2,2-二甲基-1-[5-二茂铁基-3-(三氟甲基)-1H-吡唑-1-基]丙-1-酮的合成及其电化学性质*
吴小琼,阮班锋
(1.安顺职业技术学院,贵州安顺 561000;2.合肥工业大学医学工程学院,安徽合肥 230009)
二茂铁(FcH)及其衍生物广泛应用于催化有机合成[1-3]、生物化学和医学[4-9]、电化学和光电功能材料[10-12]、有机金属化学及超分子组装和离子传感器[13-18]等研究领域。近年来,各种新型的二茂铁衍生物不断出现,极大地拓展了有机化学的研究范围和金属有机化学的研究领域[19]。

Scheme 1
由于FcH的特殊结构和高度的富电子体系、良好的热稳定性、较高的反应活性,且具有易受环境影响的可逆氧化还原电对,易发生氧化还原反应,使其在电化学方面有着广泛应用[20]。
为了扩展二茂铁衍生物的范围,本文以乙酰基二茂铁为原料,经3步反应合成了一个新型的二茂铁衍生物——2,2-二甲基-1-[5-二茂铁基-3-(三氟甲基)-1H-吡唑-1-基]丙-1-酮 (4,Scheme 1),其结构经1H NMR,ESI-MS,元素分析和 X-射线单晶衍射表征。并研究了其电化学性质。
1 实验部分
1.1 仪器与试剂
Boetius型显微熔点仪(温度未校正);VNMRS 600型核磁共振仪(CDCl3为溶剂,TMS为内标);Nicolet FT-IR 2170SX型红外光谱仪(KBr压片);Micromass GCT2MS型质谱仪(EI源);CHI660D型电化学分析仪(1 mm铂盘为工作电极,铂丝为对比电极和甘汞参比电极)。
4,4,4-三氟-1-二茂铁丁烷-1,3-二酮(2)和5-二茂铁基-3-(三氟甲基)1H-吡唑(3)按文献[21]方法合成;其余所用试剂均为分析纯,其中四丁基高氯酸铵(TBAP),使用之前用乙醇重结晶,于100℃真空干燥过夜;二氯甲烷,减压蒸馏后用P2O5干燥。
1.2 4 的合成
在反应瓶中依次加入3 3.20 g(10 mmol),三乙胺1.21 g(12 mmol),三甲基乙酰氯 1.45 g(12 mmol)和二氯甲烷15 mL,搅拌下回流反应过夜。旋蒸脱溶,残余物用乙酸乙酯溶解,用水洗涤,旋蒸脱溶后经硅胶柱层析[洗脱剂:V(石油醚)∶V(乙酸乙酯)=4∶1]纯化得棕色晶体,用混合溶剂[V(石油醚)∶V二氯甲烷)=1∶1]重结晶得棕红色单晶 4,收率 45.3%,m.p.110℃ ~112℃;1H NMR δ:1.54(s,9H),4.16(s,5H),4.44(s,2H),4.80(s,2H),6.78(s,1H);13C NMR δ:27.7,29.6,42.1,47.5,55.8,57.1,70.2,110.5,120.0,152.6,165.5;IR ν:2 925,1 733,1 533,1 400,1 318,1 286,1 149,929,817 cm-1;Anal.calcd for C19H19N2OF3Fe:C 56.74,H 4.26,N 6.97;found C 56.86,H 4.25,N 6.95。
1.3 晶体结构测定
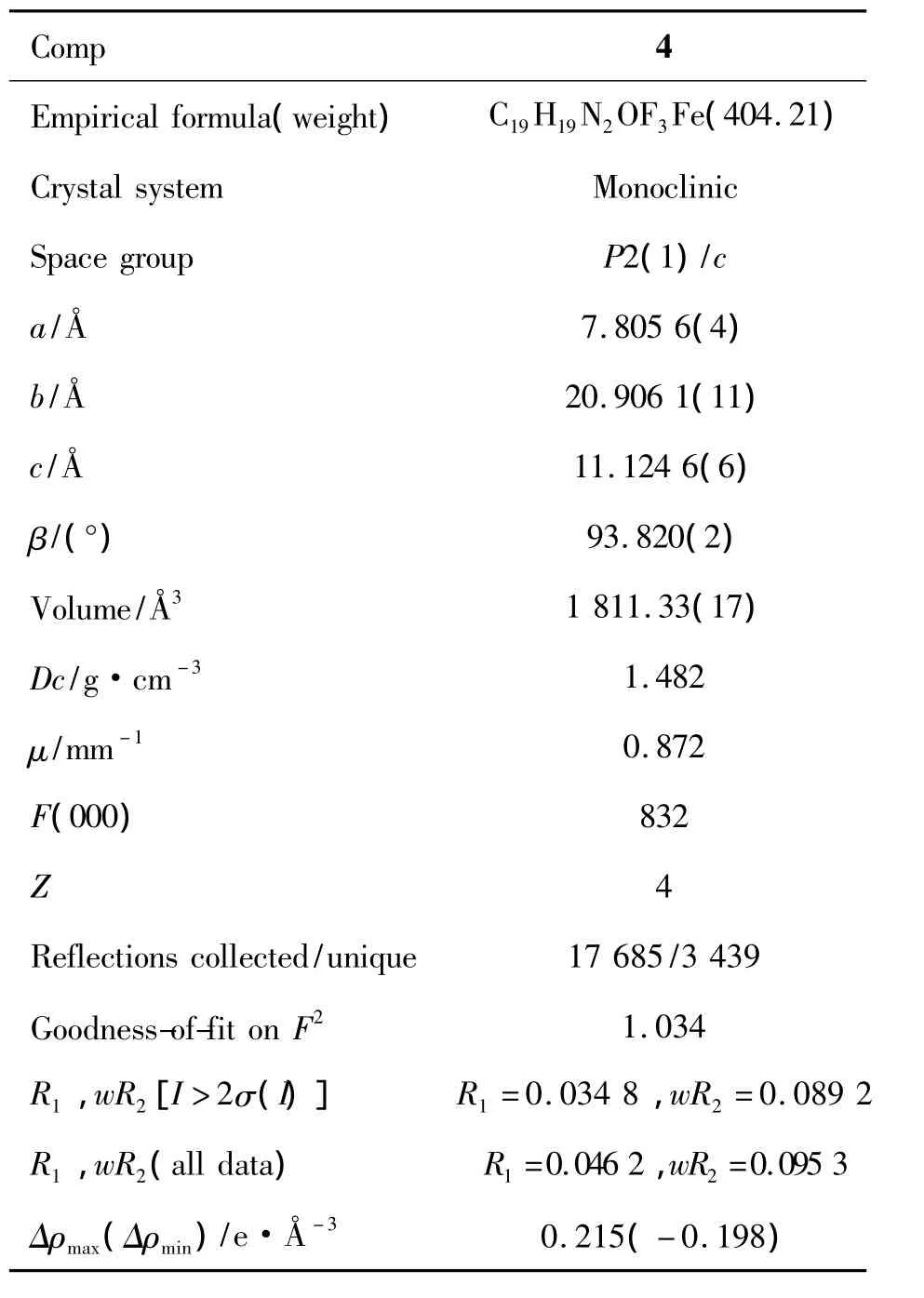
表1 4的晶体学数据Table 1 Crystal data and refinement details of 4
将单晶4(0.18 mm ×0.22 mm ×0.25 mm)置衍射仪上,在298(2)K用石墨单色化的MoKα射线(λ =0.710 73 Å)以 ω -2θ扫描方式,在2.62°< θ<25.71°内共收集衍射数据 17 685 个,其中独立衍射点3 439个。利用SHELXTL-97程序通过最小二乘法定义F2直接法解析晶体结构,氢原子坐标经差值Fourier合成得到,非氢原子坐标由直接法得到。氢原子采用各向同性热参数修正,其它原子采用各向异性热参数修正,晶体结构用全矩阵最小二乘法修正。4(CCDC:938 330)的晶体学数据见表1。
2 结果与讨论
2.1 4的晶体结构
4的分子结构见图1,键长键角见表2。从表2可见,铁原子跟二茂铁两个五元环上碳原子的平均距离分别是2.036 Å 和 2.028 Å。铁原子和五元环上碳原子的最近距离为2.020(2)Å(Fe1-C9)。铁原子和二茂铁五元环之间的距离分别为1.648 Å 和 1.633 Å。两个五元环面心与铁原子之间有所偏离(177.9°),吡唑环与五元环的夹角为 22.8°。

图1 4的分子结构Figure 1 Molecule structure of 4
2.2 4的电化学性质
4的循环伏安曲线见图2。由图2可见,在-0.5 V ~1.5 V内,4 有一对可逆的氧化还原峰(E1/2),电势值分别为 Epa=0.640 V,Epc=0.482 V和ΔE=153 mV。4的分子由二茂铁,吡唑和CF3基团组成。因此,相比于二茂铁的氧化E1/2(273 mV),4的E1/2(561 mV)的氧化还原峰发生了正移。这是由于4分子上的吡唑环连接的CF3具有强吸电子效应,使其比未取代的二茂铁更难被氧化。

表2 4的主要键长和键角Table 2 Selected bond lengths and angles of 4
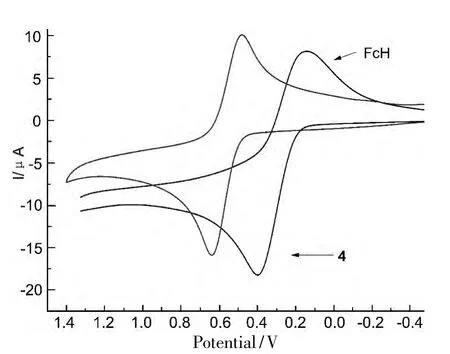
图2 4和FcH的循环伏安曲线*Figure 2 Cyclic voltammograms of 4 and FcH
3 结论
合成了一个新型的二茂铁衍生物——2,2-二甲基-1-[5-二茂铁基-3-(三氟甲基)-1H-吡唑-1-基]丙-1-酮(4);4 的 CCDC 号为938 330;电化学性质研究表明:4有一对可逆的氧化还原峰,Epa=0.640 V,Epc=0.482 V,ΔE=153 mV。
[1]Kealy T J,Pauson P L.A new type of organo-iron compound[J].Nature,1951,168:1039 -1040.
[2]Hu X P,Zheng Z.Unsymmetrical hybrid ferrocenebased phosphine-phosphoramidites:A new class of practical ligands for Rh-catalyzed asymmetric hydro-genation[J].Org Lett,2004,6:3585 -3588.
[3]Dai L X,Tu T,You S L,et al.Asymmetric catalysis with chiral ferrocene ligands[J].Acc Chem Res,2003,36:659 -667.
[4]Fiorina V J,Dubois R J,Brynes S.Ferrocenyl polyamines as agents for the chemoimmunotherapy of cancer[J].J Med Chem,1978,21:393 -395.
[5]Jiradej M,Kanjana R,Korawinwich B,et al.Novel ferrocenic steroidal drug derivatives and their bioactivities[J].J Med Chem,2010,53:3937 -3943.
[6]Blum J,Gelman D,Baidossi W,et al.Palladium-catalyzed methylation of aryl and vinyl halides by stabilized methylaluminum and methylgallium complexes[J].J Org Chem,1997,62:8681 -8686.
[7]Sun M L,Ruan B F,Zhang Q,et al.Synthesis,crystal structures,electrochemical studies and anti-tumor activities of three polynuclear organotin(Ⅳ)carboxylates containing ferrocenyl moiety[J].J Organomet Chem,2011,696:3180 -3185.
[8]Huang X F,Tang J F,Ji J L,et al.Synthesis,characterization and antitumor activity of novel amide derivatives containing ferrocenyl pyrazol-moiety[J].J Organomet Chem,2012,706 -707:113 -123.
[9]Huang X F,Wang L Z,Tang L,et al.Synthesis,characterization and antitumor activity of novel ferrocene derivatives containing pyrazolyl-moiety[J].J Organomet Chem,2014,749:157 -162.
[10]Mueller-westerhotff U T,Zheng Y,Ingram G.A simple synthesis of metallocene aldehydes from lithiometallocenes and N,N-dimethylformamide:Ferrocene and ruthenocene aldehydes and 1,1'-dialdehydes[J].J Organomet Chem,1993,463:163 -167.
[11]Kanis D R,Ratner M A,Marks T J.Design and construction of molecular assemblies with large second-order optical nonlinearities.Quantum chemical aspects[J].Chem Rev,1994,94:195 -242.
[12]Miller J S,Epstein A J,Reiff W M.Ferromagnetic molecular charge-transfer complexes[J].Chem Rev,1988,88:201 -220.
[13]Ion A C,Moutet J C,Pailleret A,et al.Electrochemical recognition of metal cations by poly(crown ether ferrocene)films investigated by cyclic voltammetry and electrochemical impedance spectroscopy[J].J Electroanal Chem,1999,464:24 -30.
[14]Ion A,Ion I,Moutet J C,et al.Electrochemical recognition of metal cations by redox-active receptors in homogeneous solution and in polymer films:Some relevant examples[J].Sensors and Actuators B:Chemical,1999,59:118 -122.
[15]Buda M,Moutet J C,Saint-Aman E,et al.Copper complexes generated from ferrocene-bipyridyl metallosynthons[J].Inorg Chem,1998,37:4146 -4148.
[16]Harriman A,Ziessel R,Moutet J C,et al.Complexation between ferrocene-based 2,2'-bipyridine ligands and copper(Ⅰ)cations[J].Phys Chem Phys,2003,5:1593-1598.
[17]Vonan W,Enseleit U,Gerlach F,et al.Conceptions,materials,processing technologies and fields of application for miniaturized electrochemical sensors with planar membranes[J].Electrochim Acta,2004,49:3745-3750.
[18]Banerjee S,Ray A,Sen S,et al.Pseudohalide-induced structural variations in hydrazone-based metal complexes:Syntheses,electrochemicalstudiesand structural aspects[J].Inorg Chim Acta,2008,361:2692-2700.
[19]Debroy P,Roy S.Recent advances in the synthesis and p roperties of ferrocenes having an unsaturated backbone[J].Coord Chem Rev,2007,251:203 -221.
[20]Stephen C,Yu C J,Cindy B,et al.Electron transfer at electrodes through conjugated“Molecular Wire”bridges[J].J Am Chem Soc,1999,121:1059 -1064.
[21]Zhang Q,Song W L,Hossain A M,et al.Synthesis,crystal structure,electrochemistry and in situ FTIR spectroelectrochemistry of a bisferrocene pyrazole derivative[J].Dalton Trans,2011,40:3510 -3516.

