Solid lipid microparticles:An approach for improving oral bioavailability of aspirin
T.H.Gugu,S.A.Chime,*,A.A.Attm
aDepartment of Pharmaceutical Technology and Industrial Pharmacy,University of Nigeria,Nsukka 410001,NigeriabDepartment of Pharmaceutics,University of Nigeria,Nsukka 410001,Nigeria
Solid lipid microparticles:An approach for improving oral bioavailability of aspirin
T.H.Gugua,S.A.Chimea,*,A.A.Attamab
aDepartment of Pharmaceutical Technology and Industrial Pharmacy,University of Nigeria,Nsukka 410001,NigeriabDepartment of Pharmaceutics,University of Nigeria,Nsukka 410001,Nigeria
ARTICLEINFO
Article history:
Received 14 February 2015
Received in revised form 13 June 2015
Accepted 24 June 2015
Available online 14 July 2015
Solid lipid microparticles
Ulcer inhibition
NSAIDs
Anti-inflammation
Lipids
The objectives of the work were to develop a lipid based delivery system for aspirin and to evaluate its physicochemical and pharmacodynamic properties.Aspirin-loaded solid lipid microparticles(SLMs)were formulated by hot homogenization and analysed for their encapsulation efficiency(EE%),in vitro release,particle size,anti-inflammatory and ulcer inhibition properties.Particle size ranged from 33.10±5.85 to 43.50±7.27µm for batches A1 to A3 SLMs loaded with 1,3 and 5%aspirin and containing Poloxamer 407,while batches B1,B2 and B3 formulated with Soluplus as surfactant had particle size range of 31.10±1.46 to 45.60±2.92µm.Batches A1 and B1 containing 1%of aspirin had the highest EE of 70 and 72%respectively.Maximum in vitro release of 95.1 and 93.2%were obtained at 8 h from batches A1 and B1 respectively.SLMs exhibited about 77.8%oedema inhibition,while the reference had 66.7%and ulcer inhibition range of 25-75%.Aspirin-loaded SLMs exhibited good properties and could be used orally twice daily for the treatment of inflammation.
©2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
1. Introduction
Aspirin is one of the oldest drugs used in the treatment of pain and inflammation;however,recently this drug has been prescribed for a host of indications.In addition to its use as an analgesic,anti-inflammatory,and antipyretic agent,it is indicated for use in the prevention and treatment of heart disease and stroke[1].Further studies are under way investigating the potentials of aspirin in boosting the immune system,treating cognitive decline,and lowering the risk of colon and ovarian cancer[1].Despite these attributes,it suffers a lot of side effects with the gastrointestinal(GI)side effect being the most prominent in patients on daily dose of aspirin.Aspirin has been regarded as a potential gastric irritant and studies have shown that the incidence of gastrointestinal side effects may in-crease with regular use[1].Because of this,aspirin is preferably formulated in the form of enteric coated formulations.
Aspirin is a moisture-sensitive drug and can hydrolyse into acetic and salicylic acids when exposed to high humidity and elevated temperatures,coating could subject aspirin tablets to both high temperatures and humidity[1].Mitrevej and Hollenbeck found that a hydrophilic field generated around aspirin crystals under high-humidity conditions and upon combining the drug with certain tablet excipients like hydrophilic disintegrants may lead to condensation of aspirin crystals[2]. Because of all these problems encountered in formulating aspirin tablets,the GI side effects and because of the importance of this drug in the treatment of ailments,a new delivery system that will prevent the GI side effects as well as reduce the rigors involved in formulation is very important so as to enhance patient acceptability of this drug,reduce or eliminate gastric irritation and enhance the efficacy of this drug[1].
Solid lipid microparticles(SLM)are micro-scale drug carriers possessing a matrix made from fatty acid,glyceride, fatty alcohol,and solid wax with high melting points[3].They combine many advantages of drug carrier systems.The amount of drug encapsulated can vary up to 95%for lipophilic and hydrophilic drugs and because they are made from physiological or physiologically related materials,they are well tolerated in living systems.The solid matrix protects loaded labile substances against degradation and it offers the possibility of controlled drug release and drug targeting[4-6].Compared to the polymer microparticles,SLMs have the advantage of better biocompatibility,which minimizes the hazards of acute and chronic toxicity;they possess solid cores which reduce the mobility of incorporated drug and drug leakage from the carriers.They can be produced on a large industrial scale and are easy to produce[7,8].They also have the ability to mask the taste of some drugs and have been shown to enhance the absorption of both hydrophilic and lipophilic drugs and have been shown to protect the GI against the gastric irritation side effects of non steroidal anti-inflammatory drugs(NSAIDs)[5-9].Because of all these quality attributes of SLMs,we decided to investigate its properties on aspirin.Therefore,the aims of the work were to formulate aspirin-loaded SLMs in order to investigate its ability to protect the drug from hydrolysis,and also to study the anti-inflammatory and anti ulcer properties of the formulations.
2. Materials and methods
2.1. Materials
The following materials were used as supplied by the local suppliers with no further modifications:Phospholipon®90H (Phospholipid GmbH,Köln,Germany),sorbic acid(Sigma®Chemical Company,USA),sorbitol(Qualikems Laboratory Reagent,India),Poloxamer®407(Synochem City,Germany), Soluplus®,sodium hydroxide,monobasic sodium phosphate (Merck,Darmstadt,Germany),goat fat(Quarter market,Awka, Nigeria),activated charcoal(Bio-Lab,UK),aspirin(Evans Pharmaceutical Ltd.,England),and distilled water(Lion Water, Nsukka,Nigeria).
2.2. Extraction of goat fat
The fat was extracted by grating the adipose tissue prior to boiling with half its weight of water on a water bath for 45 min. Molten fat was separated from the aqueous phase using a muslin cloth.Further purification was carried out by heating a 2%w/w suspension of a 1:9 ratio blend with activated charcoal and bentonite at 80-90°C for 1 h.Thereafter,the suspension was vacuum-filtered using Buchner funnel[10].
2.3. Preparation of lipid matrix(LM)
The lipid matrix was prepared by fusion using Phospholipon®90H(30 g)and purified goat fat(70 g).The lipids were weighed and melted together in a beaker placed on a magnetic stirrer hot plate(SR1 UM 52188,Remi Equip.,India)at 70°C and stirred with a glass stirrer until a transparent homogenous white melt was obtained.The lipid matrix was stirred continuously until it solidified at room temperature[6].
2.4. Formulation of the SLMs
The aspirin-loaded SLMs were prepared using the melt homogenization technique according to the formula presented in Table 1.In each case,5 g of the lipid matrix was melted at 80°C on a water bath and an appropriate amount of aspirin was incorporated into the lipidic melt.Sorbitol,Soluplus or poloxamer as the case may be were dissolved in hot distilled water at the same temperature with the lipidic melt.The hotaqueous phase was transferred into the molten lipid and immediately homogenized with Ultra-Turrax(T25 Basic,Digital, Ika Staufen,Germany)at 7200 rpm for 10 min.SLMs containing no drug(unloaded lipospheres)were also formulated[10,11].

Table 1-Formula for different batches of SLMs.
2.5. Lyophilization of liquid SLMs
A 50 ml quantity of the SLMs was lyophilized using a freezedryer(Amsco/Finn-Aqua Lyovac GT3,Germany),the sample formulation was added into 250 ml conical flask,the flask was attached to the vacuum pressure pump and the formulations were lyophilized in order to obtain water free SLMs.
2.6. Determination of percentage yield
The percentage yield was determined after lyophilization using the equation:
where W1is weight of SLM(g),W2is weight of drug loaded(g) and W3is weight of excipients(g)[12].
2.7. Differential scanning calorimetric analysis
A 1 mg quantity of the individual lipids,the lipid matrices and the SLMs were weighed into an aluminium pan,hermetically sealed and the thermal behaviour determined using a calorimeter(Netzsch DSC 204 F1,Germany),in the range of 10-400°C at a heating rate of 5°C/min.Baselines were determined using an empty pan,and all the thermograms were baseline corrected.
2.8. Particle size and morphology determination
The particle size of the SLMs was determined by computerized image analysis of at least 100 microparticles.The lyophilized SLMs were dispersed in little amount of distilled water on a microscope slide,covered with a cover slip and imaged under a binocular microscope(Leica,Germany)attached with a Motic image analyser(Moticam,China)at a magnification of×400.The particle morphologies were also observed and photomicrographs were taken.
2.9. Time-dependent pH stability studies
About 5%of the lyophilized lipospheres were prepared in distilled water and the pH of dispersions of drug loaded and unloaded lipospheres were determined in a time dependent manner:1 day,30 days and 90 days using a pH meter(pH ep®Hanna Instruments,Padova,Italy).
2.10. Drug content and encapsulation efficiency analysis
Beer-Lambert’s plot of aspirin was obtained at a concentration range of 0.1-0.8 mg%using water as the medium and at a predetermined wavelength of 300 nm.The drug content was determined in the unlyophilized SLMs immediately after preparation and also in the lyophilized formulations.A 5 ml quantity of unlyophilized SLMs from each of the batches were centrifuged at 1252×g for 30 min(Chem.Lab.Instrument,UK).The supernatant was diluted with water,filtered using a filter paper (Whatman no.1)and analysed in spectrophotometer(UNICO 2102 PC UV/Vis Spectrophotometer,USA).The actual drug content(ADC)was determined by subtracting the actual mass of aspirin in the supernatant from the total amount of aspirin incorporated in the formulation(or the theoretical drug content).
The experiment was repeated using the 0.5 g of lyophilized formulation;the SLMs were extracted by triturating with distilled water,filtered and analysed as above for drug content. The experiment was repeated for each formulation and encapsulation efficiency(EE%)was calculated from the equation below:
where,ADC is the actual drug content and TDC is the theoretical drug content.
2.11. In vitro release studies
Beer’s plot was obtained for aspirin in simulated intestinal fluid (SIF)(pH 7.2)at a concentration range of 0.1 to 0.8 mg/ml at a predetermined wavelength of 300 nm.The USP paddle method was adopted in this study.About 900 ml of SIF(pH 7.2)maintained at a temperature of 37±1°C was used for the study.The dialysis membrane containing the formulation was placed inside a tightly secured basket and the basket was placed in the bottom of the beaker.The paddle was rotated at 100 rpm. About 5 ml was withdrawn from the dissolution medium at 0.5,1.0,1.5,2.0,3,5 and 8 h,filtered with a non adsorbent filter paper(Whatman No.1)and analysed using a spectrophotometer(UNICO 2102 PC UV/Vis Spectrophotometer,USA)at 300 nm. An equal volume of the withdrawn sample was replaced with a fresh medium to maintain sink condition in each case.The amount of drug released at each time interval was determined with reference to the standard Beer’s plot.The experiment was repeated two times for each sample and the mean was calculated.
2.12. In vitro release kinetics
The dissolution data obtained were analysed to determine the in vitro release kinetic mechanism using four kinetic models including the first order,Higuchi square root equation and Ritger-Peppas empirical model as shown:
where Q is the percentage drug released at time,t and K1,K2and K3are the rate constants of first-order,Higuchi and Ritger-Peppas models,respectively.Mt/M∝is fraction of drug released at time t,n is diffusion exponent and is indicator ofthe mechanism of transport of drug through the formulation,k is kinetic constant(having units of t-n)incorporating structural and geometric characteristics of the delivery system [13-16].
2.13. Pharmacodynamic studies
2.13.1. Anti-inflammatory studies
The anti-inflammatory activity of aspirin-loaded SLMs was carried out using the rat paw oedema test[17].All animal experimental protocols were carried out in accordance with guidelines of the Animal Ethics Committee of the Faculty of Pharmaceutical Sciences,University of Nigeria,Nsukka.The phlogistic agent employed in the study was fresh undiluted egg albumin.Wistar rats of either sex(90-150 g)were divided into eight experimental groups of five rats per group.The animals were fasted and deprived of water for 12 h before the experiment.The deprivation of water was to ensure uniform hydration and to minimize variability in oedematous response[18].The aspirin-loaded SLMs(Batches A1,A2,A3,B1, B2 and B3)equivalent to 200 mg/kg body weight was administered orally to the rats(Groups A-F).The reference group received 200 mg/kg of pure sample of aspirin(Batch G),while the control group received normal saline(10 ml/kg)(Batch H). Thirty minutes post treatment,oedema was induced by injection of 0.1 ml fresh undiluted egg albumin into the sub plantar region of the right hind paw of the rats.The volumes of distilled water displaced by treated right hind paw of the rats were measured using plethysmometer before and at 30 min, 1,1.5,2,2.5,3,3.5 and 4 h after egg albumin injection.The antiinflammatory activity was calculated at each time as per cent inhibition of oedema using the relation:
where,Vt is the volume of oedema at corresponding time and Vo is the volume of oedema in control rats at the same time [11,19-21].
2.13.2. Ulcerogenicity of SLMs
The studies were carried out on healthy Wistar rats(150-210 g).The animals were divided into eight experimental groups of five animals per group.The aspirin-loaded SLMs(Batches A1,A2,A3,B1,B2 and B3)equivalent to 200 mg/kg body weight was dispersed in water and administered orally to the rats (Groups A-F)using a syringe.The reference group received 200 mg/kg of pure sample of aspirin(Batch G),the control group received normal saline(10 ml/kg)(Batch H),while the reference group received 200 mg/kg pure sample of aspirin orally. The animals were fasted for 8 h prior to a single dose of either the control or the test compounds,given free access to food and water,and sacrificed 17 h later by ether anaesthesia.The gastric mucosae of the rats were examined under microscope using a 4×binocular magnifier.The lesions were counted and divided into large(greater than 2 mm in diameter),small (1-2 mm)and punctiform(less than 1 mm).For each stomach, the severity of mucosal damage was assessed according to the following scoring system:0-no lesions or one punctiform lesion;1-two to five punctiform lesions;2-one to five small ulcers;3-more than five small ulcers or one large ulcer;4-more than one large ulcer[5,22].
2.14. Data and statistical analysis
Statistical analysis was performed using SPSSVersion 16.0(SPSS Inc.,Chicago,IL,USA).All values were expressed as mean±SD. Data were analysed by one-wayANOVA,followed by the Duncan multiple comparison test.Statistical significance was set at P<0.05.
3. Results
3.1. Differential scanning calorimetry(DSC)
The results of the DSC thermograms of aspirin,goat fat,lipid matrices and drug-loaded SLMs(A1 and B1)are shown in Fig.1 and show that aspirin exhibited sharp endothermic melting peak at 141.1°C.The thermograms of goat fat showed endothermic melting peak at 58.7°C,while the lipid matrix showed two major endothermic peaks at 59.7 and 77.4°C.The DSC thermograms of aspirin-loaded SLMs formulated with Poloxamer 407(A1)and Soluplus(B1)respectively and containing 1%of aspirin showed endothermic peaks corresponding to the composition of the SLM with the endothermic peaks of aspirin at 95.4 and 96.8°C respectively.
The results of the differential scanning calorimetry of the goat fat showed melting peak confirming that it was pure;the DSC thermograms of the lipid matrix(goat fat:P90H)showed that it had reduced crystallinity,therefore there was distortion of the chemical structures of the individual lipid thereby creating spaces for drug localization.This was confirmed by the broad endothermic peaks observed in the lipid matrix(LM). These results,however,were in agreement with the work done by Uronnachi et al.[23],who also prepared SLMs of zidovudine with goat fat and P90H as the lipid matrix.The DSC thermograms of aspirin showed sharp endothermic peak at 141°C which confirmed that the drug used was pure and crystalline and the melting peak was close to that quoted for aspirin in the official book[24].When the drug was incorporated into the SLMs the melting peaks of aspirin were reduced and this showed that the crystallinity of the drug was reduced especially in batch A1.
3.2. Particle size and morphology
The results of the particle size of aspirin-loaded and unloaded SLMs(lyophilized)are shown in Table 2 and showed that particle size ranged from 33.10±5.85 to 43.50±7.27µm for batches A1 to A3 SLMs loaded with 1,3 and 5%aspirin and containing Poloxamer 407,while batches B1,B2 and B3 formulated with Soluplus had particle size range of 31.10±1.46 to 45.60±2.92µm.The results of the particle morphology of aspirin-loaded and bland SLMs are shown in Fig.2 and show that the SLMs were spherical and smooth.The results showed that particle size was not directly proportional to drug loading. However,particle size increased significantly with EE%as shown in Table 2.Also,SLMs containing 1%of drug exhibited the highest particle size than the other formulations(A1 and B1). However,the bland SLMs containing Soluplus as the surfactant(B4)exhibited particle size greater than some of the drug loaded SLMs.This may be due to drug entrapment which may have actuated bending and tilting of the hydrocarbon chains of the lipid matrix with consequent size reduction.Therefore without drug entrapment,there is little or no stress impinged on the amphiphilic particle.Hence the observed larger particle sizes seen in the unloaded SLMs[11].The particle morphology revealed that the SLMs were spherical.The spherical shape of particles may be attributed to high shear force of the homogenizer[11].Also,the presence of a suitable lyoprotectant (sorbitol)used in the formulation resulted to the smooth nature of the SLMs,even after lyophilization.Particle size however, was not significantly affected by the nature of the surfactant used(P<0.05).

Table 2-Some physicochemical properties of aspirinloaded and unloaded SLMs.
3.3. Encapsulation efficiency(EE%)
The results of the EE%of aspirin-loaded SLMs are shown in Table 2 and show that the SLMs had EE%range of 46.7 to 72.0% in all the batches.However,batches A1 and B1 containing 1% of aspirin recorded the highest EE of 70 and 72%respectively. The results of the EE%showed that generally,the formulations exhibited high EE values;however EE varied directly with particle size and inversely with drug loading.Increase in drug loading decreased significantly the EE%of the SLMs.This may be due to saturation of the lipid matrices with increased drug content.Therefore,to reduce this problem,the lipid matrix may be increased above the amount used.
3.4. The pH of the SLMs
The results of the pH of the SLMs are shown in Fig.3 and show that the pH of aspirin-loaded SLMs increased from 2.4 to 3.5 for batchA1 SLMs,but this increase was not significant(P<0.05); however,the pH remained in the acidic region all through the study.The pH of the unloaded SLMs also increased from 5.9 to 6.6 at 14 days and later decreased at 30 and 90 days.However, the aspirin pure sample retained a stable pH throughout the study.The results of the pH of the aspirin-loaded SLMs showed an increased pH over time;however,these increases were not significant(P<0.05).The unloaded SLMs also had pH increase attesting to the fact that the increase was not due to drug degradation.Researchers over time have found that in SLM formulations,pH increases are usually due to the release of free fatty acids from the individual lipids[6,9,12].
3.5. In vitro drug release
The results of the in vitro drug release are shown in Fig.4 and show that drug release at 0.5 h was up to 60%in most of the formulations.At 3 h,about 89.1,72.4 and 80%of aspirin were released from batches A1,A2 and A3 containing Poloxamer 407 and 1,3 and 5%of aspirin,while about 88,72 and 71.2%of drug were released from aspirin-loaded SLMs formulated with Soluplus and containing 1,3 and 5%aspirin(batches B1,B2 and B3)respectively.The results show that maximum releases of 95.1 and 93.2%were obtained at 8 h from batches A1 and B1 respectively.The results of the in vitro drug release of the aspirinloaded SLMs showed that the formulations had very high drug release within the first 0.5-1 h,an effect like a burst release. However,this is often encountered in lyophilized SLMs because of the presence of unencapsulated drug or loosely bound drugin the peripheral region of the SLMs.This effect is however, advantageous in aspirin formulations especially as an analgesic,antipyretic,anti-inflammatory agent and in the management of various heart conditions.This is because an initial high concentration is reached i.e.above minimum effective concentration,before maintaining the dose for a prolonged period.Therefore,this formulation may be used for twice daily application.
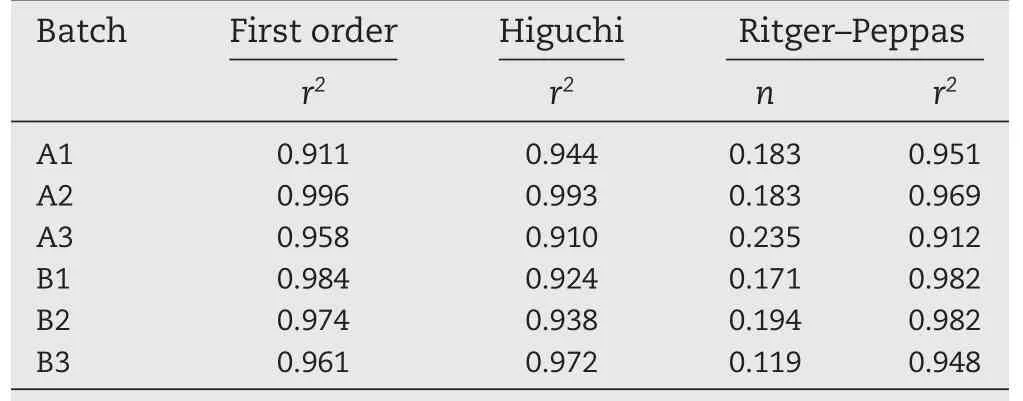
Table 3-In vitro release kinetics.
3.6. In vitro release kinetics
The results of the in vitro release kinetics of the aspirinloaded SLMs are shown in Table 3 and show that the first order plots were linear in all the batches.Also the Ritger-Peppas plots were linear and the n values were significantly less than 0.5 (P<0.05).The regression values(r2)obtained from Higuchi plots also were linear showing that diffusion was implicated as one of the mechanisms of drug release from the SLM formulations.The results showed that the formulations followed first order release kinetics.However,the results of the Higuchi plots revealed that the release mechanism involved diffusion controlled process.Therefore,the release mechanism involved not only dissolution controlled process but also,diffusion.The Ritger-Peppas mechanism revealed that the formulations followed Fickian diffusion release(n≤0.43,non swellable spheres) [14-16].

Table 4-Anti-inflammatory properties of aspirin-loaded SLMs.
3.7. The anti-inflammatory properties
The results of the anti-inflammatory properties of the aspirinloaded SLMs are shown in Table 4 and show that at T1.5h(i.e. 1.5 h)about 78.3 and 84%of oedema inhibition were obtained in rats that received batchesA3 and B1 respectively,while the reference group(G)had 84%oedema inhibition.Also,atT3h, batches A3 and B1 had oedema inhibition of 72.2 and 77.8%, while the reference had 66.7%.The results showed that the formulations exhibited significant higher anti-inflammatory properties than aspirin pure sample used as the reference drug (P<0.05).The phospholipid component of the SLM was responsible for improved anti-inflammatory activity.The phospholipid constituent(lecithin)was believed to facilitate drug absorption via lymphatic circulation.Lipids from lymph lipid precursor pool(LLPP)are known to assemble into lipoproteins which are transported from the enterocyte via the lymphatic system into the systemic circulation[11].This explains the reason for the higher anti-inflammatory properties of the SLM formulations.
3.8. Ulcer inhibition properties
The results of ulcer inhibition properties of aspirin-loaded SLMs are shown in Table 5 and show that the SLMs had per cent ulcer inhibition range of 25-75%with lesions ranging from 1 to 2 mm in diameter.The results also revealed that the formulations exhibited significantly higher ulcer inhibition than the reference drug(P<0.05).These results however,were in agreement with the work done by Obitte et al.[11],Chime et al.[5],and Momoh et al.[9],who individually found out that SLM formulations inhibited the ulcer potentials of most NSAIDs.
4. Conclusion
This research has proven that aspirin could be formulated as SLMs.The need for a new delivery system for this drug cannot be over emphasized.This is because SLM formulations of this drug significantly enhanced absorption.Other advantagesinclude elimination or inhibition of ulcers in patient on long term therapy,better disease management,ease of formulation and scale,and safety in terms of excipient used in formulation.There is a need to further study this formulation and all its aspects in order to ensure that aspirin SLMs get to the market soon so that patients would be able to benefit from them.

Table 5-The ulcer inhibitory properties of aspirinloaded SLMs.
AcAcknowledgements他是山东省章丘市食品药品监管局的负责人。在他的带领下,该局先后荣获全国食品药品监管系统先进集体、省食品药品监管系统先进集体和先进基层党组织、省级文明单位等荣誉称号。
The authors wish to thank Phospholipid GmbH,Köln,Germany for the gift of Phospholipon 90H used in this study.
REFERENCES
[1]Charles RC,Bruce RK,Laura KS.Formulation of acetylsalicylic acid tablets for aqueous enteric film coating. Pharm Tech Drug Del 2001;13(5):44-53.
[2]Mitrevej A,Hollenbeck RG.Influence of hydrophilic excipients on the interaction of aspirin and water.Int J Pharm 1983;14:243-250.
[3]Long C,Zhang L,Qian Y.Preparation and crystal modification of ibuprofen loaded solid lipid microparticles. Chinese J Chem Eng 2006;14(4):518-525.
[4]Eradel MS,Gugor S,Ozsoy Y,et al.Preparation and in vitro evaluation of indomethacin loaded solid lipid microparticles.Acta Pharm.Sci.2009;51:203-210.
[5]Chime SA,Attama AA,Builders PF,et al.Sustained release diclofenac potassium-loaded solid lipid microparticle,based on solidified reverse micellar solution(SRMS):in vitro and in vivo evaluation.J Microencapsul 2013;30(4):335-345.
[6]Umeyor EC,Kenechukwu FC,Ogbonna JD,et al.Preparation of novel solid lipid microparticles loaded with gentamicin and its evaluation in vitro and in vivo.J Microencapsul 2012;29(3):296-307.doi:10.3109/02652048.2011.651495.
[7]Milak S,Medicott N,Tucker IG.Solid lipid micro particles containing loratidine prepared using a micro mixer. J Microencapsul 2006;23:823-831.
[8]EL-Kamel HA,AL-Fagih MI,Alsarra AI.Testosterone solid lipid micro particles for transdermal drug delivery formulation and physicochemical characterization. J Microencapsul 2007;24(5):457-475.
[9]Momoh MA,Akpa PA,Attama AA.Phospholipon 90G based SLMs loaded with ibuprofen:an oral anti-inflammatory and gastrointestinal sparing evaluation in rats.Pakistan J Zool 2013;44(6):1657-1664.
[10]Attama AA,Nkemnele MO.In vitro evaluation of drug release from self micro-emulsifying drug delivery systems using a biodegradable homolipid from Capra hircus.Int J Pharm 2005;304-310.
[11]Obitte NC,Chime SA,Ibe DC,et al.Piroxicam solid lipid microparticles:in vitro and in vivo evaluation.Am J Pharm Tech Res 2013;3(3):324-336.
[12]Attama AA,Okafor CE,Builders PF,et al.Formulation and in vitro evaluation of a PEGylated microscopic lipospheres delivery system for ceftriaxone sodium.Drug Deliv 2009;16:448-616.
[13]Rawat SM,Singh D,Saraf S.Formulation optimization of controlled delivery systems for antihypertensive peptides using response surface methodology.Am J Drug Discov Dev 2011;1(3):174-187.
[14]Ritger PL,Peppas NA.A simple equation for description of solute release 1.Fickian and non-Fickian release from non swellable device in the form of slabs,spheres,cylinders and discs.J Cont Rel 1987;5:23-36.
[15]Higuchi T.Mechanism of sustained-action medication. Theoretical analysis of rate of release of solid drugs dispersed in solid matrices.J Pharm Sci 1963;52:1145-1149.
[16]Chime SA,Onunkwo GC,Onyishi IV.Kinetics and mechanisms of drug release from swellable and non swellable matrices:a review.Res J Pharm Biol Chem Sci 2013;4(2):97-103.
[17]Winter EA,Risley EA,Nuss GU.Anti-inflammatory and antipyretic activities of indomethacin.J Pharm Exp Ther 1963;141:367-376.
[18]Winter ER,Risley EA,Nuss GU.Carrageenan-induced oedema in hind paw of rats as an assay for antiinflammatory drugs.Proc Soc Exp Biol Med 1962;111:544-547.
[19]Parez GRM.Anti-inflammatory activity of Ambrosia artemisiaefolia and Rheo spathaceae.Phytomedicine 1996;3:163-164.
[20]Ahmed MM,Qureshi S,Al-Bekairi AM,et al.Antiinflammation activity of Caralluma tuberculata alcoholic extract.Fitoterapia 1993;64:359-362.
[21]Ajali U,Okoye FBC.Antimicrobial and anti-inflammatory activities of Olax viridis root bark extracts and fractions.Int J Applied Res Nat Prod 2009;2(1):27-32.
[22]Chung-Chin M,Santos JL,Oliveira EV,et al.Synthesis,ex-vivo and in vitro hydrolysis study of an indoline derivative designed as anti-inflammatory with reduced gastric ulceration properties.Molecules 2009;14:3187-3197.
[23]Uronnachi EM,Ogbonna JDN,Kenechukwu FC,et al. Properties of zidovudine loaded solidified reverse micellar microparticles prepared by melt dispersion.J Pharm Res 2012;5(5):2870-2874.
[24]British Pharmacopoeia.British Pharmacopoeia,vol.1,2. London:Her Majesty’s Stationery Office;2009.p.443.
*Corresponding author.Department of Pharmaceutics,University of Nigeria,Nsukka 410001,Nigeria.Tel.:+234 8061329790;fax:+234 42 771709.
E-mail addresses:salome.chime@unn.edu.ng;emmymarachi@yahoo.com(S.A.Chime).
Peer review under responsibility of Shenyang Pharmaceutical University.
http://dx.doi.org/10.1016/j.ajps.2015.06.004
1818-0876/©2015 The Authors.Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2015年5期
Asian Journal of Pharmacentical Sciences2015年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Lipid-albumin nanoassemblies co-loaded with borneol and paclitaxel for intracellular drug delivery to C6 glioma cells with P-gp inhibition and its tumor targeting
- The characterization and dissolution performances of spray dried solid dispersion of ketoprofen in hydrophilic carriers
- Preparation and evaluation of nattokinaseloaded self-double-emulsifying drug delivery system
- Self nano-emulsifying drug delivery system for Embelin:Design,characterization and in-vitro studies
- Sustained release donepezil loaded PLGA microspheres for injection:Preparation,in vitro and in vivo study
- Transdermal delivery of fluorescein isothiocyanate-dextrans using the combination of microneedles and low-frequency sonophoresis
