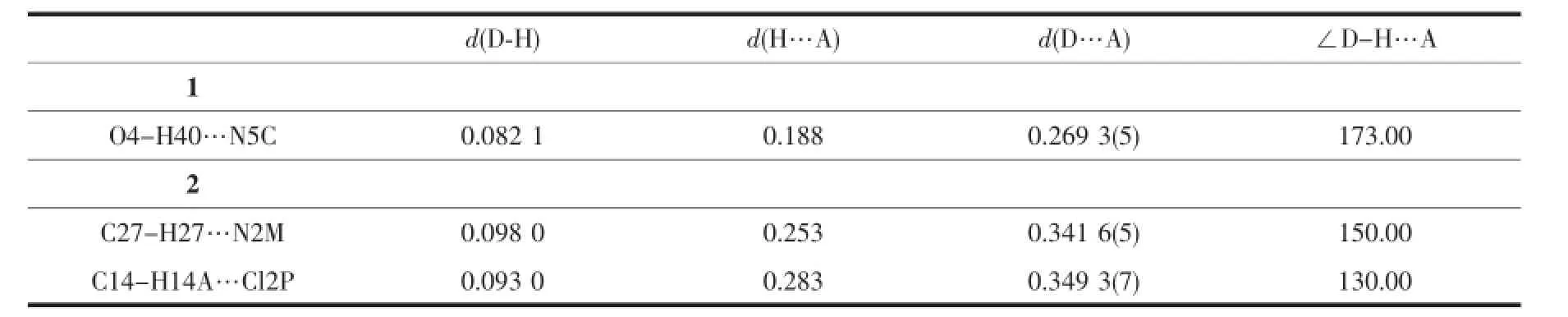
Table3 Lengths(nm)and angles(°)of hydrogen bonds for 1 and 2
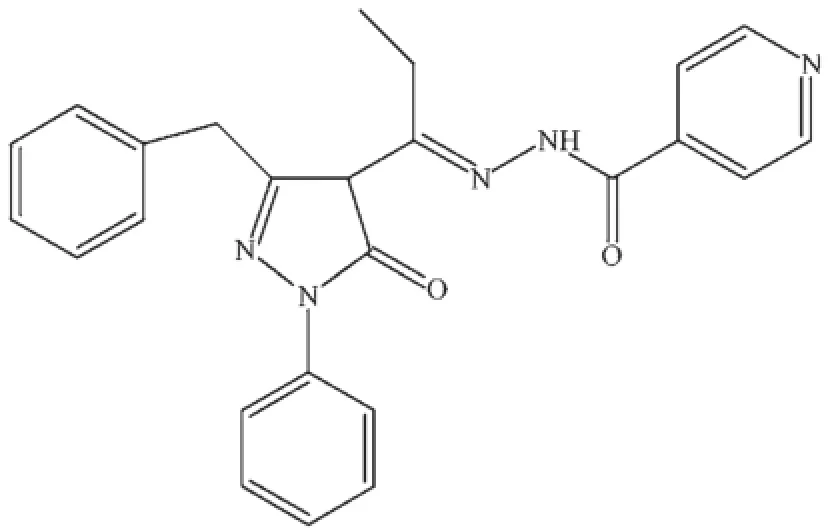
Scheme 1
2.2 IR and UV-Vis spectra
IRspectroscopyusuallyprovidesvaluable information on the coordination modes of the ligand and metal ions.The IR spectrum of the ligand showsstrong peaks at 2 967,1 623 and 1 591 cm-1,which are assigned to ν(N-H),ν(C=O)of the lateral chain and ν(C=O)of the pyrazolone-ring,respectively[15-17]. These results suggest that the free ligand in the solid state is in di-keto form.Compared with the spectrum of the free ligand,the most notable changes in the spectra of 1 and 2 are the disappearance of ν(NH) and ν(C=O)of the pyrazolone-ring and the lateral hydrazide chain.Meanwhile,new bands are observed between 1 607~1 595 cm-1and 1 346~1 360 cm-1, which are assigned to ν(-C=N-N=C-)of hydrazone and ν(C-O-),respectively.Weak bands in the region of 534~537 cm-1and 458~473 cm-1are also observed, which are assigned to ν(M-O)and ν(M-N)stretching vibrations.These results indicate that the ligand undergoes isomerization from di-keto form to the enol form during the coordination,and then lose two protons to coordinate with Mnatoms as double negative charged units.The results are coincident with the crystal structural analyses below.
The UV-Vis absorption spectra of the ligand and manganesecomplexes were recorded in solid state and displayed in Fig.1.The electronic absorption spectrum of the ligand gives bands at 219,329 and 413 nm correspond to π→π*and n→π*transitions. The spectra of manganesecomplexes 1 and 2 do not exhibit significant differences between each other and the broad bands at around 320 and 450 nm might be due to intra ligand charge transfer type and1A1g→1T1gtransitionrespectively,inanoctahedral structure[18].
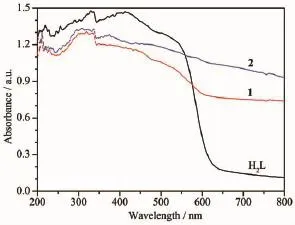
Fig.1 UV-Vis absorption spectra of the ligand,1 and 2 in solid state
2.3 Crystal structural description
2.3.1 Crystal structure of 1
The crystal structure of 1 is shown in Fig.2 with atom numbering scheme.The asymmetric unit contains two Mncenters,two ligand anions L2-,two coordinatedmethanolmoleculesandtwoμ2-bridged methanol molecules.The ligand anions act as negative bivalent tridentate chelating units and band with the Mnatoms.The two Mnatoms(Mn and MnA)are bridged by two μ2-O atoms of the methanol molecules and form a dinuclear unit.Each Mnis hexacoordinated by O1,N3 and O2 atoms from one ligand anion L2-,one O4 atom from methanol molecule,and two O atoms(O3 and O3A)from two bridging methanol molecules to form an octahedron geometry with N1O5 donor sets.The O1,N3,O2 and O3 atoms consist of the equatorial plane with the least square plane deviation of 0.008 32 nm,and the Mnion strays from the equatorial plane 0.000 97 nm,which indicates that the Mnatom nearly lies in the equatorial plane.The bond distances of Mn-O1,Mn-N3,Mn-O2 and Mn-O3 in the equatorial plane are 0.192 6(2),0.200 2(2),0.193 5(2)and 0.187 6(2)nm, respectively.The axial positions are occupied by O3A and O4 atoms with the bond distances of 0.218 3(2)and 0.224 5(2)nm.It is obvious that the axial bond distances are longer than the bond distances of the equatorial plane.The bond angels of O1-Mn-O2,O3-Mn-N3 and O3A-Mn-O4 are 170.95(7)°,171.59(9)° and 172.29(7)°,respectively,which are deviated from the theoretical value of 180°.All these observations implied that the geometry around the Mnatom in 1 should be an elongated distorted octahedron.In the dinuclear unit,two Mnatoms are bridged by two μ2-O atoms(O3 and O3A)from methanol molecules to define a planar centrosymmetric parallelogram,in which the side lengths are 0.187 6(2)and 0.218 3(2) nm.The Mn…Mn intermetallic distance is 0.312 1(8) nm.The bond angles of Mn-O3-MnA and O3A-Mn-O3 are 100.25(8)°and 79.75(8)°.On the other hand,the ligandfunctionsastridentatechelatingagent coordinated to the Mnatom with its ONO donor atoms in meridional fashion.
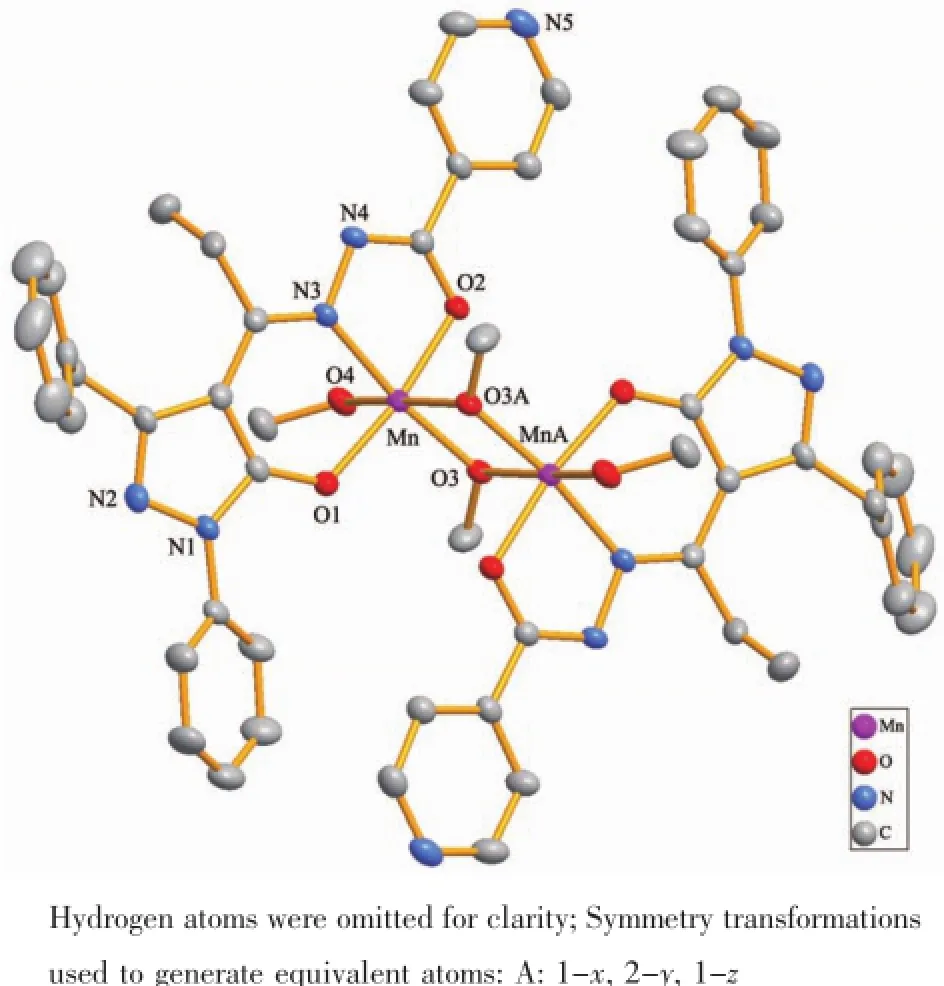
Fig.2 Crystal structure of 1 with 50%probability displacement ellipsoids
Furthermore,thedinuclearunitsarefurther linked into 1D chain via O-H…N intermolecular hydrogenbondbetweentheO4atomofthe coordinated methanol molecule and N5 atom from neighboring ligand(O4-H40…N5C,0.269 3(5)nm, 173.00°,Symmetry mode:C:2-x,1-y,1-z),as shown in Fig.3.In addition,there are weak face to face π-π interactions between the parallel pyridine rings of the ligands with a centroid-to-centroid distance of 0.373 8 nm.
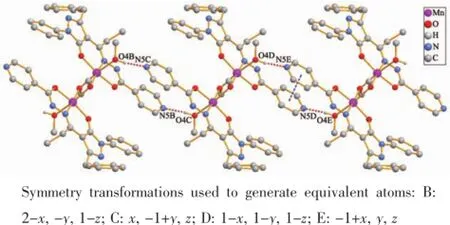
Fig.3 1D chain of 1
2.3.2 Crystal structure of 2
The crystal structure of 2 is shown in Fig.4 with atom numbering scheme.The asymmetric unit contains one crystallographically independent Mncenter, one ligand anion L2-and one μ2-bridged methanol molecule.Similar with 1,the two Mnatoms(Mn1 and Mn1A)are bridged by two μ2-O atoms of the methanolmoleculesandformadinuclearunit. However,the most interesting thing in 2 is that the ligand H2L functions as a negative bivalent tetradentate chelating-bridging unit.The pyridyl groups of the ligands take part in the coordination and bridge the neighboring dinuclear units,which give rise to a 2D network as indicated in Fig.5.Each Mnis hexa-coordinated by O1,N3 and O2 atoms from one ligand anion L2-,one N5B atom from another ligand anion, and two O atoms(O3 and O3A)from two bridging methanol molecules to form an octahedron geometry with N2O4 donor set.The O1,O2,N3,O3 atoms consist of the equatorial plane and the axial positions are occupied by O3A and N5B atoms.The Mn-O/N bond distances in equatorial plane are in the range of 0.189 8(2)to 0.199 1(2)nm,which are shorter than the axial bond lengths(Mn-O3A:0.228 4(2)nm,Mn-N5B:0.232 4(2)nm).Therefore,the local coordination geometry around the Mncenter in 2 can be described as highly distorted octahedron.

Fig.4 Crystal structure of 2 with the coordination environment around the Mncenter
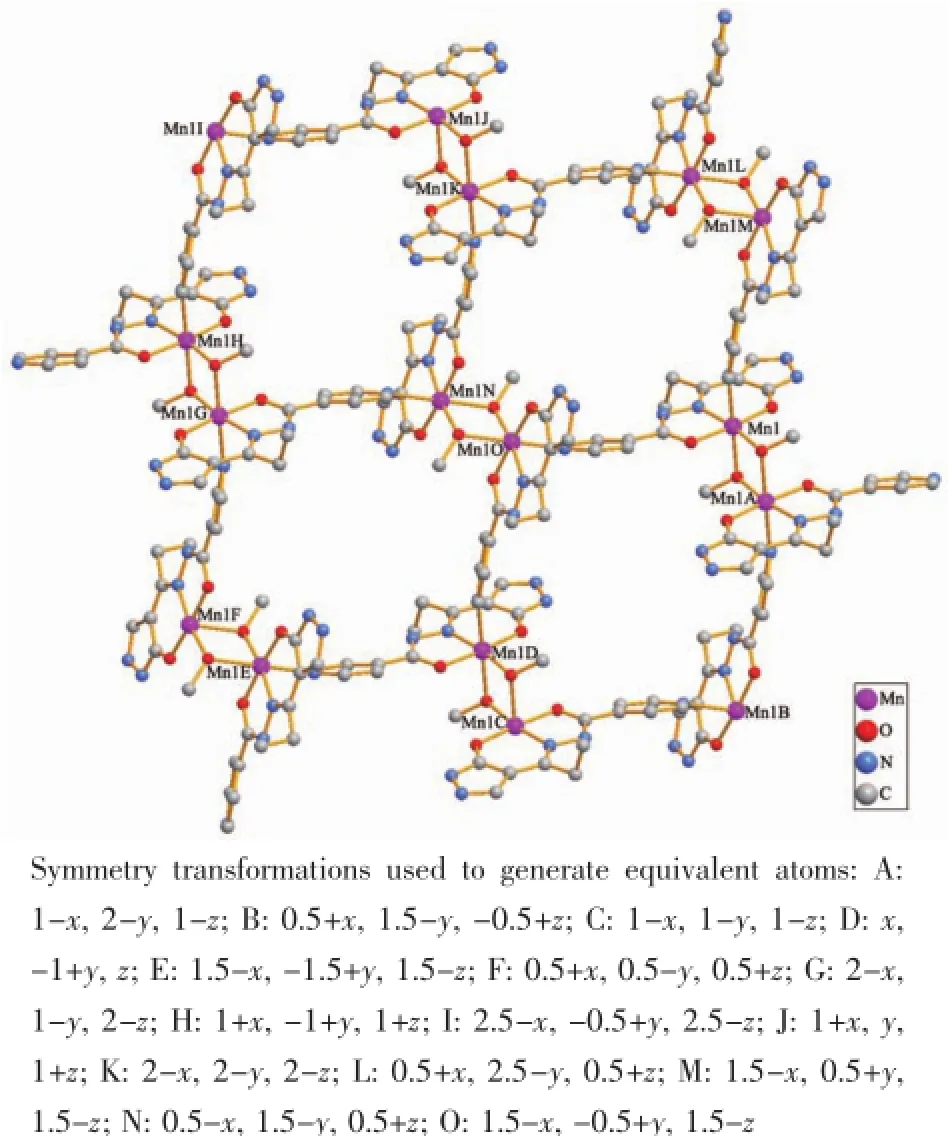
Fig.5 2D network of 2
Incomparison,thestructureof2isquite different from 1.The coordination mode of the ligand plays a vital role in the formation of multidimensional structure.In 1,the ligand functions as a tridentate chelating unit to bond with one Mnatom.While in 2,it is noteworthy to note that the pyridine N atoms of the ligands take part in the coordination,and the ligand acts as a tetradentate chelating-bridging unit coordinated with two Mnatoms to form a 2D network.The results indicated that the pH value of the solution has a certain effect on coordination mode of the ligand and structure of the resulting complex. Moreover,in both complexes the O1-C7 and O2-C20 distances are consistent with a single bond while the C17-N3 and C20-N4 are consistent with a double bond,which confirm that the ligand is in the deprotonated tautomeric enolic form to bond with Mn atom.
2.4 Thermal analyses
Toestimatethestabilityofthecomplexes, thermal gravimetric measurements for 1 and 2 were carried out.The TG curves of complex 1 and 2 are shown in Fig.6.For 1,no weight loss is observed from room temperature to 200℃.After that temperature,1 begins to decompose following a process of continuous weight loss of the coordinated methanol molecule and ligand anion.The observed weight loss is 84.0%, which is well consistent with the theoretical value of 83.9%.The decomposition completes at 430℃and the end product,estimated as MnO2,has the observed mass of 16.2%as against the calculated value of 16.1%.The TG curve of 2 shows a weight loss of 6.0%from 110 to 185℃,corresponding to the removal of one lattice MeOH molecule of each formula unit(Calcd.:4.8%).Then the decompositions oflatticechloroform,twocoordinatedmethanol molecules and the ligand were followed.Weight constancy is attained at around 430℃.The end product,estimated as MnO2,has an observed mass of 14.8%compared with the calculated value of 13.1%. The thermal gravimetric analyses show that 1 is more stable than 2.
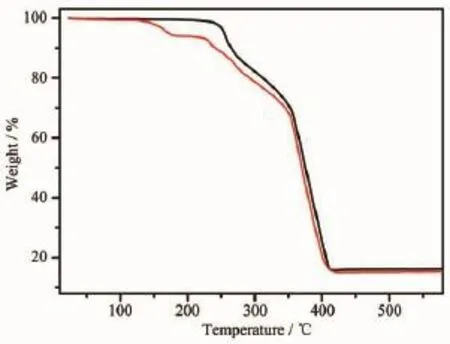
Fig.6 TG curves of 1 and 2
3 Conclusions
[1](a)Leong W L,Vittal J J.Chem.Rev.,2011,111(2):688-764
(b)Fujita M,Tominaga M,Hori A,et al.Acc.Chem.Res., 2005,38(4):369-378
(c)Stock N,Biswas S.Chem.Rev.,2012,112(2):933-969
(d)Tranchemontagne D J,Mendoza-Cortés J L,O′Keeffe M, et al.Chem.Soc.Rev.,2009,38(5):1257-1283
[2](a)Vukotic V N,Loeb S J.Chem.Soc.Rev.,2012,41(18):5896-5906
(b)Turega S,Whitehead M,Hall B R,et al.Chem.Commun., 2012,48(5):2752-2754
(c)Fang Q R,Zhu G S,Jin Z,et al.Angew.Chem.Int.Ed., 2007,46(35):6638-6642
(d)Goswami A,Sengupta S,Mondal R.CrystEngComm,2012, 14(2):561-572
[3](a)Weng D F,Wang Z M,Gao S.Chem.Soc.Rev.,2011,40(6): 3157-3181
(b)Cui Y J,Yue Y F,Qian G D,et al.Chem.Rev.,2012, 112(2):1126-1162
(c)Farha O K,Hupp J T.Acc.Chem.Res.,2010,43(8):1166 -1175
(d)Chen B L,Xiang S C,Qian G D.Acc.Chem.Res.,2010, 43(8):1115-1124
(e)Li J R,Kuppler R J,Zhou H C.Chem.Soc.Rev.,2009, 38(5):1477-1504
[4](a)Liu J,Thallapally P K,McGrail B P,et al.Chem.Soc. Rev.,2012,41(6):2308-2322
(b)Suh M P,Park J J,Prasad T K,et al.Chem.Rev.,2012, 112(2):782-835
(c)Ma Z B,Moulton B.Coord.Chem.Rev.,2011,255(15/16): 1623-1641
(d)Murray L J,Dincǎ M,Long J R.Chem.Soc.Rev.,2009, 38(5):1294-1314
[5](a)Nowicka B,Korzeniak T,Stefańczyk L,et al.Coord.Chem. Rev.,2012,256(17/18):1946-1971
(b)Zhao D,Timmons D J,Yuan D Q,et al.Acc.Chem.Res., 2011,44(2):123-133
(c)Dong Y B,Jiang Y Y,Li J,et al.J.Am.Chem.Soc., 2007,129(15):4520-4521
(d)Zhang G Q,Yang G Q,Ma J S.Cryst.Growth Des.,2006, 6(8):1897-1902
(e)Xu G C,Hua Q,Okamura T,et al.CrystEngComm,2009, 11(2):261-270
[6](a)Spokoyny A M,Kim D,Sumrein A,et al.Chem.Soc.Rev., 2009,38(5):1218-1227
(b)Allred R A,Doyle K,Arif A M,et al.Inorg.Chem.,2006, 45(10):4097-4108
(c)Garcia-Couceiro U,Castillo O,Luque A,et al.Cryst. Growth Des.,2006,6(8):1839-1847
(d)Ardizzoia G A,Angaroni I M A,Monica G,et al.Inorg. Chem.,1991,30(23):4347-4353
[7]Casas J S,García-Tasende M S,Sánchez A,et al.Coord. Chem.Rev.,2007,251(9/10):1561-1589
[8]Marchetti F,Pettinari C,Pettinari R.Coord.Chem.Rev., 2005,249(24):2909-2945
[9](a)JI Ya-Li(吉亚丽),LIU Lang(刘浪),JIA Dian-Zeng(贾殿赠),et al.Chinese J.Inorg.Chem.(无机化学学报), 2003,19(4):345-349
(b)Xu G C,Zhang L,Liu L,et al.Polyhedron,2008,27:12-24
(c)Yang Y,Zhang L,Liu L,et al.Inorg.Chim.Acta,2007, 360(1):2638-2646
[10](a)Zhang L,Xu G C,Yang Y,et al.Dalton Trans.,2013,42 (12):4248-4257
(b)Li H,Xu G C,Zhang L,et al.Polyhedron,2013,55:209-215
(c)Yi L J,Xu G C,Zhang L,et al.Inorg.Chem.Commun., 2014,45:36-39
[11]Xu G C,Zhang L,Zhang Y H,et al.CrystEngComm,2013, 15(15):2873-2880
[12](a)Zhao X,Yu H,Yu S,et al.Biochemistry,2006,45(13): 4131-4140
(b)Wengenack N L,Todorovic S,Yu L,et al.Biochemistry, 1998,37(45):15825-15834
(c)WengenackNL,LopesH,KennedyMJ,etal.Biochemistry, 2000,39(37):11508-11513
[13](a)Wang X H,Jia D Z,Liang Y J,et al.Cancer Lett.,2007, 249(2):256-270
(b)Zhang Y H,Zhang L,Liu L,et al.Inorg.Chim.Acta, 2010,363(2):289-293
[14](a)Sheldrick G M.SHELX-97:Programs for X-ray Crystallography,University of Göttingen,Göttingen,Germany, 1997.
(b)Sheldrick G M.SHELXTL,Version 6.10,Bruker Analytical X-ray Systems,Madison,WI,2001.
[15](a)Dobrzyńska D,Duczmal M,Jezierska J,et al.Polyhedron, 2002,21:2381-2385
(b)Franco E,López-Torres E,Mendiola M A,et al.Polyhedron, 2000,19:441-451
[16]Deng Y F,Zhou Z H,Cao Z X,et al.J.Inorg.Biochem., 2004,98(6):1110-1116
[17]Yang Z Y,Yang R D,Li F S,et al.Polyhedron,2000,19: 2599-2604
[18]Verma S K,Singh V K.Polyhedron,2014,76:1-9
Synthesis,Crystal Structure and Properties of Two MnComplexes with 4-Acyl-pyrazolone Derivative
YU Wan-XueZHANG Li*XU Guan-ChengZHANG Yan-HuiJIA Dian-Zen*
(Key Laboratory of Material and Technology for Clean Energy,Ministry of Education,Key Laboratory of Advanced Functional Materials,Autonomous Region,Institute of Applied Chemistry,Xinjiang University,Urumqi 830046,China)
Two manganese complexes,[Mn2L2(μ-CH3OH)2(CH3OH)2](1)and{[MnL(μ-CH3OH)]·CH3OH·CHCl3}n(2)(H2L=N-(1-phenyl-3-benzyl-4-propylene-5-pyrazolone)-isonicotinic hydrazide)were synthesized and structurally characterized by elemental analyses,IR spectra,UV-Vis spectra,thermogravimetric analyses and single crystal X-ray diffraction.The results show that the pH value of the reaction system has significant effect on the coordination mode of the ligand,which results in a dinuclear structure of 1 and a 2D network of 2.The thermal gravimetric analyses show that 1 is more stable than 2.CCDC:997907,1;997908,2.
4-acyl-pyrazolone derivative;manganesecomplex;crystal structure;thermostability
O614.81+3
A
1001-4861(2015)01-0205-08
10.11862/CJIC.2015.007
2014-07-08。收修改稿日期:2014-09-16。
新疆自治区自然科学基金面上项目(No.2012211A009)资助项目。*
。E-mail:zhangli420@xju.edu.cn,jdz0991@gmail.com

