Comparative study on SO2 release and removal under air and oxy-fuel combustion in a fluidized bed combustor
Zheng Zhimin Wang Hui Yang Li Wei Xing Guo Yongjun Guo Shuai Wu Shaohua
(School of Energy Science and Engineering, Harbin Institute of Technology, Harbin 150001, China)
Comparative study on SO2release and removal under air and oxy-fuel combustion in a fluidized bed combustor
Zheng Zhimin Wang Hui Yang Li Wei Xing Guo Yongjun Guo Shuai Wu Shaohua
(School of Energy Science and Engineering, Harbin Institute of Technology, Harbin 150001, China)
SO2release and removal were studied under both the air and oxy-fuel combustion conditions using an anthracite coal from the Jincheng mine in China on a bench-scale fluidized bed combustor (FBC). Special attention was paid to the effects of the combustion atmosphere, O2concentration, bed temperature, and limestone addition. The released amount of SO2was clearly higher under 30%O2/70%CO2than that of the air atmosphere. As the O2concentration in O2/CO2mixture increased from 21% to 40%, the released amount of SO2increased significantly, but then it decreased when the O2concentration increased up to 50%. The bed temperature from 860 to 920 ℃ has no obvious influence on the the SO2release but shows a strong influence on the desulfurization with limestone in both oxy-fuel and air conditions. The maximum SO2removal efficiency appears to be at 880 to 900 ℃ for both the air and oxy-fuel combustion conditions.
oxy-fuel combustion; fluidized bed; SO2release; limestone desulfuration
Oxy-fuel combustion is one of the most advanced technologies for carbon capture and storage(CCS).Oxy-fuel circulating fluidized bed (CFB) combustion as one type of oxy-fuel combustion has received special attention due to its wide adaptation in fuels, low NOxemissions, and, in particular, in furnace desulphurization. In recent years, a wide range of research involving oxy-fuel CFB has been carried out[1-5]. Despite that, research on the release and removal of SO2in oxy-fuel CFB is still controversial.
There have been some studies on the difference in SO2release between air and oxy-fuel combustion without desulfurization. Tan et al.[6]found that even though the concentration of SO2in oxy-fuel combustion was higher, its mass emission was usually slightly lower than that in air combustion. Wang[7]drew a similar conclusion and he proposed that the lower SO2emission in oxy-fuel combustion was due to the higher capture of fly ash towards sulfur. Duan et al.[8]showed that the release of SO2in oxy-fuel combustion was significantly higher than that in air combustion when oxygen concentration was higher than 30% in oxy-fuel combustion. The main reason for the result was that the increase in oxygen concentration led to the increase in bed temperature, and subsequently increased the combustion efficiency and sulfur conversion rate. On the other hand, Zheng et al.[9]believed that the amount of SO2released under both conditions has no obvious difference based on theoretical calculation. Therefore, the difference between SO2release in air combustion and oxy-fuel combustion is still uncertain.
In-furnace desulphurization has also been widely studied. The most popular reagent used for desulphurization is limestone. In terms of the mechanisms of limestone desulfurization in the furnace, two types of reaction mechanisms exist in the operational temperature range (i.e., 850 to 950 ℃) in oxy-fuel combustion. They are direct sulfation and indirect sulfation reaction. When the temperature is at 850 ℃, a high concentration of CO2can suppress CaCO3decomposition, which leads to a direct sulfation reaction. However, it should be mentioned that with the increase in CO2partial pressure, the calcium conversion rate decreases accordingly[10]. Jia at al.[4]found that desulfurization efficiency was only 40.1%, which was far less than 68.4% for air combustion under similar conditions (i.e. the bed temperature: 850 ℃; the Ca/S ratio: 2.5)[11].
In this paper, experiments were conducted on a bench-scale fluidized bed combustor. The effects of combustion atmosphere, oxygen concentration, the bed temperature and limestone addition on the release and removal of SO2were investigated.
1 Experimental Sections
1.1 Fuel, limestone, and bed material
An anthracite coal from the Jincheng mine in China, which is referred to JCA here, was used in the experiment. It was precrushed to granule with particle sizes less than 2.36 mm. The proximate and elemental analysis for the coal are presented in Tab.1.Prior to the experiments, coal samples were exposed to air and dried. Limestone from Shou County in China was used as the sorbent.The particle size for limestone was below 1.18 mm. The chemical compositions of limestone and the resulting ash of JCA are listed in Tab.2 and Tab.3, respectively. The particle size distributions (PSDs) of the coal and limestone are given in Fig.1. Quartz sand with a particle size range of 0.18 to 0.55 mm was used as the bed material.

Tab.1 Proximate and ultimate analyses of JCA expressed on air-dry basis %
Notes: M is the moisture; A is the ash content; V is the volatile; FC is the fixed carbon; LHV is the lower heating value.

Tab.2 Chemical composition of the limestone as sorbent %

Tab.3 Chemical composition of the resulting ash of JCA %

Fig.1 The PSDs of JCA and limestone
1.2 Experimental setup
The schematic diagram of the fluidized bed combustor(FBC) experimental set-up is given in Fig.2. The total height of the combustor is 3 500 mm, including the preheating section of 385 mm, the dense section of 315 mm, and the dilute phase section of 2 800 mm. The inner diameters of the dense section and the dilute section are 51 and 83 mm, respectively, and between them there is a transition connection with a slope of 11°. A cyclone is connected to the outlet of the combustor to capture coarse ash followed by a convective section. The length and the inner diameter of the convective section are 850 and 56 mm, respectively. Three sampling ports are arranged to measure flue gas, fly ash, and ash deposits, respectively. Following the convective section, a high temperature filter bag is incorporated to collect the fly ash. The temperature of the combustor is controlled by an electric heater. Coal is fed with a screw feeder at a steady coal-feeding rate of 5 to 20 g/min. Either air or O2/CO2can be fed into the system separately. A two-gas system makes it easy and simple to switch between air and oxy-fuel combustion.
Before the ignition, quartz sand with the weight of 150 to 200 g is introduced into the combustor. When the temperature of the reactor reaches 800 ℃, coal is introduced into the reactor. Air and oxy-fuel atmosphere can be swit-ched if necessary before ignition. During ignition, a few coal samples were introduced into the reactor. When the temperature reaches around 850 ℃, the amount of coal can be increased to the setting value.

Fig.2 Schematic diagram of the bench-scale fluidized bed combustor(unit: mm)
The difference in the streams of gas flow affected the residence time of the fluidized bed status and particles, concequently affecting the combustion and pollutant emissions. Therefore, it is more reliable to keep the same gas velocity during all the experiments. However, the fact has to be considered that when too much coal is introduced into the furnace, the furnace temperature will become out of control, particularly under the high oxygen concentration in oxy-fuel combustion. Therefore, during the test, the amount of coal and the flow of the gas should be reduced to ensure a stable bed temperature. The running time of each case is about 30 to 60 min.
For comparison among different combustion cases, the concentration of oxygen in the flue gas at the outlet for all conditions is set to be 6%.The concentration of SO2is measured on-line by the Fourier transform infrared spectroscopy (FTIR).
1.3 Data processing
In order to compare the differences in the release of SO2under different combustion conditions, the volume concentration of SO2can be converted into the amount of its release by
(1)

The removal efficiency of SO2is calculated by
(2)
whereC0is the amount of SO2release without limestone, mg/MJ;CLis the amount of SO2release with limestone, mg/MJ.
The utility of limestoneξas sorbent is calculated by
(3)
2 Results and Discussion
2.1 SO2release
2.1.1 Effect of O2concentration on SO2release
SO2release is represented by both the volume concentration and mg/MJ as shown in Fig.3(a) and Fig.3(b), respectively. It can be noted that in Fig.3(a), SO2concentration increases linearly with the O2concentration of 21% to 40% and then increases slowly with the O2concentration of 40% to 50%, deviating from the previous linear curve. The SO2concentration under air combustion is slightly higher than that in oxy-fuel combustion at the same O2concentration of 21%. In Fig.3(b), when the SO2release is represented in mg/MJ, SO2emission increases with the increase in the O2concentration from 21% to 40% in oxy-fuel combustion and then SO2emission decreases with the O2concentration from 40% to 50%. The emission of SO2under air combustion is significantly higher than that in oxy-fuel combustion under the same O2concentration of 21%.

(a)

(b)
Two reasons can be used to explain why SO2emission is so low in oxy-fuel combustion (21%O2). The high thermal capacity but low oxygen diffusivity in the CO2atmosphere reduces the surface temperature of coke particles and then leads to the decrease of SO2release. Meanwhile, the high concentration of CO2can inhibit coke combustion and lead to higher CO formation in the furnace to create a reductive atmosphere. Therefore, SO2can be reduced to COS by CO[12].The substantial increase of oxygen concentration will increase the surface temperature of the particles and enhance the oxidation rate of coke, which will increase the emission of SO2. Meanwhile, the effects of CO formation on the reduction of SO2will be weakened[13-14]. The combined impacts of the above two reasons lead to the increase in SO2emission at concentrations of O2from 20% to 40%.The SO2emission begins to decline when the O2concentration exceeds 40%. There may be two reasons for this. One is that the volume concentration of SO2increases significantly due to the reduction in the total volume flow of flue gas. SO2is easily oxidized to SO3and subsequently forms H2SO4by reacting with H2O at the low temperature of flue gas below 1 000 ℃[15-16]. The other reason is that SO2may react with alkaline earth metal in fly ash[8,15]. Therefore, SO2emission in the oxy-fuel combustion depends highly on O2concentration.
2.1.2 Effect of bed temperature on SO2release
The effect of the bed temperature on SO2release rate is shown in Fig.4 under air and 30%O2/70%CO2combustion. The bed temperature ranges from 840 to 920 ℃. It can be found that SO2does not show any obvious change except to rise slightly above 900 ℃. Generally, SO2release is not influenced by the bed temperature regardless whether in air combustion or oxy-fuel combustion below 900 ℃. As described in Refs.[17-18], there are three main forms of sulfur in coal, including pyrite, organic sulphur, and sulfates. Pyrite will decompose at around 600 ℃, sulfates will decompose over 1 000 ℃, and organic sulfur will decompose at different temperatures ranging from 400 to 1 000 ℃. Part of organic sulfur can be bonded to the coal matrix and retained in the ash due to the incomplete burnt condition. However, the increased temperature will weaken this retention of sulphur in ash as well as increasing the conversion of char[8].

Fig.4 Effect of bed temperature on SO2 release under air and oxy-fuel combustion
2.2 SO2removal
2.2.1 The effect of bed temperature
Fig.5 shows the SO2removal as a function of the bed temperature under both air and oxy-fuel combustion conditions during anthracite combustion with a Ca/S ratio of 2.5.Fig.5(a) shows the effect of bed temperature on SO2emissions using mass concentration, and Fig.5(b) shows the effect of bed temperature on desulfurization efficiency, which are calculated by Eq.(2). It can be seen from Fig.5(a) that the bed temperature has significant effects on SO2emissions under both air and oxy-fuel combustion. The SO2emissions are similar when the temperature varies from 860 to 900 ℃. The SO2emission in oxy-fuel combustion is high at 840 and 920 ℃. Fig.5(b) shows that the desulfurization efficiency increases with the increase in the temperature from 880 to 900 ℃ and then decreases under both air and oxy-fuel combustion. The desulfurization efficiency in air is higher than that in oxy-fuel combustion at 840 and 920 ℃, but a little lower within 870 to 920 ℃. According to the thermodynamic equilibrium curve of CaCO3calcination[9], for oxy-fuel combustion, the CO2concentration can be enriched up to a value as high as 90%. Therefore, the limestone can be surrounded by high CO2concentrations ranging from 40% to 90%. Under such high CO2concentrations, the sorbent can behave in two ways depending on the temperature. At 840 ℃, direct sulphation may be dominant and indirect sulphation may be dominant at temperatures higher than 840 ℃. The sulphation conversions achieved the under indirect sulphation are normally higher than those achieved under the direct sulphation optimum temperature for sulphur[19]. At 840 ℃, direct sulphation is dominant for oxy-fuel combustion, and indirect sulphation for air combustion. Therefore, the desulfurization efficiency for air combustion is higher than that for the oxy-fuel combustion. The reaction is gradually transformed into indirect sulphation as the temperature increases; as a consequence, the maximum desulfurization efficiency can be reached at about 890 ℃. The desulfurization efficiency under the oxy-fuel combustion at this temperature is higher than that under air combustion. The possible reason is that, for air combustion, the long-term heating at high temperatures causes the sintering of the calcined CaO.

(a)
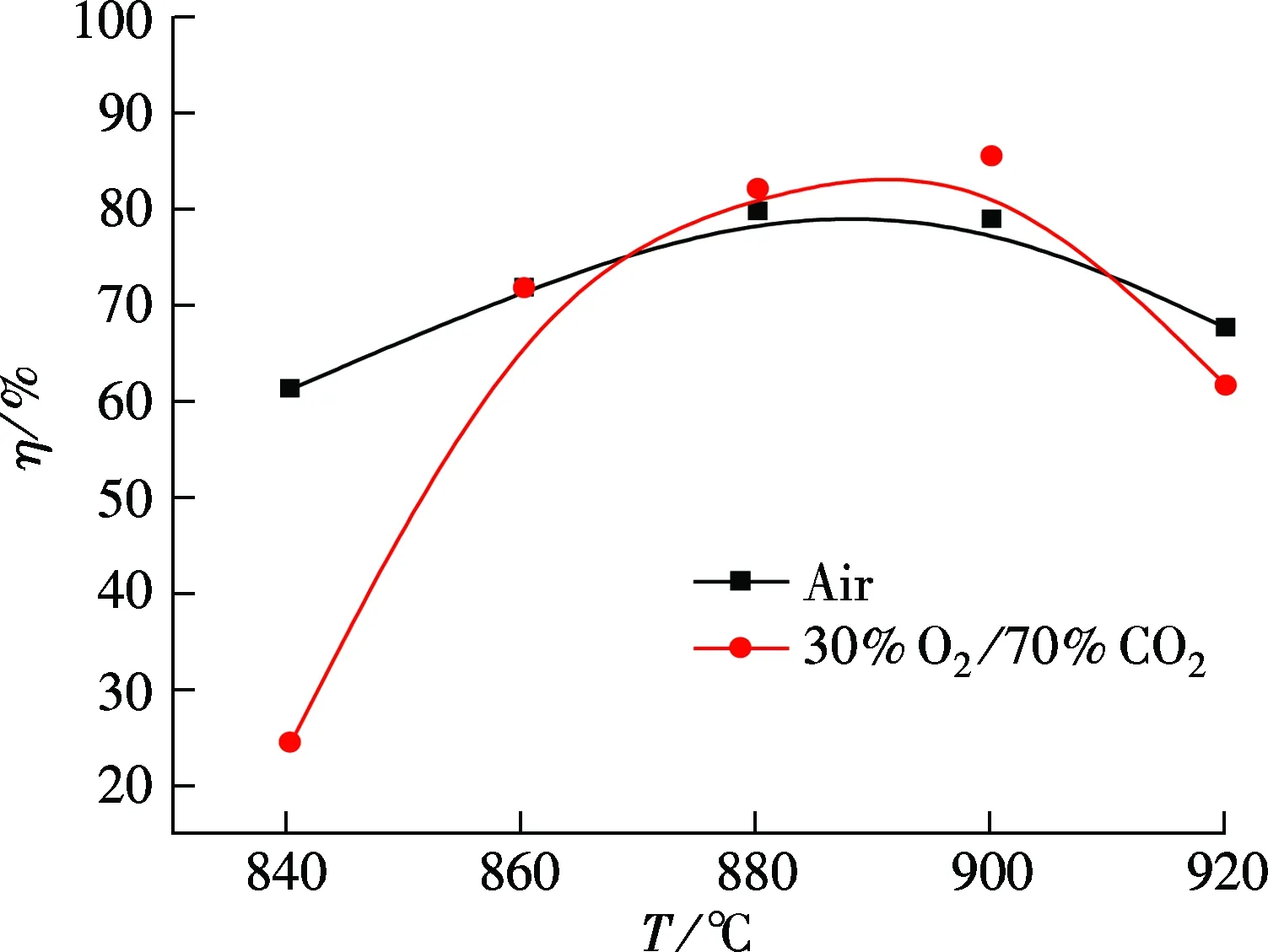
(b)
At 920 ℃, the desulfurization efficiency under the oxy-fuel combustion is lower than that under air combustion, and it may be caused by the intensive sintering of calcined CaO[20-21]. Above all, it is clear that temperature is one of the most important parameters that affect the SO2removal process under both air combustion and oxy-fuel combustion.The maximum desulfurization efficiency is shifted to a higher temperature from air combustion to oxy-fuel combustion.
2.2.2 The effect of Ca/S ratio
The calcium to sulfur ratio is an important parameter in the limestone desulfurization system of a fluidized bed because it has a significant impact on boiler safety and economy. As given in Fig.6, the SO2removal under the same calcium to sulfur ratio is different under air and oxy-fuel combustion, and the difference is most significant when the calcium to sulfur ratio is 1.5. The SO2emission under oxy-fuel is significantly lower than that under the air combustion atmosphere. As shown in Fig.7, the SO2removal efficiency is 51.5% and 77.2%, respectively when the Ca/S ratio is 1.5 under the air and oxy-fuel combustion at 900 ℃.When the calcium to sulfur ratios are 2.5 and 3.5, the removal efficiency are closer under both conditions. This agrees with previous findings[4,8]. According to Fig.8, with the increase in the Ca/S ratio, the utilization of calcium has a downward trend. When the Ca/S ratio is 2.5, limestone utilization is the maximum under the oxy-fuel combustion atmosphere. The low calcium to sulfur ratio is preferable under the air combustion atmosphere, while the Ca/S ratio of 2.5 is appropriate for JCA at 900 ℃ under an oxy-fuel combustion atmosphere.

Fig.6 The effect of Ca/S ratio on SO2 emission at the bed temperature of 900 ℃ under air and oxy-fuel combustion atmosphere

Fig.7 The effect of Ca/S ratio on SO2 removal efficiency at the bed temperature of 900 ℃ under air and oxy-fuel combustion atmosphere

Fig.8 The effect of Ca/S ratio on limestone utilization at the bed temperature of 900 ℃ under air and oxy-fuel atmosphere
3 Conclusion
For JCA, when the oxygen concentration is between 21% and 40%, increasing the oxygen concentration can significantly increase SO2emission. When the oxygen concentration is increased to 50%, SO2has a downward trend. Under the air combustion, SO2emission is slightly higher than that in oxy-fuel combustion (Here, 21%O2/79%CO2), and far lower than these under higher O2concentrationsin oxy-fuel combustion. A bed temperature (860 to 920 ℃) has no significant effect on the emission of SO2. When the Ca/S ratio is 2.5, the effect of bed temperature on SO2removal is the same as that under the air and oxy-fuel combustion atmosphere, but at 840 ℃, the removal efficiency under oxy-fuel combustion is much lower than that under air combustion. Under the air combustion atmosphere, with the increase in the Ca/S ratio (1.5 to 3.5), the utilization ratio of limestone decreases.When the Ca/S ratio is 2.5, the limestone utilization ratio is quite high at the bed temperature of 900 ℃ under the oxy-fuel combustion.
[1]Kuivalainen R, Pikkarainen T, Leino T, et al. Development of CFB technology to provide flexible air/oxy operation for a power plant with CCS[C]//The34thInternationalTechnicalConferenceonCoalUtilization&FuelSystems. Clearwater, FL, USA, 2009.
[2]Nsakala N, Liljedahl G N, Turek D G, et al. Oxygen-fired circulating fluidized bed boilers for greenhouse gas emissions control and other applications [C]//TheSecondAnnualNationalConferenceonCarbonSequestration. Alexandria, VA, USA, 2004.
[3]Jia L, Tan Y, Anthony E J, et al. Emissions of SO2and NOxduring oxy-fuel CFB combustion tests in a mini-circulating fluidized bed combustion reactor [J].Energy&Fuels, 2009, 24(2): 910-915.
[4]Jia L, Tan Y, Wang C, et al. Experimental study of oxy-fuel combustion and sulfur capture in a mini-CFBC [J].Energy&Fuels, 2007, 21(6): 3160-3164.
[5]Duan L, Zhao C, Zhou W, et al. O2/CO2coal combustion characteristics in a 50 kWth circulating fluidized bed [J].InternationalJournalofGreenhouseGasControl, 2011, 5(4): 770-776.
[6]Tan Y, Croiset E, Douglas M A, et al. Combustion characteristics of coal in a mixture of oxygen and recycled flue gas [J].Fuel, 2006, 85(4): 507-512.
[7]Wang L. Experimental and modeling study of SO2behavior during oxy combustion in fluidized beds [D]. Salt Lake City, USA: Department of Chemical Engineering of the University of Utah, 2012.
[8]Duan L, Zhou W, Li H, et al. Sulfur fate during bituminous coal combustion in an oxy-fired circulating fluidized bed combustor [J].KoreanJournalofChemicalEngineering, 2011, 28(9): 1952-1955.
[9]Zheng L, Furimsky E. Assessment of coal combustion in O2+CO2by equilibrium calculations [J].FuelProcessingTechnology, 2003, 81(1): 23-34.
[10]Mao Y, Fang M, Luo Z, et al. Calcination and desulfurization of limestone under O2/CO2atmosphere [J].JournalofFuelChemistryandTechnology, 2004, 32(3):323-328.
[11]Liu H, Katagiri S, Kaneko U, et al. Sulfation behavior of limestone under high CO2concentration in O2/CO2coal combustion [J].Fuel, 2000, 79(8):945-953.
[12]Dong X, Wang H, Liu H, et al. Study on SO2emission under various atmospheres during coal combustion [J].JournalofEnvironmentalSciences, 2003, 23(3):322-326.
[13]Du Y, Wang J, Wang X, et al. Analysis of pollutant discharge of coal combustion in oxygen-enriched atmosphere [J].CoalConversion, 2011, 34(3):75-78.
[14]Liu H, Qiu J, Xu Z, et al. Release of NO and SO2in high-concentration CO2atmosphere during coal combustion [J].JournalofEngineeringThermophysics, 2008, 29(2): 354-356.
[15]Fleig D, Normann F, Andersson K, et al. The fate of sulphur during oxy-fuel combustion of lignite [J].EnergyProcedia, 2009, 1(1): 383-390.
[16]Ahn J, Okerlund R, Fry A, et al. Sulfur trioxide formation during oxy-coal combustion[J].InternationalJournalofGreenhouseGasControl, 2011, 5(S1): 127-135.
[17]Anthony E J, Granatstein D L. Sulfation phenomena in fluidized bed combustion systems [J].ProgressinEnergyandCombustionScience, 2001, 27(2): 215-236.
[18]Miura K, Mae K, Shimada M, et al. Analysis of formation rates of sulfur-containing gases during the pyrolysis of various coals [J].Energy&Fuels, 2001, 15(3): 629-636.
[19]de Diego L F, Rufas A, García-Labiano F, et al. Optimum temperature for sulphur retention in fluidized beds working under oxy-fuel combustion conditions [J].Fuel, 2013, 114:106-113.
[20]Chen C, Zhao C, Liang C, et al. Calcination and sintering characteristics of limestone under O2/CO2combustion atmosphere [J].FuelProcessingTechnology, 2007, 88(2):171-178.
[21]Borgwardt R. Calcium oxide sintering in atmospheres containing water and carbon dioxide [J].Industrial&EngineeringChemistryResearch, 1989, 28(4): 493-500.
流化床空气和富氧燃烧气氛下SO2的释放和脱除特性比较研究
郑志敏 王 辉 杨 利 魏 星 郭永军 郭 帅 吴少华
(哈尔滨工业大学能源科学与工程学院,哈尔滨150001)
在一个小型流化床试验台上比较了晋城无烟煤在空气和富氧燃烧下SO2的释放和脱除特性,考察了燃烧气氛、氧浓度、床温和石灰石的添加等因素的影响.研究结果表明:在30% O2的富氧燃烧条件下SO2的释放量明显高于其在空气燃烧条件下的释放量;随着富氧燃烧气氛中O2浓度的增加(从21%增至40%),SO2的释放量显著增加,当氧浓度达到50%时,SO2的释放量有下降的趋势.床温860~920 ℃对SO2的释放没有明显的影响,但对其脱除有显著的影响.在空气和30% O2富氧气氛下,SO2的最佳脱硫温度均为880~900 ℃.
富氧燃烧;流化床;SO2释放;石灰石脱硫
TK224.1
Foundation items:The National Natural Science Foundation for Young Scholars of China (No.51106038), the National Key Technology R&D Program of China during the 12th Five-Year Plan Period (No.2012BAA02B01-04).
:Zheng Zhimin, Wang Hui, Yang Li, et al. Comparative study on SO2release and removal under air and oxy-fuel combustion in a fluidized bed combustor[J].Journal of Southeast University (English Edition),2015,31(2):232-237.
10.3969/j.issn.1003-7985.2015.02.013
10.3969/j.issn.1003-7985.2015.02.013
Received 2015-01-10.
Biographies:Zheng Zhimin(1983—), male, graduate; Wang Hui(corresponding author), male, associate professor, wanghui_hb@hit.edu.cn.
 Journal of Southeast University(English Edition)2015年2期
Journal of Southeast University(English Edition)2015年2期
- Journal of Southeast University(English Edition)的其它文章
- Construction of crash prediction model of freeway basic segment based on interactive influence of explanatory variables
- Experimental studies on gas-phase mercury oxidation removal and denitration of coal combustion with NH4Br addition
- CO2 capture using dry TiO2-doped Na2CO3/Al2O3 sorbents in a fluidized-bed reactor
- Effect of sulfation during carbonation on CO2 capture in calcium looping cycle
- Synthesis of highly reactive sorbent from industrial wastes and its CO2 capture capacity
- CO2 capture by carbonated carbide slag seriflux after drying in calcium looping cycles
