Preparation of polyimide/LDH nanocomposites and characterization of their properties
GUO Bing-zhi(郭冰之), ZHAO Yun(赵芸), LI Han-sheng(黎汉生), QIU Wei-zhen(丘伟贞), JIAO Qing-ze(矫庆泽),
(1.School of Chemical Engineering and Environment, Beijing Institute of Technology, Beijing 100081, China; 2.Beijing Institute of Technology, Zhuhai, Zhuhai 519088, China)
Preparation of polyimide/LDH nanocomposites and characterization of their properties
GUO Bing-zhi(郭冰之)1,2, ZHAO Yun(赵芸)1, LI Han-sheng(黎汉生)1, QIU Wei-zhen(丘伟贞)2, JIAO Qing-ze(矫庆泽)1,2,
(1.School of Chemical Engineering and Environment, Beijing Institute of Technology, Beijing 100081, China; 2.Beijing Institute of Technology, Zhuhai, Zhuhai 519088, China)
Pre-exfoliated organic layered double hydroxides(LDHs) modified with N-Lauroyl-glutamate (LDH-LG) was prepared in the O/W type microemulsion. Then exfoliated polyimide(PI)/LDH nanocomposites were prepared by in situ polymerization. The high resolution transmission electron microscope showed that the nanoscale organic LDH particles were homogeneously dispersed in the polymer matrix. The X-ray diffraction analysis showed that the addition of LDH-LG improved the crystallinity of PI. Mechanical testing showed that the mechanical properties of the PI/LDH nanocomposite films were significantly enhanced by nano-LDH sheets and 0.5% (wt)of LDH-LG led to improvement of the tensile strength by 32.5%.
nanocomposites; polyimide; layered double hydroxides
Because of the controllable physical and chemical properties of layered double hydroxides (LDHs), polymer/LDH nanocomposites have been widely studied. The versatility of polymer/LDH nanocomposites are expected to be applied in the fields of catalysis, flame retardant and biomedicine[1-3]. However, compared to montmorillonite, the strong electrostatic interaction between highly charged hydroxide layers and the intercalated anions hinders the exfoliation of LDH layers. There are two main methods to prepare polymer/LDH nanocomposites: one is direct intercalation of polymer chains such as polystyrene, poly(ethylene oxide) and polyamide into LDH interlayer[4-5]; the other is to insert polymer monomer such as methyl methacrylate and styrene between the LDH interlayer, then achieve exfoliated polymer/LDH nanocomposites by in situ polymerization[6-7].
Polyimide(PI) is widely used in aerospace, electronics, microelectronics and chemical industries[8-10]due to its excellent chemical stability, thermal stability and mechanical properties. Uniformly dispersed layered inorganic nanomaterials in the PI matrix could further improve the mechanical properties and heat resistance of PI, thus broaden the application areas of PI[11-12].
However, up to now, most studies have been focused on exfoliated PI/montmorillonite nanocomposites[11-14], with only a few reports of exfoliated PI/LDH nanocomposites in recent years. Hsueh and Chen[3]reported the preparation of LDHs/PI nanocomposites. Lü et al.[15]prepared organic ZnAl-LDH/PI electromagnetic shielding composites. Chen et al.[16]prepared PI precursor/LDH ultrathin films through a layer-by-layer self-assembly method.
In this study, we propose a new approach to prepare the exfoliated PI/ LDH nanocomposites. Firstly, LDHs modified with N-Lauroyl-glutamate (LDH-LG) was prepared using a microemulsion method. Then, the pre-exfoliated LDH-LG layers were mixed with N, N-Dimethylformamide (DMF), pyromellitic dianhydride (PMDA) and 4,4’-oxydianiline (ODA). Finally, a completely exfoliated PI/LDH nanocomposite was obtained by in situ polymerization. The microstructure, thermal and mechanical properties of PI/LDH nanocomposites were examined in details.
1 Experimental
1.1 Materials
N-Lauroyl-glutamate sodium(LG-Na),was from AoKe New Material Technology Co., Ltd. (Shanghai, China). Mg(NO3)2·6H2O, Al(NO3)3·9H2O, sodium carbonate, sodium hydroxide and acetone were all purchased from Tianjin Damao Chemical Reagent (Tianjin, China). N-octane was from Tianjin Fuchen Chemical Reagents Factory (Tianjin, China). Calcium hydride was from Tianjin Beidouxing Fine Chemical Co.,Ltd. PMDA, ODA, DMF, xylene and anhydrous methanol were obtained from Alfa Aesar. PMDA and ODA were recrystallized from xylene and anhydrous methanol respectively prior to use. DMF was purified by reduced pressure distillation over calcium hydride and stored over a molecular sieve prior to use.
1.2 Preparation of LDH-LG
The LDH-LG was achieved in a microemulsion that was prepared as follows[17]: A mixture of 50.0 g octane and 4.5 g LG-Na was added into 58.5 mL of de-ionized water, and simultaneously maintained pH value at 10.0 by drop-wise addition of 2 mol·L-1NaOH solution. An aqueous solution containing magnesium nitrate (Mg(NO3)2) and aluminum nitrate (Al(NO3)3) with Mg2+/Al3+molar ratio of 2∶1 was added drop-by-drop into the microemulsion. To maintain the pH value of 10.0 of the system, an aqueous solution of 2 mol·L-1of NaOH was added simultaneously. The obtained slurry was stirred and kept at 85 ℃ for 24 h. The precipitates were filtered and washed with de-ionized water repeatedly to eliminate the unreacted LG-Na, and further washed three times with acetone. Most of the wet product was added to acetone to form the LDH-LG acetone suspension. A part of the wet product was dried under vacuum overnight and ground into white powders. The magnesium/aluminum layered double hydroxides (MgAl-LDHs) with molar ratio of Mg2+/Al3+=2 were synthesized by the nucleation crystallization separation method[18].
1.3 Preparation of PI/LDH nanocomposites
The suspension of LDH-LG in anhydrous DMF was sonicated for 3 h in an ultrasonic bath (KQ-300E,300 W, 40 kHz) at room temperature after removing acetone by rotary evaporation. Then ODA (0.500 0 g, 0.002 5 mol) was added with magnetic stirring and nitrogen protection until ODA was completely dissolved. Then PMDA (0.555 5 g, 0.002 55 mol) was added. The whole polymerization was carried out at 15 ℃ for 4 h. The mixture was cast onto a clean glass sheet using smear casting and dried at 60 ℃ for 4 h in a vacuum drying chamber to produce polyamic acid (PAA)/LDH composite membrane. Subsequently, the membrane was cured at 80, 100, 150, and 200 ℃ respectively for 1 h and at 250, 300 ℃ for 0.5 h in argon to obtain solvent-free PI/LDH nanocomposite. According to this procedure, the LDH-LG was added into the system with the respective concentration of 0.05 to 2.5%(wt) of PI. The resulting samples were named as PI, PI/LDH-1, PI/LDH-2, PI/LDH-3, PI/LDH-4 and PI/LDH-5.
1.4 Characterization
Fourier transform infrared (FTIR) spectra were performed on a Bruker VERTEX-70 spectrometer with a disc of KBr. The X-ray diffraction patterns were recorded on a Shimadzu XRD-6000 X-ray powder diffractometer (Cu Karadiation,λ=0.154 06 nm), the scan ranges of specimens were collected from 2θ=0.5°-70°. The morphology of LDH-LG in PI matrix was obtained using JEOL JEM-2100 transmission electron microscopy (TEM) with an accelerating voltage of 200 kV. The thermogravimetric analysis (TGA) was performed on a Shimadzu TGA-50H thermoanalyzer, the samples were examined under nitrogen atmosphere at a heating rate of 10°C/min. The tensile properties of the nanocomposites were measured on a DEW-30, AGS-J Mechanical Testing System. Sample length and width were 70 mm and 10 mm, and five specimens per measurement were averaged to obtain final value, the strain rate was 5 mm·min-1.
2 Results and discussion
2.1 Structure of the LDH-LG
The structure of LDH-LG synthesized using the microemulsion method was identified by XRD patterns shown in Fig. 1 and Fig. 2. It can be seen that (003) characteristic diffraction peak of LDH materials is not observed in the range of 2°-70° and 0.5°-2°, which is usually at 2θ=11.7°. XRD data suggest that the LDHs nanoplatelets composed of a limited number of layers (1-5) would exhibit a weak and significantly broadened (003) reflection[19]. The resulting LDH-LG with no (003) reflection but the other resolved in-plane reflections shows that the LDH-LG possibly has a monolayer thickness. A similar phenomenon was also reported by Gang Hu et al[19]. In addition to the (003) peak, there is a broad reflection at 2θof around 20°, which is absent in carbonate-intercalated LDHs. This peak is attributed to the scattering of the X-rays by the carbon chain of LG[20].
Fig.3 reveals the TGA curves of MgAl-LDHs and LDH-LG at a heating rate of 10 ℃/min. Two distinguishable weight losses of the MgAl-LDHs are observed in the range of 100-230 ℃ and 300-580 ℃. In the first one, a weight loss of 14.5% is attributed to the evaporation of the physically adsorbed and intercalated water[21]. The second step of weight loss of about 27.5% can be ascribed to the dehydroxylation of the hydroxide layers and elimination of intercalated carbonate anion[21]. The TGA data of LDH-LG exhibits similar tendency, and the temperature of weight loss is almost at the same range as that of MgAl-LDHs. In the first step, the weight loss of 14.5% from room temperature to 230 ℃ can be attributed to the loss of water. The main weigh loss stage occurred at the temperature of 300-580 ℃ is due

Fig.1 Wide-angle XRD pattern of LDH-LG

Fig.2 Narrow-angle XRD pattern of LDH-LG

Fig.3 TG profiles of MgAl-LDHs and LDH-LG
to the thermal decomposition of LG chain on the LDH layer and dehydroxylation of the LDH sheets. The remaining weight percent of the inorganic compositions of the MgAl-LDHs and LDH-LG are 54.3% and 37.8%, respectively. The total weight loss for the same region of LDH-LG is 16.5% which is higher than that for MgAl-LDHs. These results are in agreement with the XRD measurements.
2.2 Structure of PI/LDH nanocomposites
Fig.4 gives the XRD patterns of PI/LDH films with different LDH-LG contents. The pure PI film shows only a single broad peak at 2θ=17.6°, which is corresponding to the literature data[22-23]. Unlike pure PI, XRD patterns of PI/LDH nanocomposites with different content of LDH-LG exhibit the characteristic reflections of PI at 2θof 13.8° and 16.7°. The peak at 2θof 13.8 ° corresponds to diffraction from (101) plane of PI[8].

a-PI/LDH-1;b-PI/LDH-2; c-PI/LDH-3;d-PI/LDH-4; e-PI/LDH-5; f-PIFig.4 XRD patterns of PI and PI/LDH nanocomposites
No diffraction peaks for LDH-LG are observed. The intensity of peaks for PI/LDH nanocomposites varies with increasing LDH-LG contents. Because PI macromolecule is linear rigid macromolecule containing certain flexible groups, in the casting molding process, PI macromolecular chain arraying along the LDH lamellar orientation is easy to spread and orientation arrangement occurs in the external force. The percentage of crystallinity(C) is calculated using the following relation[2]:
C=A1/A×100%
whereAis the total area of the peaks, andA1is the total area under the diffraction pattern. The values of percentage of crystallinity of PI/LDH-1, PI/LDH-2, PI/LDH-3, PI/LDH-4, and PI/LDH-5 nanocomposite films are 39.75%, 39.86%, 40.46%, 39.95%, 38.56%, respectively. It is observed that the crystallinity of PI/LDH composites increases at first and then decreases with increasing the contents of LDH-LG nanosheets.
TEM instrument can be directly employed to visualize the exact intercalation or exfoliation degree of filler in the polymer matrix. Fig.5 shows the HR-TEM micrograph of PI/LDH-4 nanocomposite film. The dark lines present LDH-LG platelets. The HR-TEM observation indicates the formation of completely nanoplatelet-like LDHs. Therefore the exfoliated PI/LDH nanocomposites have been successfully prepared.

Fig.5 HR-TEM micrograph of PI/LDH-4 nanocomposite
2.3 Thermal properties of the PI/LDH nanocomposites
In order to better understand the effect of LDH-LG on the thermal stability of polymer matrix, TGA was used to investigate the thermal degradation of pure PI and PI/LDH nanocomposites. Typical thermogravimetric profiles of weight loss for pure PI and PI/LDH nanocomposites at a heating rate of 10 ℃/min are illustrated in Fig. 6. When 5% weight loss is selected as a point of comparison, the decomposition temperatures for pure PI, and PI/ LDH nanocomposites with 0.05, 0.25, 0.5, 1.5, and 2.5%(wt) of LDH-LG loading are determined to be 558 ℃, 558 ℃, 559 ℃, 563 ℃, 555 ℃ and 550 ℃, respectively. These results show a first increased and then decreased trend. In general, when nanoparticles are added to the polymer matrix, the thermal stability of polymer is improved. When the amount of LDH-LG is less than 0.5%(wt), the dispersion of the LDH-LG nanolayers in the PI matrix enhance the thermal resistance of the PI/LDH nanocomposites because the inorganic Mg/Al nanolayers have much higher thermal resistance than the organic PI molecules[3]. However, when the amount of LDH-LG is more than 0.5%(wt), it reduces the thermal stability of the composite material.
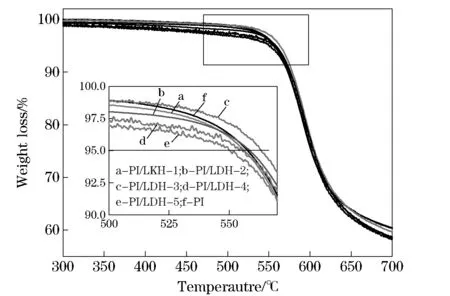
a-PI/LDH-1; b-PI/LDH-2; c-PI/LDH-3; d-PI/LDH-4; e-PI/LDH-5; f-PI
2.4 Tensile properties of PI/LDH nanocomposite film
The tensile properties of nanocomposite films are listed in Tab.1. The tensile strength increases with increasing amount of LDH-LG up to a certain content. The maximum degree of tensile strength is observed at a LDH-LG loading of 0.5%(wt), corresponding to 32.5% increase in tensile strength compared to pure PI. This is as expected since the LDH-LG nanolayers inherently is rigid and, the nanoscale dispersion should also increase the tensile strength of polymeric systems[16]. The LG anion, grafted on the surface of the LDH nanolayers, is the connection between the LDH nanolayers and the PI matrix, increasing the compatibility between these two inorganic and organic phases. Thus, the well dispersed LDH-LG nanolayers effectively enhance the tensile strength of the PI/LDH nanocomposites. When the LDH-LG content exceeds 0.5%(wt), the tensile strength at break decreases but remains higher than that of pure PI. In this case, a few amount of LDH-LG nanolayers were aggregated to form a defect in the nanocomposites and reduce tensile strength[3].

Tab.1 Tensile properties of PI and PI/LDH nanocomposites
3 Conclusion
Pre-exfoliated LDH nanoplatelets modified with N-Lauroyl-glutamate was prepared by a microemulsion method. Then LDH-LG was mixed with DMF, PMDA and ODA to obtain a completely exfoliated PI/LDH nanocomposite by in situ polymerization. HR-TEM observation confirmed that the LDH-LG particles were homogeneously dispersed in the PI matrix. The addition of LDH-LG into PI improved the crystallinity and tensile strength of PI/LDH nanocomposite film.
[1] Wang Q, Feng Y J, Feng J T, et al. Enhancedthermal-and photo-stability of acid yellow 17 by incorporation into layered double hydroxides[J]. J Solid State Chem, 2011, 184:1551-1555.
[2] Ram L, Bhupendra S R, Gaur M S. Structural and polarization properties of polyimide/TiO2nanocomposites[J]. Ionics, 2012,18:565-572.
[3] Hsueh H B, Chen C Y. Preparation andproperties of LDHs/polyimide nanocomposites[J]. Polymer, 2003,44:1151-1161.
[4] Ma J,Yu Z Z,Zhang Q X,et al. Anovel method for preparation of disorderly exfoliated epoxy/clay nanocomposite[J]. Chem Mater, 2004,16:757-759.
[5] Gemeay A H, Mansour I A, El-Sharkawy R G, et al. Preparation andcharacterization of polyaniline/manganese dioxide composites via oxidative polymerization: effect of acids[J]. Eur Polym J, 2005,41(11):2575-2583.
[6] Chiang M F, Wu T M. Synthesis andcharacterization of biodegradable poly(L-lactide)/layered double hydroxide nanocomposites[J]. Compos Sci Technol, 2010, 70:110-115.
[7] Chan Y N, Juang T Y, Liao Y L, et al. Preparation ofclay/epoxy nanocomposites by layered-double-hydroxide initiated self-polymerization[J]. Polymer, 2008,49:4796-4801.
[8] Jin Liang, Zhang Qinghua. Synthesis andcharacterization of polyimides prepared in ionic liquids[J]. Polym Mater Sci & Eng, 2010,26(3):23-26.(in Chinese)
[9] Pu Y P, Lu G S, Zhao P, et al. Effects of forming pressure on the porosity of polyimide porous materials[J]. Journal of Beijing Institute of Technology, 2008, 17(3): 351-354.
[10] Pu Y P, Lu G S, Li X J, et al. Tribological property of polyimide porous materials[J]. Journal of Beijing Institute of Technology, 2006,15(4):483-487.
[11] Yano K, Usuki A, Okada A, et al. Synthesis andproperties of polyimide-clay hybrid[J]. J Poly Sci, Part A:Polym Chem, 1993,31:2493-2498.
[12] Li Guiying, Zhang Qizhen, Yang Wenhong. Synthesis andcharacterization of polyimide/montmorillonite nanoeomposites[J]. J Shandong Univ, 2003, 38(5):105-108.(in Chinese)
[13] Gu A J, Kuo S W, Chang F C. Syntheses andproperties of PI/clay hybrids[J]. J Appl Polym Sci, 2001,79(10): 1902-1910.
[14] Hsiao S H, Liou G S, Chang L M. Synthesis andproperties of organosoluble polyimide/clay hybrids[J]. J Appl Polym Sci, 2001,80(11):2067-2072.
[15] Lü F Z, Wu Y Y, Zhang Y H,et al. Structure and magnetic properties of soft organic ZnAl-LDH/polyimide electromagnetic shielding composites[J]. J Mater Sci, 2012, 47:2033-2039.
[16] Chen D, Huang S, Zhang C,et al. Layer-by-layer self-assembly of polyimide precursor/layered double hydroxide ultrathin films[J]. Thin Solid Films, 2010, 518:7081-7085.
[17] Xi Huan, He Jing, Evans D G,et al. Delamination of layered double hydroxides in microemulsion[J]. Chinese J Inorg Cherm, 2004, 20(10):1217-1222.(in Chinese)
[18] Duan Xue, Jiao Qingze, Li Lei.New synthetic methods of uniformly dispersed ultrafine anionic layered material: CN 1288 078[P].2001-03-21.(in Chinese)
[19] Hu G, Wang N, O’Hare D,et al. Synthesis of magnesium aluminium layered double hydroxides in reverse microemulsions[J]. J Mater Chem, 2007, 17:2257-2266.
[20] Gunawan P, Xu R. Synthesis ofunusual coral-like layered double hydroxide microspheres in a nonaqueous polar solvent/surfactant system[J]. J Mater Chem, 2008,18:2112-2120.
[21] Zhao Y, Li F, Zhang R, et al. Preparation of layered double-hydroxide nanomaterials with a uniform crystallite size using a new method involving separate nucleation and aging steps[J]. Chem Mater, 2002, 14:4286-4291.
[22] Shi H G, Li Y, Guo T Y. Insitu preparation of transparent polyimide nanocomposite with a small load of graphene oxide[J]. J Appl Polym Sci, 2013:3163-3169.
[23] Lei Yong, Liu Yufeng, Jiang Luxia, et al. Research on the synthesis and properties of polyimide/montmorillonite nanocomposite film[J]. Insulating Materials, 2001,1:5-8.(in Chinese)
(Edited by Wang Yuxia)
10.15918/j.jbit1004-0579.201524.0119
O 631.1 Document code: A Article ID: 1004- 0579(2015)01- 0133- 06
Received 2014- 02- 15
Supported by the National Natural Science Foundation of China(21071017; 21376029)
E-mail: jiaoqz@bit.edu.cn
 Journal of Beijing Institute of Technology2015年1期
Journal of Beijing Institute of Technology2015年1期
- Journal of Beijing Institute of Technology的其它文章
- Investigation of the optimum differential gear ratio for real driving cycles by experiment design and genetic algorithm
- Effect of juglone on immunity response and oxidative stress in mice
- Tracking algorithm of BPSK signal in low bit SNR and high dynamic scenarios
- Birkhoff symmetry and Lagrange symmetry
- New scheme of dynamic traitor tracing against the immediate rebroadcast attack
- Construction method of Chinese sentential semantic structure
