Influence of Ammonium Ion on Nitrolysis of 3,7-Dinitro-1,3,5,7-tetraazabicyclo[3,3,1]nonane (DPT)
HUANG Xiao-chuan, YU Tao, QIU Shao-jun, GUO Tao, TANG Wang, GE Zhong-xue, MENG Zi-hui, XU Zhi-bin
(1. Xi′an Modern Chemistry Research Institute, Xi′an, 710065,China; 2. Department of Applied Chemistry, School of Chemical Engineering & Environment, Beijing Institute of Technology, Beijing 100081,China)
1 Introduction
Octahydro-1,3,5,7-tetranitro-1,3,5,7-tetrazocine (HMX) is one of the most popular explosive compounds with high density, high energy and excellent thermal stability. Reported manufacturing process for HMX involves the nitrolysis of hexamine in the ternary systems including nitric acid, ammonium nitrate and acetic anhydride, among which the Bachmann process is the most classic method[1]. However the expensive acetic anhydride leads to a relatively high cost of HMX in Bachmann process, and then its application was greatly limited in high energetic explosive. Owing to the above reason,a new preparation process of HMX through the key precursor, 3,7-dinitro-1,3,5,7-tetraazabicyclo[3,3,1]nonane(DPT)[2-3], has attracted great attention due to the low cost, high security and convenient performance. The nitrolysis of DPT is the committed step in DPT process. Different nitrolysis methods for DPThave been explored intensively, among which the HNO3-NH4NO3method is most promising[4-8]. Compared to the Bachmann process, the cost of HMX is significantly reduced by employing the HNO3-NH4NO3method.


Scheme 1 The nitrolysis of DPT in nitric acid-ammonium nitrate or pure nitric acid

2 Experiment section
2.1 Reagents and Instruments
DPT was prepared by Xi′an Modern Chemistry Research Institute[3], with the purity over 99%. HNO3was purified before use. Others are analytical reagents and commercially available without further purification.
Melting points were determined microscopically on an X-5 precision micro melting point apparatus.1H NMR spectra were recorded on a Bruke AV-400 spectrometer, FT-IR (KBr) spectra were recorded on a PerkinElmer FT-IR spectrometer, and mass spectra were collected on a HP5989B mass spectrometer. The purity of HMX was checked on Shimadzu LC-2010 HPLC.
2.2 The nitrolysis of DPT in nitric acid-ammonium salt or nitric acid-nitrate
The preparation process of HMX as used in this research was a modification on the original HNO3-NH4NO3method for the nitrolysis of DPT[5]. In a three necked round bottomed flask equipped with magnetic stirring assembly, 10.0 mL of HNO3and a certain amount of ammonium salt or nitrate were mixed. The temperature of the nitrolysis system was maintained at (5.0±1.0) ℃. 2.0 g of DPT was added slowly into flask, and the system temperature would rise to 7-8 ℃. After DPT addition, the reaction was continued for another 30 min and then the temperature was increased to and kept at 25 ℃ for 40 min. Then, the reaction mixture was poured into ice water. The solution was neutralized by ammonia and pyrolyzed at 90 ℃.HMX was washed several times with water and then dried.
The sample was characterized. Melting point: 279-281 ℃; FT-IR (ν/cm-1): 1559.28, 1461.88, 1394.96, 1201.94, 1143.63, 1086.52, 945.72, 760.31 and 599.70;1H NMR (DMSO-d6),δ=6.02 (8H, singlet); MS:[M+Cl]-~331, [M+HCOO]-~341, [M+NO3]-~358. Purity: 98.6%.
2.3 Calculation methods
The optimized geometries and correlative energies of the reactants(R), transition states (TS), and products (P) were generally calculated at B3LYP/6-31G (d,p) level. The exchange-correlation potential in the B3LYP DFT method was constructed from Becke’s three parameter formula (B3) foe exchange[13]along with the Lee-Yang-Parr parameterization for correlation (LYP)[14]. 6-31G (d,p) was a split-valence double-zeta plus polarization basis set[15]. The structures and imaginary frequencies of transition states were confirmed by the calculation of both vibration analysis and the intrinsic reaction coordinate (IRC)[16-17]at the same level. All compounds had true minima on their potential energy surface without imaginary frequency and the transition state had only one imaginary frequency. All calculations were performed on a Lenovo T350 server with two Intel Xeron 5620 processors using the Gaussian 09 software package[18]in our laboratory.
3 Results and discussions
3.1 The nitrolysis of DPT via nitric acid-ammonium salt

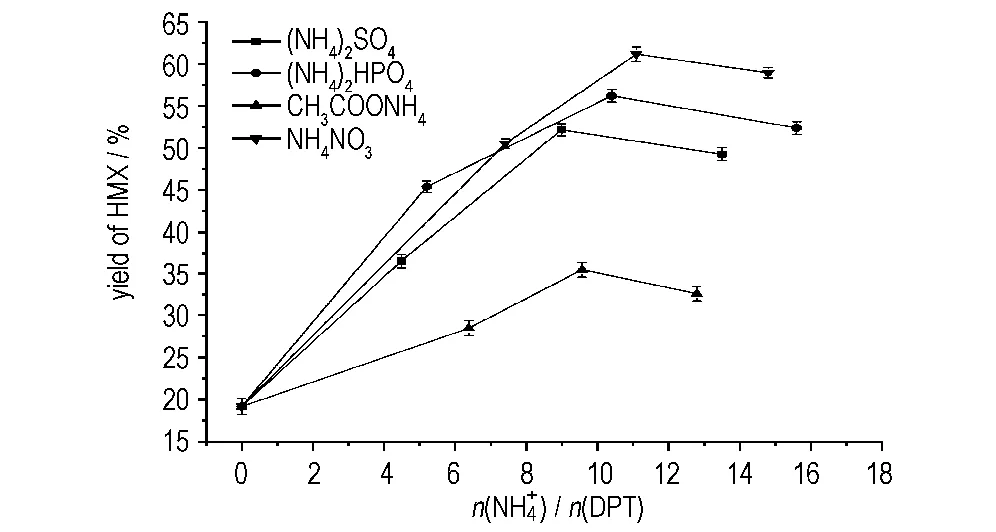


3.2 Comparison of the role of and in DPT nitrolysis




3.3 Mechanism of in the nitrolysis of DPT




4 Conclusions



[1] Bachmann W E, Sheehan J C. A new method of preparing the high explosive RDX[J].JournaloftheAmericanChemicalSociety, 1949, 71(5): 1842-1845.
[2] Radhakrishnan S, Talawar M B, Venugopalan S, et al. Synthesis, characterization and thermolysis studies on 3, 7-dinitro-1, 3, 5, 7-tetraazabicyclo [3, 3, 1] nonane (DPT): A key precursor in the synthesis of most powerful benchmark energetic materials (RDX/HMX) of today[J].Journalofhazardousmaterials, 2008, 152(3): 1317-1324.
[3] SONG Hong-yan, WANG Peng, QIN Guang-ming, et al. Reaction mechanism of one-pot synthesis of dinitro pentamethylene tetramine[J].ChineseJournalofOrganicChemistry, 2010 (3): 414-418.
[4] CHEN Li, CHEN Ze-min, CHEN Xin-hu. The exploration of DPT nitrolysis in nitrate - nitrate system[J].ChineseJournalofExplosives&Propellants, 1986 (3):1-5.
[5] CHEN Li, FANG Zhi-jie, CHEN Zhe-min. The exploration of DPT nitrolysis kinetics in nitric acid - ammonium nitrate system[J].ChineseJournalofExplosives&Propellants, 1987 (3): 1-6.
[6] LI Quan-liang, CHEN Jun, WANG Jian-long. Synthesis craft of HMX from 1,5-Meth-ylene-3,7-dintrio-1,3,5,7-tetraazacyclooctane[J].ChineseJournalofEnergeticMaterials(HannengCailiao), 2007, 15(5): 509-510.
[7] HE Zhi-yong, LUO Jun, Lü Chun-xu, et al. Mechanisms and by-products of nitrolysis of 3,7-dinitro-1,3,5.7-tetrazabicyclo[3.3.1]nonane[J].ChineseJournalofEnergeticMaterials, 2012, 20(1): 5-8.
[8] HE Zhi-yong, LUO Jun, Lü Chun-xu, et al. Synthesis of HMX from DPT by green nitrolysis with dinitrogen pentoxide[J].ChineseJournalofExplosives&Propellants, 2010 (2): 1-4.
[9] Mckay A F, Richmond H H, Wright G F. Nitrolysis of hexamethylenetetramine.Ⅱ.nitrolysis of 1,5-endomethylene-3,7-dinitro-1,3,5,7-tetrazacyclooctane(DPT)[J].CanadaJournalofResearch, 1949, 27(B): 23-27.
[10] Bachmann W E, Horton W J, Jenner E L, et al. Cyclic and linear nitramines formed by nitrolysis of hexaminel[J].JournaloftheAmericanChemicalSociety, 1951, 73(6): 2769-2773.
[11] Yu T, Chang H B, Lai W P, et al. Computational study of esterification between succinic acid and ethylene glycol in the absence of foreign catalyst and solvent[J].PolymerChemistry, 2011, 2(4): 892-896.
[12] Klapötke T M, Krumm B, Scherr M, et al. Experimental and theoretical studies on some energetic functionalized trimethylamine derivatives[J].Chemistry-AEuropeanJournal, 2009, 15(42): 11341-11345.
[13] Becke A D. Density-functional thermochemistry. III. The role of exact exchange[J].TheJournalofChemicalPhysics, 1993, 98: 5648-5652.
[14] Lee C, Yang W, Parr R G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density [J].PhysicalReviewB, 1988, 37(2): 785-789.
[15] Hehre W J, Radom L, Schleyer P.AbInitio Molecular Obital Theory[M]. New York: Wiley, 1986.
[16] Gonzalez C, Schlegel H B. An improved algorithm for reaction path following[J].TheJournalofChemicalPhysics, 1989, 90: 2154.
[17] Gonzalez C, Schlegel H B. Improved algorithms for reaction path following: Higher-order implicit algorithms[J].TheJournalofChemicalPhysics, 1991, 95(8): 5853-5860.
[18] Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09, Revision A. 02, Gaussian[CP]. Inc.,Wallingford, CT.
[19] Si Zhenmei. Study on the hydrolysis-nitration mechanism of amide compounds[D]. Beijing: Beijing Institute of Technology, 2011.

