Fe/SAPO-34 Catalytic Performance on Dehydration of Ethanol to Ethylene∗
LI Guo-feng,ZHU Gui-dan,LI Na,LU Jiang-yin
(College of Chemistry and Chemical Engineering,Key Laboratory of Oil and Gas Fine Chemicals of Ministry of Education,
Xinjiang University,Urumqi Xinjiang 830046,China)
0 Introduction
Ethylene is the cock tap of petroleum and chemical industry.It is also the basic raw material of the three largest syntheticmaterials(resin,rubber, fiber).Traditionally,ethylenewasproducedthroughpetroleumhydrocarboncracking by naphtha,kerosene,heavy oil,ethane,propane,butane and so on.However,as oil prices rising in recent years,the cost of petroleum-based ethyleneis increasing.Hence the using of ethanol from renewable biomass to produce ethylene becomes an economic development trend.Over the high efficiency catalysts,dehydration of ethanol to ethylene not only has high purity,low cost of separation and puri fication,but also possesses low investment and fast gains.This prompted it to be an alternative proposal[1−4].
At present,ZSM-5 is widely used as the catalyst for the dehydration of ethanol to produce ethylene[5−16].However,because of the acid strength of ZSM-5,coke forms easily during the dehydration of ethanol.Thus the problem of maintaining the stability of ZSM-5 remains.In contrast,SAPO-34 is a kind of micropore molecular sieve with the average pore size of 0.4 nm and weaker strength acid.Recently,the study on SAPO-34 mainly focuses on its synthesis and modi fication[17−19].The main application field of SAPO-34 was catalytic reaction of MTP/MTO,but application to the catalysis for the dehydration of ethanol to ethylene has been rarely studied.In this work,we prepared Fe/SAPO-34 catalyst used for the dehydration of ethanol by impregnating SAPO-34 into iron nitrate.
1 Experimental
1.1 Catalyst preparation.
The Fe/SAPO-34 catalyst was prepared by impregnation method.Commercial SAPO-34(SiO2:Al2O3:P2O5=1:1:1,Nankai University,China)was used.An mixed solution of SAPO-34 molecular sieve and iron nitrate was put into the rotary evaporation fl ask,dipping for 12 h at 40◦C.After impregnation,the mixture was distilled by vacuum distillation,and the wet sample was dried at 120◦C for 4 h and then the dried catalyst was calcined in a fl ow of air at 600◦C for 6 h.The loadings of Fe were 0.0%,0.5%,1.0%,1.5%,2.0%,3.0%,4.0%and 5.0%on SAPO-34,respectively.Before using the modi fied SAPO-34 catalysts,the catalyst was pelletized,crushed and sieved,and the 0.5-1.0 mm fraction was taken.
1.2 Catalyst characterization.
The phase identi fication of the parent and modi fied catalysts were carried out by X-ray diffraction(Bruker-D8)with Cu target and Kα radiation source operated at 35 kV and 25 mA.XRD patterns were collected over a 2θ value in the range of 5∼60◦C at the rate of 2◦C/min.
The nitrogen adsorption-desorption measurements were carried out at–196◦C in a ASAP 2020 instrument.High purity liquid nitrogen as adsorption medium,the samples were pretreated under a stable vacuum of 5×10−3Torr at 200◦C for 1 h and then measured using the static method.
NH3-temperature-programmed desorption(TPD)and H2-temperature-programmed reduction(TPR)experiments were performed in an Autosorb-TP-5080 apparatus(Xianquan,China).In the NH3-TPD experiment,100 mg of the sample was placed in the quartz tube.The samples were pretreated at 400◦C for 0.5 h in a fl ow of helium first,and then the temperature was cooled to room temperature.The adsorption of NH3was performed at room temperature for 30 min.Physical adsorbed NH3was removed at 120◦C.NH3-TPD curves were recorded under the condition of helium fl ow with heating the sample at the rate of 10◦C/min from 120◦C to 850◦C.
The reduction behaviors of Fe/SAPO-34 catalysts were characterized by H2-TPR.50 mg sample was placed in the adsorption tube and preheated in fl owing N2at 200◦C for 0.5 h,then cooled down to room temperature.After adsorption of H2for 0.5 h,TPR pro files were obtained under the conditions of N2fl ow with the temperature varying from 30◦C to 900◦C at a heating rate of 10◦C/min.
1.3 Activity measurements
The catalytic evaluation of dehydration of ethanol to ethylene was performed in a continuous fl ow fixed bed quartz tubular reactor with an inner diameter of 6 mm.About 0.3 g of catalyst was put in the reactor.Previous activation in situ was done in nitrogen fl ow(48 mL/min)for 30 min at 470◦C with a heating rate of 2◦C/min.Pure ethanol(≥99.7%)was syringed by a metering pump(Agilent P200Ⅱseries)into a nitrogen stream,preheated to 120◦C by heating taps before entering the reactor.The total pressure was kept at 0.1 MPa.The catalyst bed was heated electrically.A thermocouple reaching into the catalyst bed was used to measure the temperature during the reaction.The heated gaseous products were analyzed by an online gas chromatography(SP-3420)equipped with hydrogen- fl ame ionization detector(FID)using a Porapak Q packed column for products.
2 Results and discussion
2.1 Catalyst Characterization
2.1.1 Characterization of Nitrogen adsorption
Nitrogen adsorption results are listed in Table 1.Compared to the SAPO-34 molecular sieve,the Fe-modi fied catalyst has larger speci fic surface area and pore volume,which are bene ficial to improve the catalytic activity and stability of catalysts.
In addition,with the increase of Fe loadings,the speci fic surface area and pore volume of the Fe/SAPO-34 was increased initially and then decreased slightly.When the Fe loading was 1.5%,the speci fic surface area and pore volume reached a maximum of 488.25 m2/g and 0.241 cm3/g while the average pore diameter had the minimum 19.78 Å.Furthermore,when the Fe loading exceeded 2.0%the speci fic surface area and pore volume decreased.This is possibly attributed to the deposition of Fe2O3on the catalyst surface.

Table 1 Speci fic surface area(SBET),pore volume and pore diameter of Fe/SAPO-34 samples with the different weight of Fe
2.1.2 XRD
XRD patterns of Fe/SAPO-34 and supporter samples are shown in Fig.1.Fe-modi fied SAPO-34 molecular sieve maintained a complete crystal structure from the diffraction peaks(2 θ=9.8 ˚,12.8 ˚,15.9 ˚and 20.6 ˚).It indicated that Fe-modi fication did not destruct the crystal structure of SAPO-34 molecular sieve.With the increase of Fe loading,the intensity of the diffraction peaks is slightly reduced.However,there was no peak of Fe2O3in the patterns.This indicated that,Fe nanoparticles well dispersed in the pores instead of the surface of the molecular sieves.
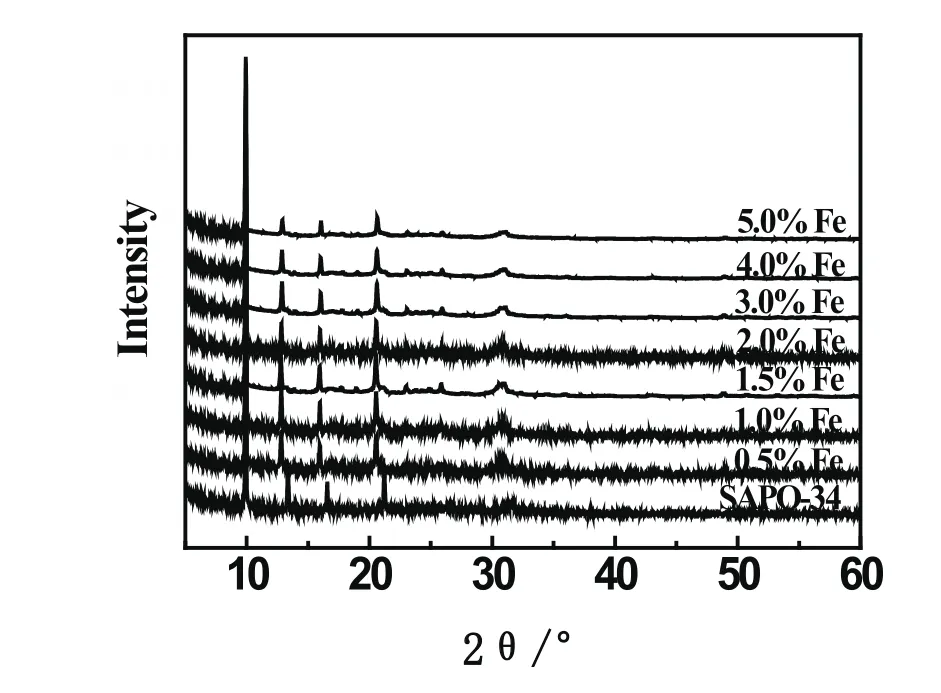
Fig 1 XRD patterns of Fe modi fied SAPO-34 catalysts

Fig 2 NH3-TPD pro files of Fe-modi fied SAPO-34 catalysts
2.1.3 Acidic property of samples
NH3-TPDpatternsofzeolitesupportandFe-modi fiedcatalystsinthetemperaturerangeof50∼600◦Careshownin Fig.2.The desorption temperature indicates the acid strength of samples.The samples showed two peaks from 130◦C to 300◦C and 300◦C to 500◦C which were assigned to weak acid sites and moderately strong acid sites respectively.The acid amounts corresponding to the amounts of adsorbed NH3were estimated from the areas below the NH3-TPD curves.Table 2 is the acid distribution results of samples by Fig.2.From Table 2,the peaks of the weak acid and moderatelystrongacidshiftedtolowertemperatureasFeloadingsrising,indicatingthedecreaseoftheacidstrength.In addition,the moderately strong acid strength dropped obviously.Table 2 showed that desorption peak area decreased corresponding to the reduction of the acid amount of Fe-modi fied catalyst.With the Fe loading of 1.5%,the weak acid amount had the minimum value while the moderately strong acid had the maximum value.This may be due to the deposition of Fe2O3on molecular sieve which reduced the weak acid amount and the substitution of Fe3+into molecular sieves which reduced the amount of moderately strong acid.
2.1.4 H2-TPR
The TPR pro files of Fe-modi fied catalysts were showed in Fig.3.It can be found that Fe/SAPO-34 catalysts with different Fe loadings at 400◦C have obvious reduction peaks I,and the reduction peak corresponded to Fe3+→ Fe2+reaction progress over Fe/SAPO-34 catalysts.As the Fe loading increasing,the intensity of the peak I become stronger.Especially,as the Fe loading exceeded 2.0%,reduction peaks emerge at 700◦C and 800◦C,marked as peak II and peak III,respectively.The peak II probably corresponded to the Fe2+→Fe reduction.The peak III was ascribed to Fe3+into framework of SAPO-34 molecular sieve and reduced.The results of speci fic surface area and pore volume show that the reduction peaks III are not obvious with low Fe loadings,while they become stronger with high Fe loadings,which may ascribe to the entry of Fe3+into the molecular sieve framework[20].

Table 2 Acid distribution characterization results of Fe-modi fied SAPO-34 catalysts
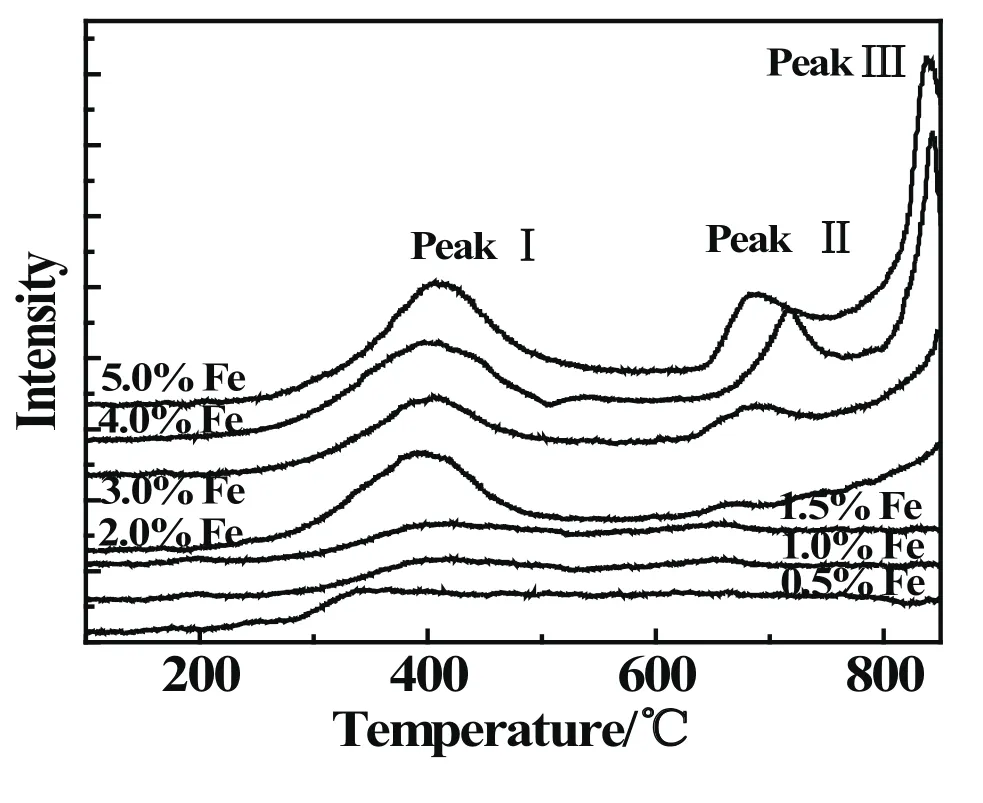
Fig 3 TPR pro files of Fe-modi fied SAPO-34 catalysts
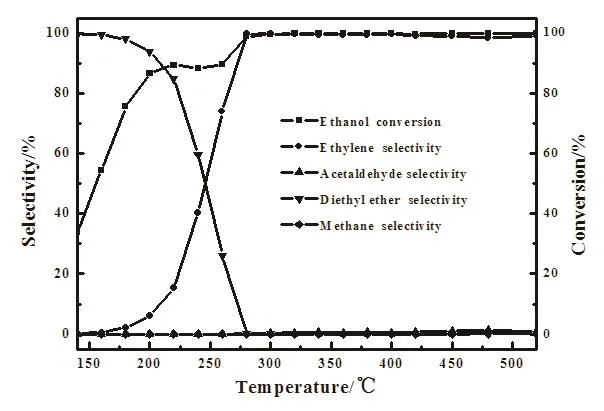
Fig 4 Catalytic performance of Fe/SAPO-34 at different temperatures Reaction conditions:Fe loading 2.0%,WHSV 3.16 h−1,0.3 g catalysts,feed 100%ethanol
2.2 Catalyst performance
2.2.1 Effect of reaction temperature
The temperature effect on Fe/SAPO-34 catalyst for the ethanol dehydration is extremely signi ficant.It can be summarized from Fig.4 that the conversion of ethanol increased with the temperature.The ethylene turned the main product when temperature exceeded 300◦C,the conversion of ethanol and the selectivity of ethylene were more than 99%.While at lower temperature,ethanol dehydration was incomplete and the main dehydration product is diethyl ether.
There were two kinds of processes during ethanol dehydration[21],intermolecular reaction occurred mainly in the low temperature region,in which the diethyl ether is the main product while intramolecular reaction occurred in the high temperature region which produces ethylene.During the initial dehydration process,the ethanol would generate the active C+.C+possessed a long lifetime in low temperature and its probability of encounter with ethanol molecules increased,thus generated diethyl ether.As the temperature increased,the probability of collision of C+with each other increased,which formed ethylene with the lost of protons[22].
2.2.2 Effect of reaction time
Fig.5 showed the effect of reaction time on Fe-modi fied catalyst and SAPO-34 for dehydration of ethanol.The ethanol conversion and ethylene selectivity reduced and the diethyl ether selectivity increased as the reaction time went on.This phenomenon indicated that with the increase of reaction time,coke on the catalysts formed and covered the activity sites which resulted in the ethanol conversion declining;in addition,as a result of the reduce of activity sites,the encounter probability of C+and ethanol molecules increased which led to the selectivity of ethylene decreases and vice products diethyl ether selectivity rises.
Moreover,Fig.5 showed that the activity of SAPO-34 was lower than Fe/SAPO-34 at the same reaction time.Especially,forthereactiontimeof7h,theethanolconversionandtheethyleneselectivityovertheFe/SAPO-34catalyst had been reached for 99%.This may be due to the strength and amount of acidity over Fe-modi fied catalyst reduced,therefore reduced the formation rate of carbon on the catalyst and prolonged the service life of the catalyst.
2.2.3 Effect of loading amount
The ethanol conversion and the ethylene selectivity with Fe loading over SAPO-34 are plotted in Table 3.The Fe loading varied from 0.5%to 5.0%.The results in Table 3 indicated that the catalytic activity over Fe-modi fied SAPO-34 increased signi ficantly compared with SAPO-34 and the value of both the ethanol conversion and the ethylene selectivity achieved more than 95%.In addition,with the Fe loading increased,the ethanol conversion and the ethylene selectivity initially increased and then decreased;when the Fe loading reached 1.5%,the ethanol conversion and the ethylene selectivity achieved the best values of 99.41%and 99.71%,respectively.Accordingly,it can be seen the catalytic activity increased over Fe-modi fied catalyst.However,when the Fe loading exceeded 1.5%,the iron compounds packed on the surface or hole of the molecular sieve and covered a portion of the activated sites,which prevented the dehydration of ethanol and led to the conversion of ethanol and ethylene selective drop.Thus,there existed an optimal Fe loading of 1.5%.

Table 3 Catalytic performance of Fe/SAPO-34 catalysts with the loading amount of metal
2.2.4 Effect of space velocity
When the Fe loading was 1.5%,Fe/SAPO-34 catalyst obtained the preferable ethanol conversion and the ethylene selectivity,therefore the space velocity(3.16-15.82 h−1)were investigated at 300◦C on the catalytic dehydration of ethanol to ethylene.
Fig.6 showed that space velocity had an important effect on Fe/SAPO-34 catalyst for dehydration of ethanol to ethylene.Under the experimental conditions,with space velocity increasing,the ethanol conversion displayed a slow downward trend,in 15.82 h−1dropped to about 89%;the selectivity to ethylene fell fast,the by-product diethyl ether selectivity increased rapidly and its selectivity reached 54%in 15.82 h−1.
The phenomenon is due to at the low space velocity(≤6.33 h−1),the ethanol of raw material had a long contact time with the catalyst and reacted completely,the ethylene selectivity was high(>98%).When the space velocity further increased,the residence time of raw material on the surface of catalyst is shortened,resulting in incomplete reaction of ethanol,which causes the rapidly rise of diethyl ether selectivity,and the reduction of ethylene selectivity.
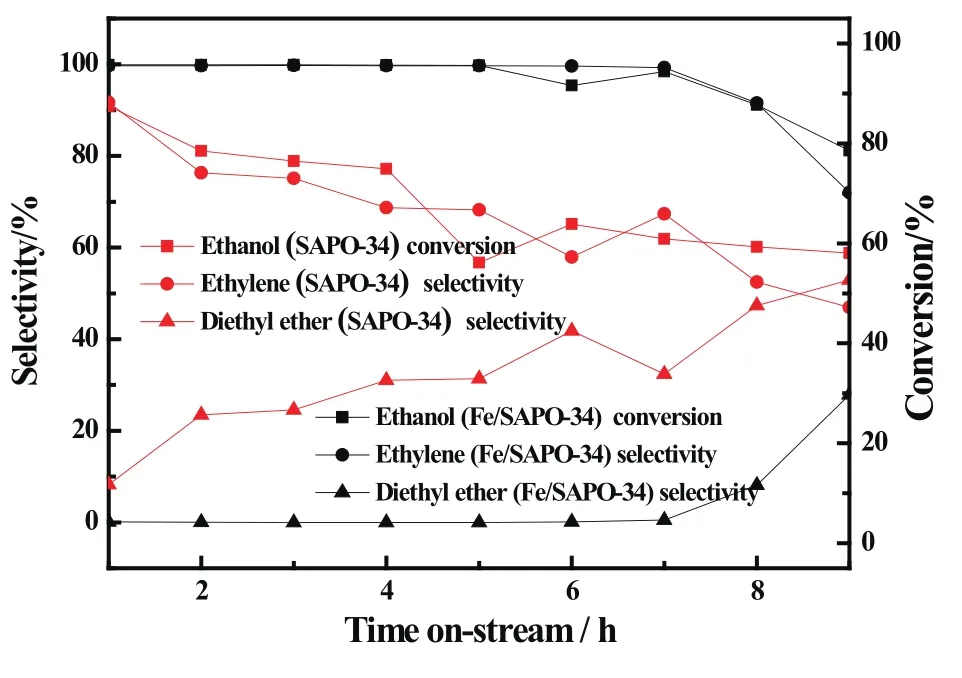
Fig 5 Catalytic performance of Fe/SAPO-34 catalysts with different reaction time(T:300◦C,WHSV:3.16 h−1)
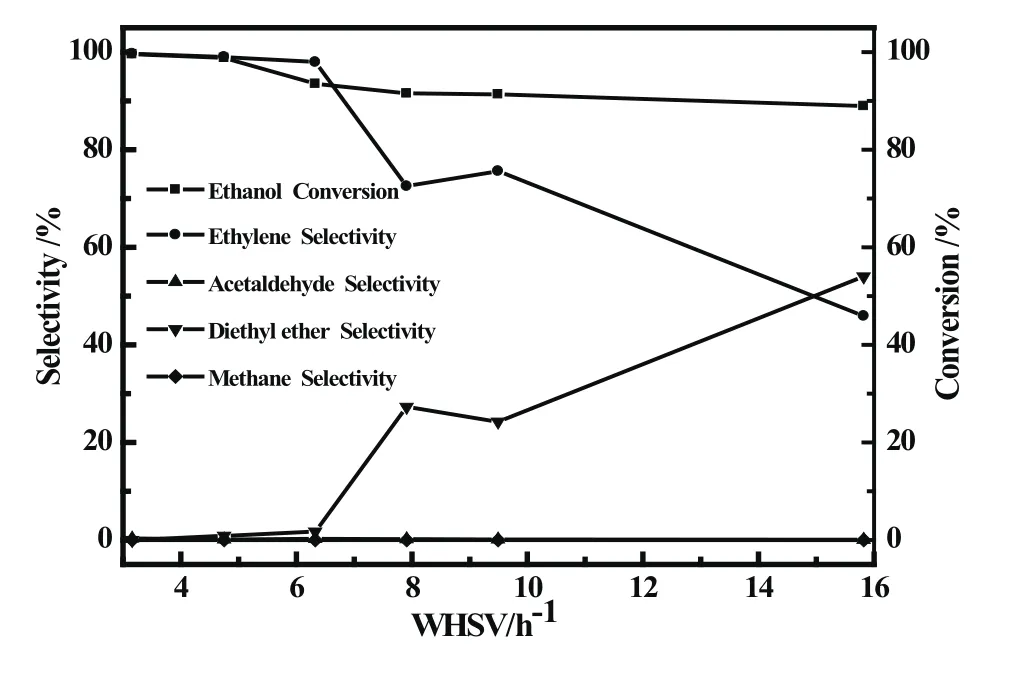
Fig 6 Catalytic performance of A/SAPO-34 catalysts at different space velocity
3 Conclusion
Fe/SAPO-34 was prepared by impregnation method and dehydration of ethanol to ethylene was studied in this work.Characterization showed that Fe-modi fication increased the surface area and decreased the strength and amount of the acid over SAPO-34 and further improved its catalytic activity and stability.The best result with the ethanol conversion and the selectivity to ethylene are respectively 99.88%and 99.76%when the Fe loading of 1.5%,temperature 300◦C,space velocity 3.16 h−1.
References:
[1]Juergen S.Conversion of ethanol over zeolite H-ZSM-5[J].Chem Eng Technol,1986,17:179-186.
[2]Le Van Mao R,Nguyen T M and Mclaugh Lin G P.The bioethanol to ethylene(B.E.T.E)process[J].Appl catal,1989,48:265-277.
[3]Kochar N K,Meeims R and Padia A S.Ethylene from ethanol[J].Chem Eng Progress,1981,77:66-70.
[4]Goldemberg J,Coelho S T and Guardabassi P.The sustainability of ethanol production from sugarcane[J].Energy Policy,2008,36:2086-2097.
[5]Kochar N K,Merims R and Padia A S.Ethylene from ethanol[J].Chem Eng Proc,1981,77:66-70.
[6]Pearson D E.Process for catalytic dehydration of ethanol vapor to ethylene[P].U S,4268606,1983-12-27.
[7]Chang C D,Slivestri A J.The conversion of methanol and other O-compounds to hydrocarbons over zeolite catalysts[J].J Catal,1977,47:249-259.
[8]Kaeding W,Butter S A.Production of chemicals from methanol:I.Low molecular weight ole fins[J].J Catal,1988,61:155-164.
[9]Jacobs J M,Jacobs P A and Uytterhoeven J B.Process for obtaining ethylene from ethanol[P].U S,4727214,1988-12-23.
[10]William R.Silicon-rich HZSM-5 catalyzed conversion of aqueous to ethylene[J].J Catal,1989,117:19-32.
[11]Sawane B,Satoru N and Shigeru T,et al.Ethanol conversion over ionexchanged ZSM-5 zeolites[J].Appl Catal,1990,59:13-29.
[12]Nguyen T M.Conversion of ethanol in aqueous solution over ZSM-5 zeolites[J].Appl Catal,1990,58:119-129.
[13]Le Van Mao R,Nguyen T M and Yao J.Conversion of ethanol in a aqueous solution over ZSM-5 zeolites:in fl uence of reaction parameters and catalyst acidic properties as studied by ammonia TPD technique[J].Appl Catal,1990,61:161-173.
[14]Phillips C B,Datta R.Production of ethylene from hydrous ethanol on HZSM-5 under mild conditions[J].Ind Eng Chem Res,1997,36:4466-4475.
[15]Takahaha I,Saito M and Inaba M,et al.Dehydration of ethanol into ethylene over solid acid catalysts[J].Catal Lett,2005,105:249-252.
[16]Juergen S.Conversion of ethanol over zeolite H-ZSM-5[J].Chem Eng Technol,1994,17:179-186.
[17]Hocevar S,Levec J.Acidity and catalytic activity of MeAPSO-34(Me=Co,Mn,Cr),SAPO-34,and H-ZSM-5 molecular sieves in methanol dehydration[J].J Catal,1992,135:518-532.
[18]Niekerk J V,Fletcher J C and Cornor C T.Effect of catalyst modi fication on the conversion of methanol to light ole fins over SAPO-34[J].Appl Catal A:General,1996,138:135-145.
[19]Inoue M,Dhupatemiya P and Phatanasri S,et al.Synthesis course of the Ni-SAPO-34 catalyst for methanol to ole fin conversion[J].Microporous Mesoporous Mater,1999,28:19-24.
[20]Chunlei Zhang,Zhiyun Wu.The study of Fe chemical environment of Fe-ZSM-5 molecular sieve[J].Journal of Molecular Catalysis,1995,9:165-171.
[21]Inoue M,Dhupatemiya P and Phatanasri S,et al.Synthesis course of the Ni-SAPO-34 catalyst for methanol to ole fin conversion[J].Microporous Mesoporous Mater,1999,28:19-24.
[22]Auprˆetre F,Duprez D.Bio-ethanol catalytic steam reforming over supported metal catalysts[J].Catal Commun,2002,3:7-263.
- 新疆大学学报(自然科学版)(中英文)的其它文章
- WSNs中基于Chebyshev多项式的可认证密钥协商方案∗
- 新疆双峰驼乳清蛋白组分对人宫颈癌HeLa细胞增殖的抑制作用∗
- 新疆加曼特金矿与斑岩型金矿的对比研究∗
- 具有非倍测度的参数型Marcinkiewicz积分交换子在Hardy空间的估计∗
- Periodic Solution of a Two-species Competitive Model with State-Dependent Impulsive Replenish the Endangered Species∗
- Permanence and Extinction for Nonautonomous SIRS Epidemic Model with Density Dependence∗

