Observation on the quality of life before and after the injection of antiangiogenic drug in the vitreous cavity of patients with wet age-related macular degeneration☆
Dan-Dan Wanga, Pei-Ying Xub, Tian-Yu Wanga, Xiao-Xia Chena, Qing Penga
a Department of Ophthalmology, Xinhua Hospital, Shanghai Jiaotong University School of Medicine, Shanghai 200092, Chinab Shanghai Minhang Hospital, Fudan University, Shanghai 201199, China
Observation on the quality of life before and after the injection of antiangiogenic drug in the vitreous cavity of patients with wet age-related macular degeneration☆
Dan-Dan Wanga, Pei-Ying Xub, Tian-Yu Wanga, Xiao-Xia Chena, Qing Penga*
aDepartmentofOphthalmology,XinhuaHospital,ShanghaiJiaotongUniversitySchoolofMedicine,Shanghai200092,ChinabShanghaiMinhangHospital,FudanUniversity,Shanghai201199,China

1. Introduction
Age-related macular degeneration (AMD) is one of the major causes of blindness in the elderly. AMD usually occurs in people over the age of 45. Both eyes can develop the disease at the same time or successively. The vision damage is progressive and can seriously affect the quality of life of older persons.1,2Along with social progress and living standard improvements, people are requiring an increasingly high demand for a quality life; therefore, the demand for understanding early AMD and its effective treatment are becoming urgent. Clinically, AMD is divided into two types: (1) atrophic AMD, also known as dry-AMD, which is characterized by progressive retinal pigment epithelium (RPE) atrophy that leads to the degeneration of photoreceptor cells and causing central vision loss, and (2) exudative AMD, also known as wet AMD or senile discoid macular degeneration, which is characterized by the formation of abnormal choroidal neovascularization under RPE that causes varying degrees of edema, exudation, hemorrhage and scar-like changes in the macular region of the fundus. In June 2006, the FDA approved the use of ranibizumab (Lucentis) for the treatment of wet-AMD patients by intravitreal injection. Ranibizumab is a recombinant humanized monoclonal antibody fragment (Fab), targeting vascular endothelial growth factor A (VEGF-A), thus inhibiting the binding of VEGF-A with its receptors VEGFR-1 and VEGFR-2, preventing proliferation of vascular endothelial cells and angiogenesis of new vessels3-5. In December 2011, it was approved by FDA for clinical use, with a very significant clinical treatment effect. However, the surgery has certain risks and affecting factors. Patient care and education during pre-, intra- and post-operation also play a very important role in the success of the surgery; our experience is reported as follows.
2. Material and methods
2.1. Survey subjects
This study is a retrospective analysis regarding the basic situation of the first 20 patient cases that were diagnosed as suffering from wet-AMD in a single eye from 2012 to 2013. In the 20 cases, 11 were males, accounting for 55% of the cases, and 9 were females accounting for 45% of the cases; the patients were aged from 41 to 68 years, with an average age of 57.68 years. Three cases with vision ranging from 0.01 to 0.05 accounted for 15% of the cases; 15 cases with vision ranging from 0.05 to 0.3 accounted for 75% of the cases; and 2 cases with vision ranging from 0.3 to 0.5 accounted for 10% of the cases. Patients' eyes had no other anterior segment or posterior segment diseases (such as glaucoma, diabetic retinopathy, retinal detachment, etc.) or systemic diseases. Patients understood that they needed to complete the VFQ-25 questionnaire carefully before and after vitreous cavity injection surgery.
2.2. Methods
2.2.1. Eye examination
During the initial diagnosis, the Snellen international standard distant vision charted with a lamp house was applied to measure patients' best corrected visual acuity of both eyes. Additionally, a non-contact tonometer (NIDEK, NT-2000) was used to measure intraocular pressure and a slit lamp was used to examine the anterior segment. The standard 90-degree pre-focusing mirror was used to examine the fundus, in addition to the 3D-OCT (Topcon, 3D OCT-1000) and HRA2 fundus fluorescence angiography for clear diagnosis. In total, 16 cases were diagnosed with typical wet-AMD, and 4 with latent wet-AMD.
2.2.2. Completion of the VFQ-25 questionnaire
After the eye examination, all of the subjects had to fill out a VFQ-25 questionnaire and measure the VRQL score. The VFQ-25 questionnaire consisted of three parts, namely: (1) overall health condition and vision: including the degree of self- satisfaction toward systemic disease and binocular vision correction, (2) activities: including reading newspapers and street signs, finding items, participating in outdoor activities and driving; (3) consequences of the vision problem: including limited mobility, inability to do what the patient wanted, and psychological feelings resulting from vision. The higher the VFQ-25 questionnaire score, the worse the VRQL.
2.2.3. Method of intravitreal injection of ranibizumab
Currently, the most common and most effective way to cure wet-AMD is intravitreal injection of anti-vascular endothelial growth factor (anti-VEGF) drugs. After a definite diagnosis for the above patients, ranibizumab was injected into the vitreous cavity. The operation requires a strictly sterilized operating room. After at least 3 times of ocular surface anesthesia, the area around the eyes was sterilized and readied with a towel. A sterile eye speculum was used, and the roots of the eyelashes are disinfected. Then, the conjunctival sac is once again disinfected with PVP. Using a 1 mL sterile syringe, 0.05 mL of the drug was extracted. Then, the needle was replaced with a special needle for a 30 g intravitreal injection. The air was emptied from the needle tip. The needle should be injected 3.5-4.0 mm back from the limbus, aligning with the eye center and perpendicular to the sclera in the vitreous cavity. However, the entry point on the bulbar conjunctiva and scleral surface should not be at the same site to reduce the possibility of postoperative infection of the eye. Then, slowly the 0.05 mL solution for injection was pushed into the vitreous cavity. Before that, 0.05 mL aqueous liquid should be extracted from patients who have had cataract surgery and have been implanted with an intraocular lens to prevent the ranibizumab injection from permeating through the aqueous layer as a result of intraocular lens implantation. After injection, press the injected eye for 3-5 minutes, cover with TobraDex eye ointment and bind up with sterile gauze. For patients needing treatment in both eyes, a new injection is required for the other eye. In addition, the sterile towel, syringe, gloves, surgical drape, speculum, and injection needles are all required to be changed before injecting the other eye.
2.3. Nursing before and after intravitreal injection
First, ask the patients to self-apply antibiotic drops three days before the injection, 4 times per day, in order to eliminate infection in the conjunctiva and circumocular area of the eye. Second, because the price of ranibizumab is quite expensive (9 800 Yuan/injection), a detailed explanation is required before the injection to educate patients that the disease can be controlled, but that vision will not necessarily improved. Third, circumocular and systemic conditions (such as intraocular pressure, blood pressure, blood glucose, liver and kidney function and ECG) are required to be controlled in the normal range; patients should not have drug allergies. Fourth, anti-inflammatory eye drops should be used for 7 to 10 days consecutively. Patients should not take a bath within 24 hours, and no vigorous eye rubbing should occur within one week. In particular, coal mining, high-altitude operation, long drives and other hazardous work cannot be performed within one week. Fifth, inform patients that if red eyes, pain, subconjunctival hemorrhage (Fig.1), increasingly blurry vision, eye swelling or general discomfort occur, they should come to the hospital for timely treatment to avoid serious complications that may occur. The nursing care we described after injection is also consistent with the report of Li Yong and other researchers. During the one-year follow-up, BCVA, IOP, 3D-OCT and fundus angiography in the patients' eyes should be checked again, and the patients were asked to complete a detailed VFQ-25 questionnaire.
3. Results
Scores of the 20 wet-AMD patients in the initial completion of the VFQ-25 questionnaire are as follows. Therapeutic visual acuity is divided into three ranges: 0.01-0.05,0.05-0.3 and 0.3-0.5. In each range, the higher the BCVA for the contralateral non-treated eye, the lower the score of the VFQ-25 questionnaire, i.e., the patient has a higher VRQL. Patients with a range of 0.05 to 0.3 have the lowest average VFQ-25 questionnaire score, i.e., they have a higher VRQL, which corresponds to the highest BCVA average value for the non-treated eye. Patients with a range of 0.01 to 0.05 have the highest average VFQ-25 questionnaire score, i.e., they have a lower VRQL, which corresponds to the lowest BCVA average value for the non-treated eye. Patients with a range of 0.3 to 0.5 have an average VFQ-25 score, which is also matched with the fact that the BCVA average value for the non-treated eye is in between these values.
The results of the one-year follow-up are as follows. Twelve patients had improved visual acuity by more than 3 lines, accounting for 60.0% (Fig.2a,2b) of cases. Five patients had no significant visual acuity improvement and accounted for 25.0% of the cases. Three patients had decreased visual acuity compared with the baseline value because of various complications, accounting for 15.0% of the cases. The reasons for diminution of vision in these three cases included vitreous hemorrhage, atrophic scarring in macular lesions (Fig.3a,3b), etc..5During the one-year follow-up after injection of ranibizumab, the improvement of vision care and the patient care as well as patient education from doctors had a significant influence on the final score of the VFQ-25.
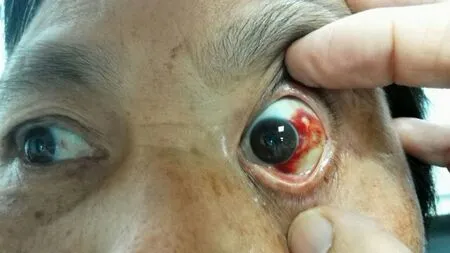
Fig. 1. Subconjunctival hemorrhage after intravitreal injection in the left eye.
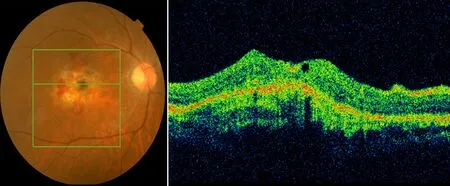
Fig. 2a. Color Fundus and OCT picture pre-intravitreal injection with wet-AMD; hemorrhage and edema in the macula were noted.

Fig. 2b. Color fundus and OCT picture post-intravitreal injection of wet-AMD after 1 month; hemorrhage and edema in the macula area almost disappeared.

Fig. 3a. Color fundus and OCT picture pre-intravitreal injection with wet-AMD accompanied by retinal hemorrhage and edema of the macula.
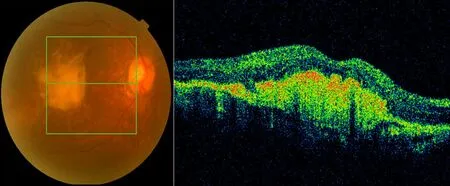
Fig. 3b. Color fundus and OCT picture post-intravitreal injection after 3 months; retinal hemorrhage and edema disappeared but obviously a scar formed in the macula.
4. Discussion
Wet-AMD is more rapidly progressed compared with dry-AMD, causing oozing, bleeding, swelling and scarring of the macula and seriously affects patients' central vision. Clinically, for patients with wet-AMD, all of the different treatment methods may result in the desired effect, but each treatment has risks, e.g., although photodynamic therapy (PDT) can effectively control disease progression, it also effects the restoration of visual function,6,7requiring active and effective communication and explanation with patients prior to treatment. Another treatment method is intravitreal injection of an anti-VEGF drug; this treatment can improve vision without forming obvious scars in the macula.8Thereby, it has an important role in restoring visual function and improving visual quality; however, this method also has disadvantages. Approximately 0.05% of the patients have endophthalmitis9and approximately 0.01% of the patients will have a stroke or cerebrovascular accident.10,11From our research, 3 patients had a poor therapeutic effect because of the following reasons: complications occurred after a variety of drug injections, such as vitreous hemorrhage, lesions, and obvious scarring. Additionally, latent wet-AMD patients have poor sensitivity toward anti-VEGF drugs.12
This clinical trial studied the main factors affecting VRQL before and after the intravitreal injection of ranibizumab. Prior to the treatment, patients relied on their good eye during daily life; Therefore, the better the distance vision, the higher the VRQL, which is consistent with the research result from Zou Haidong and Immonen, et al..2,13-15After the intravitreal injection of the drug on the eye with poor vision, the subjective experience of patients can significantly affect the VRQL because the patients are looking forward to the benefits of the treatment. A mental and financial burden exists, so the work of doctors and nurses to educate the patients also affects patients' VRQL. This phenomenon is also consistent with a previous observation16.
5. Conclusion
Prior to treatment, for wet-AMD patients, the distance vision of the good eye is crucial to the vision-related quality of life; after the intravitreal injection of the drug. The main factors affecting the vision-related quality of life include the accuracy of diagnosis and the injection skill, as well as patient care and education, and the recovery of the poor eye. Therefore, after the injection of the anti-VEGF drug, the improvement of visual function and quality of life depend on the doctor's skill, nurses' care and the understanding and cooperation of the patients.
Conflicts of interest
All contributing authors declare no conflicts of interest.
1. Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012; 379(5):1728-1738.
2. Zou HD,Bai L,Liu HY,Zhang X.Effect of age-related maculopathy on the quality of life .Chinese Journal of Ocular Fundus Diseases.2004; 20(5):303-306[In Chinese].
3. Barquet LA. Role of VEGF in diseases of the retina. Arch Soc Esp Oftalmol. 2015;90(1):3-5.
4. Kinnunen K, Ylä-Herttuala S.Vascular endothelial growth factors in retinal and choroidal neovascular diseases. Ann Med. 2012;44(1):1-17.
5. Mitchell P1.A systematic review of the efficacy and safety outcomes of anti-VEGF agents used for treating neovascular age-related macular degeneration: comparison of ranibizumab and bevacizumab.Curr Med Res Opin. 2011;27(7):1465-1475.
6. Nowak-Sliwinska P1, van den Bergh H, Sickenberg M, Koh AH.Photodynamic therapy for polypoidal choroidal vasculopathy.Prog Retin Eye Res.2013;37(10):182-199.
7. Figurska M1, Wierzbowska J, Robaszkiewicz J.Severe decrease in visual acuity with choroidal hypoperfusion after photodynamic therapy.Med Sci Monit.2011;17(6):CS75-79.
8. Au Eong KG, Sanjay S, Lim SC.Treatment of age-related macular degeneration.Lancet. 2006;368(7):1235.
9. Haddock LJ, Ramsey DJ, Young LH.Complications of subspecialty ophthalmic care: endophthalmitis after intravitreal injections of anti-vascular endothelial growth factor medications.Semin Ophthalmol. 2014 ;29(5-6):257-262.
10. Semeraro F1, Morescalchi F, Duse S, Gambicorti E, Romano MR, Costagliola C.Systemic thromboembolic adverse events in patients treated with intravitreal anti-VEGF drugs for neovascular age-related macular degeneration: an overview. Expert Opin Drug Saf. 2014;13 (6):785-802.
11. Wieberdink RG1, Ho L, Ikram MK, Koudstaal PJ, Hofman A, de Jong PT, Vingerling JR, Breteler MM.Age-related macular degeneration and the risk of stroke: the Rotterdam study.Stroke. 2011;42(8):2138-2142.
12. Reich O, Bachmann LM, Faes L, Bohni SC, Bittner M, Howell JP, Thiel MA, Rapold R, Schmid MK.Anti-VEGF treatment patterns and associated health care costs in Switzerland: findings using real-world claims data.Risk Manag Healthc Policy. 2015;21(8):55-62.
13. Finger RP, Guymer RH, Gillies MC, Keeffe JE.Finger RP, Guymer RH, Gillies MC, Keeffe JE.The impact of anti-vascular endothelial growth factor treatment on quality of life in neovascular age-related macular degeneration. Ophthalmology. 2014;121(6):1246-1251.
14. Riusala A,Sarna S.Immonen I. Visual function index (VF-14)in exudative age —related macular degeneration of longduration. Am J Op hthalmol,2003;l35(2):206-2l2 .
15. Maekenzie P J, Chang TS, Scott IU, Linder M, Hay D, Feuer WJ, Chambers K. Assessment of vision—related function in patients with age-related macular degeneration.Ophthalmology. 2002;109(4):720-729.
16. Lamoureux EL, Hassell JB, Keeffe JE. The determinants of participation in activities of daily living in people with impaired vision. Am J Ophthalmol. 2004;137:265-270.
☆This work was supported by National Natural Science Fund Project (No.81470029) and Science and Technology Commission of Shanghai Natural fund Project (No.11JC410602).
*Corresponding author. E-mail address: DrQingPeng@126.com (Q. Peng). Authors Dan-Dan Wang, Pei-Ying Xu, Tian-Yu Wang, and Xiao-Xia Chen, equally contributed to this study. Peer review under responsibility of Shanxi Medical Periodical Press. http://dx.doi.org/10.1016/j.cnre.2015.02.002
- Frontiers of Nursing的其它文章
- Predicting Pressure Ulcer Risk with the Braden Q Scale in Chinese Pediatric Patients in ICU
- The Application of Multimedia Messaging Services via Mobile Phones to Support Outpatients: Home Nursing Guidance for Pediatric Intestinal Colostomy Complications☆
- Study Weariness of Vocational College Students and Reform of the Teaching Mode in Nursing Basic Technology Course☆
- A Research Report on the Prescription Rights of Chinese Nurses☆
- GUIDE FOR AUTHORS

