Effect of Dietary Lipid on the Growth, Fatty Acid Composition and Δ5 Fads Expression of Abalone (Haliotis discus hannai Ino) Hepatopancreas
LI Mingzhu, MAI Kangsen, AI Qinghui HE Gen XU Wei ZHANG Wenbing ZHANG Yanjiao ZHOU Huihui and LIUFU Zhiguo
1)The Key Laboratory of Aquaculture Nutrition and Feed of Ministry of Agriculture;The Key Laboratory of Mariculture of Ministry of Education,Ocean University of China,Qingdao266003,P. R.China
2)College of Agriculture,Ludong University,Yantai264025,P. R. China
Effect of Dietary Lipid on the Growth, Fatty Acid Composition and Δ5 Fads Expression of Abalone (Haliotis discus hannai Ino) Hepatopancreas
LI Mingzhu1),2), MAI Kangsen1),*, AI Qinghui1), HE Gen1), XU Wei1), ZHANG Wenbing1), ZHANG Yanjiao1), ZHOU Huihui1), and LIUFU Zhiguo1)
1)The Key Laboratory of Aquaculture Nutrition and Feed of Ministry of Agriculture;The Key Laboratory of Mariculture of Ministry of Education,Ocean University of China,Qingdao266003,P. R.China
2)College of Agriculture,Ludong University,Yantai264025,P. R. China
This study investigated the effect of dietary lipid on the growth, fatty acid composition and Δ5 fatty acyl desaturase genes (Fads) expression of juvenile abalone (Haliotis discus hannaiIno) hepatopancreas. Six purified diets were formulated to contain tripalmitin (TP), olive oil (OO, 72.87% 18:1n-9), grape seed oil (GO, 68.67% 18:2n-6), linseed oil (LO, 70.48% 18:3n-3), ARA oil (AO, 41.81% ARA) or EPA oil (EO, 44.09% EPA and 23.67% DAH). No significant difference in survival rate was observed among abalone fed with different diets. Weight gain rate (WGR) and daily growth rate of shell length (DGRSL) were significantly increased in abalone fed with diets containing OO, AO and EO, but decreased in abalone fed with LO diet (P〈 0.05) in comparison with those fed with TP. High level of dietary 18:2n-6 resulted in higher content of n-6 polyunsaturated fatty acids (PUFAs) in abalone fed with GO than those fed with TP, OO, LO and EO (P〈 0.05). n-3 PUFAs in abalone fed with LO was significantly higher than those in abalone fed with TP, OO, GO and AO (P〈 0.05). The highest contents of 20:1n-9 and 22:1n-9 were observed in abalone fed with OO. The expression of Δ5Fadsin hepatopancreas of abalone was enhanced by high concentration of 18:3n-3 and suppressed by dietary LC-PUFAs; however it was not affected by dietary high concentration of 18:1n-9 or 18:2n-6. These results provided valuable information for understanding the synthesis of LC-PUFAs and nutritional regulation of Δ5Fadsexpression in abalone.
desaturase; fatty acid; gene expression; fish oil; vegetable oil
1 Introduction
For environmental and economic considerations, marine fish oil (FO) in aquafeed is reduced and substituted with alternative oils, such as vegetable oils (VOs) (Olsen, 2011; Pickova and Morkore, 2007). VOs are known to be rich in C18 polyunsaturated fatty acids (PUFAs), such as 18:2n-6 and 18:3n-3, but deficient of long chain polyunsaturated fatty acids (LC-PUFAs). Such shift in feed formulation resulted in progressively reducing content of LC-PUFAs, which are benefit for human health (Riedigeret al., 2009), in the flesh of cultured fish species (Rosenlundet al., 2011). For overcoming this problem, elevating LC-PUFAs biosynthetic capability from C18 precursors is attracting much attention in many important cultured species. This has renewed interest in the pathways of endogenous synthesis of LC-PUFAs in aquatic species.
Biosynthesis of LC-PUFAs from C18 precursors invertebrates is mediated by fatty acyl desaturases (Fads) and elongases (Tocher, 2003). The production of ARA is achieved by Δ6 desaturation of 18:2n-6 to produce 18:3n-6 that is elongated to 20:3n-6 followed by Δ5 desaturation. Biosynthesis of EPA from 18:3n-3 depends on enzymatic catalyses of Δ6Fad, elongase and Δ5Fadsuccessively. Synthesis of DHA from EPA requires the elongation of EPA to 22:5n-3 and 24:5n-3, which is then converted to DHA by Δ6 desaturation and a peroxisomal chain shortening step. The extent to which any species can convert C18 PUFAs to LC-PUFAs varies, depending on their repertoire ofFads and elongases (Liet al., 2010). In recent years, many advances have been made in cloning these critical enzymes and studying their expression in response to different nutritional regulation in many aquatic species. Δ6Fadsand elongases have been successfully characterized from different marine fishes (González-Roviraet al., 2009; Gregoryet al., 2010; Seiliezet al., 2003; Tocheret al., 2006; Zhenget al., 2009), freshwater fishes (Agabaet al., 2005; Renet al., 2012; Seiliezet al., 2001), diadromous fishes such as Atlantic salmon (Hastingset al., 2004; Monroiget al., 2010;Moraiset al., 2009; Zhenget al., 2004, 2005a, b) and shellfishes such as abalone (Mateoset al., 2011, 2012a, b). In contrast, barely information was available on cloning and nutritional regulation of Δ5Fadexpression in aquatic animals, especially in marine species (Seiliezet al., 2003; Tocheret al., 2006; Zhenget al., 2009). To date, desaturase with Δ5 activity has only been found in zebra fish (Danio rerio) (Hastingset al., 2001), Atlantic salmon (Salmon salar) (Hastingset al., 2004), white-spotted rabbitfish (Siganus canaliculatus) (Liet al., 2010) and octopus (Octopus vulgaris) (Monroiget al., 2012). Lack of Δ5 gene or activity is believed to be responsible for low LC-PUFAs biosynthesis in many marine species (Mourente and Tocher, 1993; Seiliezet al., 2003; Tocher and Ghioni, 1999; Tocheret al., 2006; Zhenget al., 2009).
The nutritional regulation on the expressions ofFads/ elongase genes have been reported in many species. Some studies have found that the increased transcript abundance ofFads/elongase genes occurred when dietary FO was replaced by VO (González-Roviraet al., 2009; Mateoset al., 2011, 2012a, b; Renet al., 2012; Tocheret al., 2006; Zhenget al., 2005b). However, it has been also suggested in some fishes that dietary VOs inclusion had no effect on the expressions ofFads/elongase genes (González-Roviraet al., 2009; Tocheret al., 2006). Furthermore, it is still unclear whether the desaturase nutritional modulation is due more to desaturase product reduction or to the increase of substrate supply, or both (Vagner and Santigosa, 2011; Zhenget al., 2004).
Abalone is one of the most valuable LC-PUFAs suppliers for human beings in the world (Sales and Janssen, 2004; Suet al., 2004). As far as we know, there is no information on the effects of dietary lipid on LC-PUFAs biosynthesis as well as Δ5Fadgene expression in abalone. Recently, we have successfully identified two isoforms of Δ5Fads (Hdhfad1 and Hdhafad2) in abalone (Haliotis discus hannaiIno) and showed that their enzymatic activities were very stringent (Liet al., 2013b). Our long-term objective is to increase the LC-PUFAs biosynthesis capacity in abalone. For this purpose, we must understand the regulation of LC-PUFAs biosynthesis by different dietary fatty acids (C18PUFA or LC-PUFA) and the molecular basis of its biosynthesis. Therefore, the present study examined the effect of dietary lipid on fatty acid composition, Δ5Fadgene expression as well as growth performance of abalone.
2 Materials and Methods
2.1 Materials (Chemicals)
Tripalmitin (TP) was obtained from Sigma-Aldrich (St. Louis, MO). Grape seed oil (GO) was purchased from HaiLongDa Chemical Reagent Co. (Qingdao, China). Olive oil (OO), linseed oil (LO), ARA oil (AO) and EPA oil (EO) were purchased from FuLin Chemical Reagent Co. (Qingdao, China). Trizol reagent was obtained from Invitrogen (Carlsbad, CA). PrimeScript®1st Strand cDNA Synthesis Kit, PrimeScript®RT reagent Kit with gDNA Eraser (Perfect Real Time) and 2×SYBR Green Real-time PCR Master Mix were obtained from TaKaRa (Shiga, Japan). pGEM-T Vector system was purchased from Promega (Madison, WI).
2.2 Experimental Diets
Procedures for diets preparation were modified from the method described by Maiet al. (1995). Casein and gelatin were used as protein source while dextrin was used as the major carbohydrate source. The compositions of mineral mix and vitamin mix were modified from those used by Maiet al. (1995). Six experimental diets were formulated to contain 3.5% different lipids including TP, OO, GO, LO, AO and EO. All ingredients were ground into fine powder through 120 μm mesh and then they were mixed to a paste by adding water (about 120%). The paste was shaped into sheets with 0.5 mm thickness, and then cut into 1 cm × 1 cm pellets. Then the pellets were soaked in an aqueous solution of CaCl2(5%) for 2 min. The surplus solution was drained naturally, and then thepellets were sealed in a sample bag and stored at -20℃until use. Abalone fed with TP diet served as control group. The nutrient composition of the diets was analyzed following the procedures, Association of Official Analytical Chemists (AOAC, 1995). Crude protein was determined by measuring nitrogen (N × 6.25); crude lipid was measured by ether extraction using Soxhlet method; ash was tested by combustion at 550℃ for 16 h. Ingredients and proximate compositions of the experimental diets were in Table 1 and 2. Dietary fatty acid composition was listed in Table 3. TP, OO, GO and LO diets were extremely rich in 16:0, 18:1n-9, 18:2n-6 and 18:3n-3, respectively. AO diet was rich in ARA while EO diet was enriched with EPA and DHA.
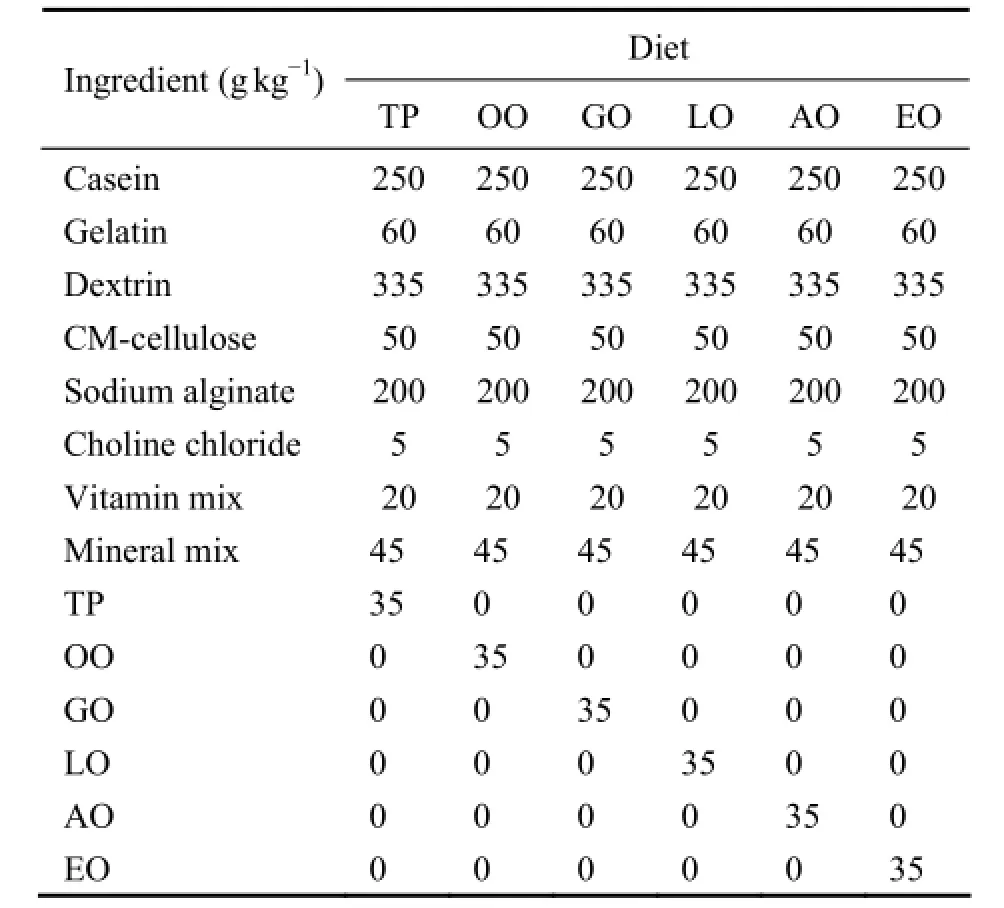
Table 1 Formulation of experimental diets
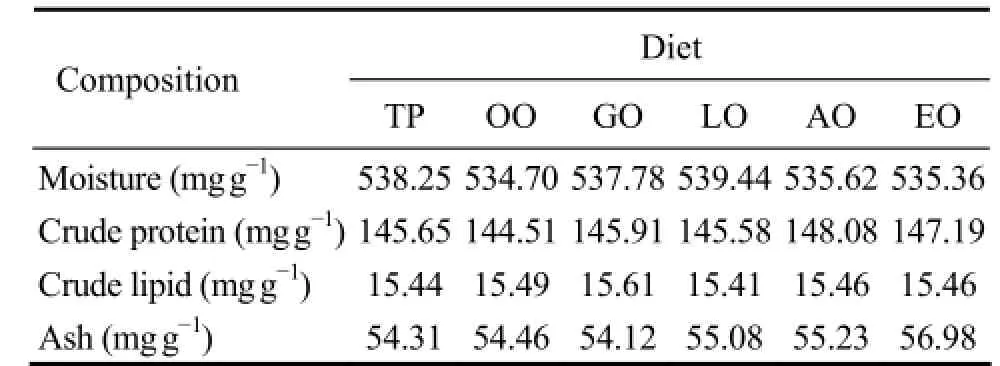
Table 2 Proximate compositions of experimental diets†

Table 3 Fatty acid compositions of experimental diets expressed as the percentage of total fatty acids
2.3 Experimental Procedure
Juvenile abalones (H. discus hannaiIno) with an initial weight of 0.38 g ± 0.01 g and an initial shell length of 15.06 mm ± 0.21mm were obtained from Aoshanwei fishery Co. Qingdao, China. The abalones were acclimated for 1 week prior to the feeding experiment, and then randomly distributed into 18 rectangular-shaped glass aquaria with the stocking density of 50 abalones/aquaria. There were three replicates for each of the six dietary treatments. Abalones were hand-fed with experimental feeds at a rate of 2%-5% equal to wet body weight at 18:00 once daily. Faces and excess feeds were removed in the next morning at 8:00 for good water quality. Aquariums and corrugated boards were cleaned three times a week for cleaning the alga possibly eaten by abalone. During the 120-day feeding experiment, temperature was registered at 16℃ to 18℃, salinity 31 to 35, and pH 7 to 8. Meanwhile, dissolved oxygen was kept at 7 mg L-1.
2.4 Sample Collection
At the end of the feeding experiment, abalones were starved for 2 days prior to sample collection. Then they were individually weighed, measured and dissected after cleaning the water by medical gauze. Hepatopancreas tissue was used to analyze the fatty acid composition and expression of Δ5Fadgenes. Samples for determining the fatty acid composition were stored at -20℃. Other samples for gene expression analysis were immediately frozen in liquid nitrogen and then stored in -80℃ refrigerator.
2.5 Analysis and Measurement
2.5.1 Growth performance
The growth parameters of abalone were calculated as follows:
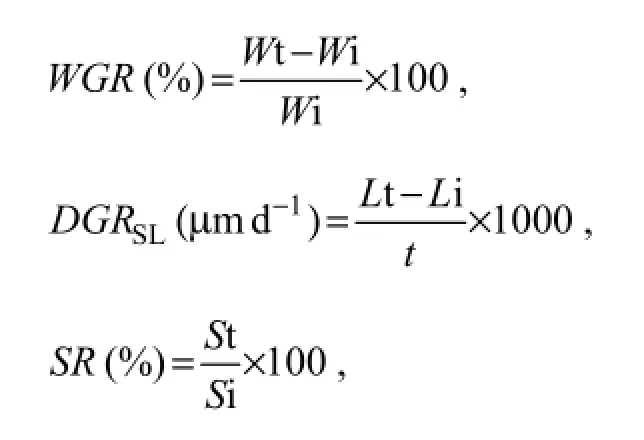
where,WGRis weight gain rate, andWt andWi are terminal and initial mean weight (g);DGRSLis daily growth rate of shell length, andLt andLi are terminal and initial mean shell length (mm);SRis survival rate, andSt andSi are terminal and initial abalone number, whiletwas dura-tion of experimental days (120).
2.5.2 Analysis of lipid and fatty acid composition
Lipid from hepatopancreas was measured according to AOAC methods (1995). The fatty acid profiles were analyzed using the procedures described by Metcalfeet al. (1966) with some modification. The detailed methodology for fatty acid extraction and the measurement for fatty acid methyl esters (FAME) were described in our previous study (Liet al., 2013a). Previous tests were conducted to make sure that all fatty acids could be esterified following the procedures above (Aiet al., 2008; Xuet al., 2004).
2.5.3 Expression of Δ5Fadsgenes in hepatopancreas of abalone fed with different diets
Hepatopancreas from abalone fed with different diets was respectively grinded in liquid nitrogen. Total RNA was extracted using Trizol reagent, then quality and concentration were determined with NanoDrop®ND-1000 (Nano-Drop Technologies, Wilmington, DE, USA). One microgram of total RNA was subjected to PrimeScript®RT reagent Kit with gDNA Eraser for reverse transcription and DNA erasability. The expression of Δ5Fadsgene was measured by quantitative real-time PCR (qRTPCR) with 2-ΔΔCT(Livak and Schmittgen, 2001). The qRT-PCR primer pairs (Table 4) were designed to guarantee the primer amplification efficiencies of target gene and reference gene (ribosomal protein S9, RPS9) approximately equal. Expression levels of reference gene (RPS9) were tested to be unresponsive to different diets (Fig.1), which suggested that RPS9 is qualified to normalize Δ5Fadsgene expression. The real-time PCR was carried out in a quantitative thermal cycler (Mastercyclereprealplex, Eppendorf, German) in a final volume of 25 μL containing 2×SYBR Green Real-time PCR Master Mix (TaKaRa, Japan), primer pairs and cDNA. The program was 95℃ for 30 s followed by 35 cycles of 95℃ for 5 s, 58℃ for 15 s and 72℃ for 20 s. Melting curve wasperformed after the amplification phase. Each sample was run in triplicate, and reactions without templates were used as negative control.
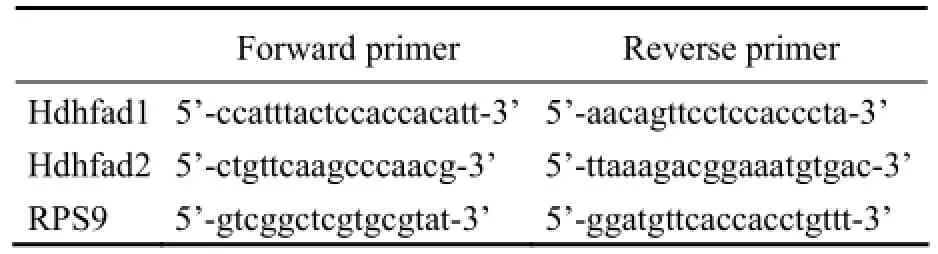
Table 4 Primer pairs used in gene expression analysis
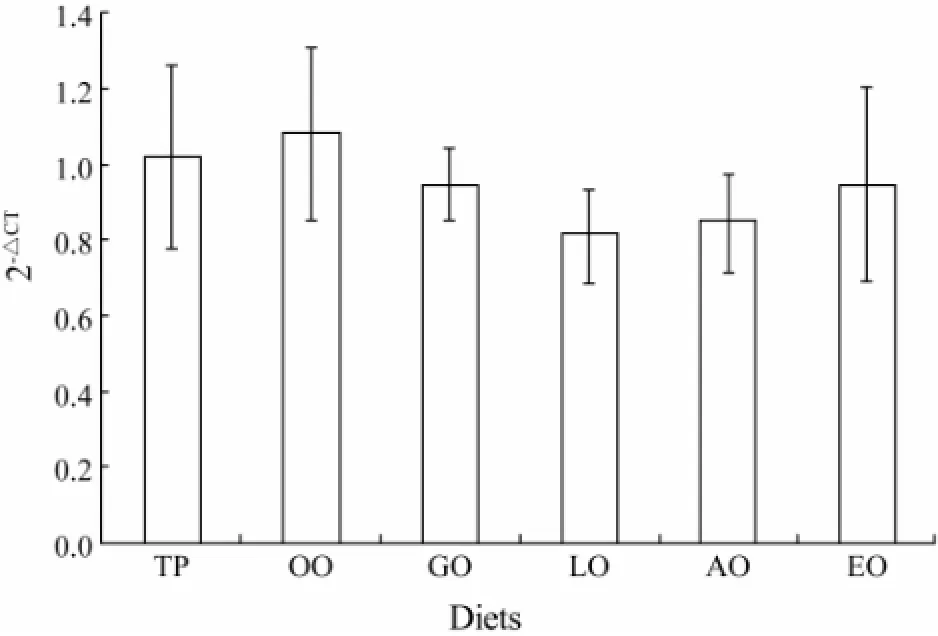
Fig.1 The effect of dietary lipid on expression level of RPS9 in hepatopancreas of abalone.
2.6 Statistical Analysis
All data are presented as means ± S.D. and analyzed by one-way analysis of variance (ANOVA) after homogeneity test using SPSS 13.0. Turkey test was used for multiple comparisons andP〈 0.05 was considered statistically significant.
3 Results
3.1 Growth Performance of Abalone
There is no significant difference inSR(ranging from 82.67 to 88.66) of abalone fed with different diets (Table 5). However, dietary fatty acids significantly affected theWGRandDGRSLof juvenile abalone. Abalone fed OO (rich in 18:1n-9), AO (rich in ARA) and EO (rich in EPA and DHA) diets had significantly higherWGRandDGRSLthan those of other groups (P〈 0.05). No significant difference was observed inWGRandDGRSLamong OO, AO and EO groups (P〉 0.05). TheWGRandDGRSLof abalone fed the diet containing high level of 18:2n-6 was not significantly different from those of the control group (P〉 0.05), which was shown in our previous study (Liet al., 2013a). The lowestWGRandDGRSLwere both found in abalone fed the diet included LO, rich in18:3n-3.

Table 5 Effects of different dietary lipids on the growth and survival of juvenile abalone
3.2 Lipid and Fatty Acid Analyses in Hepatopancreas of Cultured Abalone
The lipid level in hepatopancreas of abalone was not significantly affected by the test diets. However, fatty acid composition in hepatopancreas of abalone was significantly influenced by the diets (Table 6). Abalone fed TP diet accumulated the highest level of saturated fatty acids (SFAs) in particular 16:0 in hepatopancreas (P〈0.05). Abalone receiving high content of 18:1n-9 was found to contain the highest content of monounsaturated fatty acids (MUFAs), especially for 18:1n-9 and its elongation products 20:1n-9 and 22:1n-9 (P〈 0.05). The concentrations of n-6 PUFAs such as 18:2n-6, 20:2n-6, 20:4n-6 and 22:2n-6 in GO-fed group were significantly higher than those of other groups, except for AO-fed group. LO diet significantly increased (P〈 0.05) the hepatopancreas n-3 PUFAs (such as 18:3n-3, 20:3n-3, 20:5n-3, 22:5n-3 and 22:6n-3) through the high level of dietary 18:3n-3. The highest level of ARA was observed in hepatopancreas of abalone fed AO diet. The hepatopancreas of abalone fed EO diet accumulated the highest level of EPA and DHA.

Table 6 Lipid and fatty acid compositions (% of total fatty acids) in hepatopancreas of abalone fed diets with different lipid sources
3.3 Expression of Δ5Fadsgenes in Hepatopancreas of Abalone
The expression levels of Δ5Fadsin hepatopancreas of abalone fed different diets are shown in Fig.2. Compared to those of abalone fed the control diet (TP diet), expression levels of Hdhfad1 and Hdhfad2 were increased to 170.34% ± 0.11% and 179.71% ± 0.20% in abalone fed with LO diet, but decreased to 49.28% ± 0.02% and 45.89% ± 0.07% in abalone fed with AO diet and 38.47% ± 0.04% and 34.87% ± 0.03% in group fed with EO diet (P〈 0.05). No significant difference was found in abalone fed TP, OO and GO diets in terms of Δ5Fadsexpression in hepatopancreas (P〉 0.05).
4 Discussion
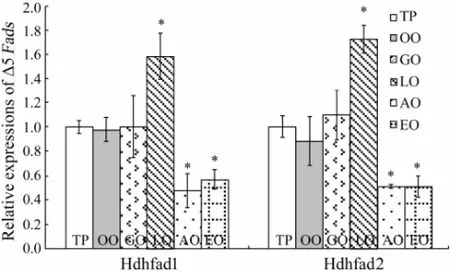
Fig.2 Effects of dietary lipid on expression levels of Δ5Fads(Hdhfad1 and Hdhfad2) in hepatopancreas of abalone. Different letters above the columns indicate significant difference (P〈 0.05) between dietary treatments, for each transcript.
Linoleic acid (LA, 18:2n-6), α-linolenic acid (ALA, 18:3n-3), ARA and EPA are essential fatty acid (EFA) forH. discus hannaiand diets containing EFA contributed to the fast growth of abalone (Bautista-Teruelet al., 2011; Durazo-Beltránet al., 2003; Maiet al., 1996; Xuet al., 2004). In the present study, the best growth performance was observed in abalone fed with AO and EO diets, which confirmed the stimulating effects of ARA and EPA on the growth of abalone (Maiet al., 1996; Xuet al., 2004). Interestingly, abalone fed OO diet also acquired comparable growth performance with those fed AO and EO. Similar reports have shown that partial or total substitution of FO by OO or palm oil, rich in 18:1n-9 (Oleic acid, OA), does not affect or even stimulate the growth of some fish and shellfish (Bahurmiz and Ng, 2007; Bellet al., 2002; Durazo-Beltránet al., 2003; Kamarudinet al., 2012; Nget al., 2003). Dietary fatty acids are known to be energy sources of organisms throughβ-oxidation (Mishra and Samantaray, 2004). Moreover, SFAs and MUFAs are preferred in this process than PUFAs (Limet al., 2001). Therefore, high level of OA in OO diet, instead of protein, may be oxidized to provide energy for abalone. This probably resulted in better growth performance in abalone fed OO diet. Likewise, high level of PUFA may have consequences on the protein sparing effect, using protein instead of PUFA for energy generation, and eventually resulted in the growth retardation of abalone fed GO and LO diets. Similar result was reported in the study of Kamarudinet al. (2012).
Relatively higher levels of LC-PUFAs such as ARA, EPA and DHA in hepatopancreas of abalone fed OO, GO or LO diet, with regard to non-detection of these fatty acids in the diets, confirmed the occurrence of desaturation and elongation in abalone (Durazo-Beltránet al., 2003; Maiet al., 1996; Xuet al., 2004). The contents of 20:1n-9 and 22:1n-9, elongation products of 18:1n-9, in abalone fed OO diet, were significantly higher than those of other groups. This suggested that high level of 18:1n-9 in OO diet may increase the elongase activity, and eventually increase the biosynthesis of n-9 series fatty acids in abalone. No desaturation products from OA, such as 18:2n-9, were detected, possibly due to the competition for further desaturation of C18 PUFAs that is known to be in the order of n-3〉n-6〉n-9 (Durazo-Beltrán,et al., 2003; Henderson and Tocher, 1987). Similarly, dietary 18:2n-6 and 18:3n-3 also increased the biosynthesis of n-6 and n-3 series PUFA, respectively, especially the Δ5 desaturation products ARA and EPA. These findings are useful for better understanding the fatty acid metabolism in abalone. Furthermore, this information may be taken into consideration when designing the aquafeed formulation.
The activity ofFadsand elongases are responsible for the biosynthesis of LC-PUFAs from C18 PUFAs (Liet al., 2010). Previous studies have proved the regulation effect of dietary fatty acid on expression levels ofFadsor elongase genes. In present study, expression of Δ5Fadsgenes was significantly decreased in abalone fed AO and EO diets (rich in LC-PUFAs), but increased in abalone fed LO diet (rich in ALA) in comparison with those of TP group. This suggested that mRNA levels of Δ5Fadsgenes in abalone were positively regulated by substrate fatty acid ALA, and negatively regulated by product fatty acids, such as ARA, EPA and DHA. Our result is similar with those of some early studies,i.e., transcription levels of desaturase or elongase genes increase when dietary FO is replaced by VO (González-Roviraet al., 2009; Izquierdoet al., 2008; Mateoset al., 2012a, b; Monroiget al., 2010; Seiliezet al., 2003; Zhenget al., 2004, 2005a). Never- theless, other studies have different results. For example, Tocheret al. (2006) found that dietary VO blends (rapeseed oil, linseed oil and palm oil) has no effect on the expression of Δ6Fadgene in comparison with that of Atlantic cod (Gadus morhuaL) fed FO diet. High dietary ALA intake did not increase the transcript abundance of Δ6Fad, Δ5Fadand elongase 5 genes in hippocampus of pig (Sminket al., 2011). Dietary OA, LA and ALA significantly decreased the expression of Δ6Fadin HepG2 genes (liver hepatocellular cells) (Portolesiet al., 2008). The exact reason for such different regulation effects in different animals is unclear at present. It may relate to the regulation of some transcriptional factors, such as PPARs and SREBP, involved in fatty acid metabolism (Matsuzakaet al., 2002; Zhenget al., 2004). Further investigations are needed to illuminate such differences.
In conclusion, abalone has the capacity of synthesizing LC-PUFAs from C18 precursors. In hepatopancreas of abalone, dietary LA stimulated the biosynthesis of n-6 LC-PUFAs in particular ARA, while dietary ALA contributed to the accumulation of n-3 LC-PUFAs, especially EPA. Expression of Δ5Fadsgenes in hepatopancreas of abalone were increased by dietary ALA, decreased by dietary LC-PUFAs (ARA, EPA and DHA), but not affected by OA or LA in diets.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 30871930). We thank Drs. J. Wang and R. T. Zuo for their help in feeding experiment.
Agaba, M. K., Tocher, D. R., Zheng, X., Dickson, C. A., Dick, J. R., and Teale, A. J., 2005. Cloning and functional characterisation of polyunsaturated fatty acid elongases of marine and freshwater teleost fish.Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 142: 342-352.
Ai, Q. H., Zhao, J. Z., Mai, K. S., Xu, W., Tan, B. P., Ma, H. M., and Liufu, Z. G., 2008. Optimal dietary lipid level for largeyellow croaker (Pseudosciaena crocea) larvae.Aquaulture Nutrition, 14: 515-522.
Association of Official Analytical Chemists (AOAC), 1995.Official Methods of Analysis of Official Analytical Chemists International. 16th edition. Association of Official Analytical Chemists, Arlington, VA, USA.
Bahurmiz, O. M., and Ng, W. K., 2007. Effects of dietary palm oil source on growth, tissue fatty acid composition and nutrient digestibility of red hybrid tilapia,Oreochromissp., raised from stocking to marketable size.Aquaculture, 262:382-392.
Bautista-Teruel, M. N., Koshio, S. S., and Ishikawa, M., 2011. Diet development and evaluation for juvenile abalone,Haliotis asininaLinne: Lipid and essential fatty acid levels.Aquaculture, 312: 172-179.
Bell, J. G., Henderson, R. J., Tocher, D. R., McGhee, F., Dick, J. R., Porter, A., Smullen, R. P., and Sargent, J. R., 2002. Substituting fish oil with crude palm oil in the diet of Atlantic salmon (Salmo salar) affects hepatopancreas fatty acid composition and hepatic fatty acid metabolism.The Journal of nutrition, 132: 222-230.
Durazo-Beltrán, E., D’Abramo, L. R., Toro-Vazquez, J. F., Vasquez-Peláez, C., and Viana, M. T., 2003. Effect of triacylglycerols in formulated diets on growth and fatty acid composition in tissue of green abalone (Haliotis fulgens).Aquaculture, 224: 257-270.
González-Rovira, A., Mourente, G., Zheng, X., Tocher, D. R., and Pendón, C., 2009. Molecular and functional characterization and expression analysis of a Δ6 fatty acyl desaturase cDNA of European Sea Bass (Dicentrarchus labraxL.).Aquaculture, 298: 90-100.
Gregory, M. K., See, V. H., Gibson, R. A., and Schuller, K. A., 2010. Cloning and functional characterisation of a fatty acyl elongase from southern bluefin tuna (Thunnus maccoyii).Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 155: 178-185.
Hastings, N., Agaba, M., Tocher, D. R., Leaver, M. J., Dick, J. R., Sargent, J. R., and Teale, A. J., 2001. A vertebrate fatty acid desaturase with Δ5 and Δ6 activities.Proceedings of the National Academy of Sciences, 98: 14304-14309.
Hastings, N., Agaba, M., Tocher, D., Zheng, X., Dickson, C., Dick, J., and Teale, A., 2004. Molecular cloning and functional characterization of fatty acyl desaturase and elongase cDNAs involved in the production of eicosapentaenoic and docosahexaenoic acids from α-linolenic acid in Atlantic salmon (Salmo salar).Marine Biotechnology, 6: 463-474.
Henderson, R., and Tocher, D. R., 1987. The lipid composition and biochemistry of freshwater fish.Progress in Lipid Research, 26: 281-347.
Izquierdo, M., Robaina, L., Juárez-Carrillo, E., Oliva, V., Hernández-Cruz, C. M., and Afonso, J. M., 2008. Regulation of growth, fatty acid composition and delta 6 desaturase expression by dietary lipids in gilthead seabream larvae (Sparus aurata).Fish Physiol Biochem, 34: 117-127.
Kamarudin, M., Ramezani-Fard, E., Saad, C., and Harmin, S., 2012. Effects of dietary fish oil replacement by various vegetable oils on growth performance, body composition and fatty acid profile of juvenile Malaysian mahseer,Tor tambroides.Aquaculture Nutrition, 18: 532-543.
Li, M. Z., Mai, K. S., Ai, Q. H., He, G., Xu, W., Zhang, W. B., Zhang, Y. J., and Zhou, H. H., 2013a. Effects of dietary grape seed oil and linseed oil on growth, muscle fatty acid composition and expression of putative Δ5 fatty acyl desaturase in abaloneHaliotis discus hannaiIno.Aquaculture, 406-407: 105-114.
Li, M. Z., Mai, K. S., He, G., Ai, Q. H., Zhang, W. B., Xu, W., Wang, J., Liufu, Z. G., Zhang, Y. J., and Zhou, H. H., 2013b. Characterization of two Δ5 fatty acyl desaturases in abalone (Haliotis discus hannaiIno).Aquaculture, 416-417: 48-56.
Li, Y., Monroig, O., Zhang, L., Wang, S., Zheng, X., Dick, J. R., You, C., and Tocher, D. R., 2010. Vertebrate fatty acyl desaturase with Δ4 activity.Proceedings of the National Academy of Sciences, 107: 16840-16845.
Lim, P. K., Boey, P. L., and Ng, W. K., 2001. Dietary palm oil level affects growth performance, protein retention and tissue vitamin E concentration of African catfish,Clarias gariepinus.Aquaculture, 202: 101-112.
Livak, K. J., and Schmittgen, T. D., 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCTmethod.Methods, 25: 402-408.
Mai, K., Mercer, J. P., and Donlon, J., 1995. Comparative studies on the nutrition of two species of abalone,Haliotis tuberculataL.and Haliotis discus hannaiIno. III. Response of abalone to various levels of dietary lipid.Aquaculture, 134:68-80.
Mai, K., Mercer, J. P., and Donlon, J., 1996. Comparative studies on the nutrition of two species of abalone,Haliotis tuberculataL. andHaliotis discus hannaiIno. V. The role of polyunsaturated fatty acids of macroalgae in abalone nutrition.Aquaculture, 139: 77-89.
Mateos, H. T., Lewandowski, P. A., and Su, X. Q., 2011. Dietary fish oil supplements increase tissue n-3 fatty acid composition and expression of delta-6 desaturase and elongase-2 in Jade Tiger hybrid abalone.Lipids, 46: 741-751.
Mateos, H. T., Lewandowski, P. A., and Su, X. Q., 2012a. Effects of dietary fish oil replacement with flaxseed oil on tissue fatty acid composition and expression of desaturase and elongase genes.Journal of the Science of Food and Agriculture, 92: 418-426.
Mateos, H. T., Lewandowski, P. A., and Su, X. Q., 2012b. The effect of replacing dietary fish oil with canola oil on fatty acid composition and expression of desaturase and elongase genes in Jade Tiger hybrid abalone.Food Chemistry, 131: 1217-1222.
Matsuzaka, T., Shimano, H., Yahagi, N., Amemiya-Kudo, M., Yoshikawa, T., Hasty, A. H., Tamura, Y., Osuga, J. I., Okazaki, H., and Iizuka, Y., 2002. Dual regulation of mouse Δ5-and Δ6-desaturase gene expression by SREBP-1 and PPARα.Journal of Lipid Research, 43: 107-114.
Metcalfe, L. D., Schmitz, A. A., and Pelka, J. R., 1966. Rapid preparation of fatty acid esters from lipids for gas chromatographic analysis.Analytical Chemistry, 38: 514-515.
Mishra, K., and Samantaray, K., 2004. Interacting effects of dietary lipid level and temperature on growth, body composition and fatty acid profile of rohu,Labeo rohita(Hamilton).Aquaculture Nutrition, 10: 359-369.
Monroig, Ó., Navarro, J. C., Dick, J. R., Alemany, F., and Tocher, D. R., 2012. Identification of a Δ5-like fatty acyl desaturase from the cephalopodOctopus vulgaris(Cuvier 1797) involved in the biosynthesis of essential fatty acids.Marine Biotechnology, 14: 411-422.
Monroig, Ó., Zheng, X., Morais, S., Leaver, M. J., Taggart, J. B., and Tocher, D. R., 2010. Multiple genes for functional Δ6 fatty acyl desaturases (Fad) in Atlantic salmon (Salmo salarL.): Gene and cDNA characterization, functional expression, tissue distribution and nutritional regulation.Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1801: 1072-1081.
Morais, S., Monroig, O., Zheng, X., Leaver, M. J., and Tocher, D. R., 2009. Highly unsaturated fatty acid synthesis in Atlantic salmon: Characterization of ELOVL5-and ELOVL2-like elongases.Marine Biotechnology, 11: 627-639.
Mourente, G., and Tocher, D. R., 1993. Incorporation and metabolism of 14 C-labelled polyunsaturated fatty acids in juvenile gilthead sea breamSparus aurataL.in vivo.Fish Physiol Biochem, 10: 443-453.
Ng, W. K., Lim, P. K., and Boey, P. L., 2003. Dietary lipid and palm oil source affects growth, fatty acid composition and hepatopancreas α-tocopherol concentration of African catfish,Clarias gariepinus.Aquaculture, 215: 229-243.
Olsen, Y., 2011. Resources for fish feed in future mariculture.Aquaculture Environment Interactions, 1: 187-200.
Pickova, J., and Morkore, T., 2007. Alternate oils in fish feeds.European Journal of Lipid Science and Technology, 109: 256-263.
Portolesi, R., Powell, B. C., and Gibson, R. A., 2008. Δ6 desaturase mRNA abundance in HepG2 cells is suppressed by unsaturated fatty acids.Lipids, 43: 91-95.
Ren, H. T., Yu, J. H., Xu, P., and Tang, Y. K., 2012. Influence of dietary fatty acids on hepatopancreas fatty acid composition and expression levels of Δ6 desaturase-like and Elovl5-like elongase in common carp (Cyprinus carpiovar. Jian).Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 163: 184-92.
Riediger, N. D., Othman, R. A., Suh, M., and Moghadasian, M. H., 2009. A Systemic Review of the Roles of n-3 Fatty Acids in Health and Disease.Journal of the American Dietetic Association, 109: 668-679.
Rosenlund, G., Corraze, G., Izquierdo, M., and Torstensen, B. E., 2011. The effects of fish oil replacement on nutritional and organoleptic qualities of farmed fish. In:Fish Oil Replacement and Alternative Lipid Sources in Aquaculture Feeds.Turchini, G. M.,et al., eds., Boca Raton, FL, USA: CRC Press; Taylor and Francis group, 487-522.
Sales, J., and Janssens, G., 2004. Use of feed ingredients in artificial diets for abalone: A brief update.Nutrition Abstracts and Reviews: Series B, 74: 13N-21N.
Seiliez, I., Panserat, S., Corraze, G., Kaushik, S., and Bergot, P., 2003. Cloning and nutritional regulation of a Δ6-desaturaselike enzyme in the marine teleost gilthead seabream (Sparus aurata).Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 135: 449-460.
Seiliez, I., Panserat, S., Kaushik, S., and Bergot, P., 2001. Cloning, tissue distribution and nutritional regulation of a Δ6-desaturase-like enzyme in rainbow trout.Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 130: 83-93.
Smink, W., Gerrits, W., Gloaguen, M., Ruiter, A., and van Baal, J., 2011. Linoleic and α-linolenic acid as precursor and inhibitor for the synthesis of long-chain polyunsaturated fatty acids in liver and brain of growing pigs.Animal, 1: 1-9.
Su, X., Antonas, K., and Li, D., 2004. Comparison of n-3 polyunsaturated fatty acid contents of wild and cultured Australian abalone.International Journal of Food Sciences and Nutrition, 55: 149-154.
Tocher, D. R., 2003. Metabolism and functions of lipids and fatty acids in teleost fish.Reviews in Fisheries Science, 11:107-184.
Tocher, D. R., and Ghioni, C., 1999. Fatty acid metabolism in marine fish: Low activity of fatty acyl Δ5 desaturation in gilthead sea bream (Sparus aurata) cells.Lipids, 34: 433-440.
Tocher, D. R., Zheng, X., Schlechtriem, C., Hastings, N., Dick, J. R., and Teale, A. J., 2006. Highly unsaturated fatty acid synthesis in marine fish: Cloning, functional characterization, and nutritional regulation of fatty acyl Δ6 desaturase of Atlantic cod (Gadus morhuaL.).Lipids, 41: 1003-1016.
Vagner, M., and Santigosa, E., 2011. Characterization and modulation of gene expression and enzymatic activity of delta-6 desaturase in teleosts: A review.Aquaculture, 315:131-143.
Xu, W., Mai, K. S., Zhang, W. B., Liufu, Z. G., Tan, B. P., Ma, H. M., and Ai, Q. H., 2004. Influence of dietary lipid sources on growth and fatty acid composition of juvenile abalone,Haliotis discus hannaiIno.Journal of Shellfish Research, 127:29-40.
Zheng, X., Ding, Z., Xu, Y., Monroig, O., Morais, S., and Tocher, D. R., 2009. Physiological roles of fatty acyl desaturases and elongases in marine fish: Characterisation of cDNAs of fatty acyl Δ6 desaturase and elovl5 elongase of cobia (Rachycentron canadum).Aquaculture, 290: 122-131.
Zheng, X., Tocher, D. R., Dickson, C. A., Bell, J. G., and Teale, A. J., 2004. Effects of diets containing vegetable oil on expression of genes involved in highly unsaturated fatty acid biosynthesis in liver of Atlantic salmon (Salmo salar).Aquaculture, 236: 467-483.
Zheng, X., Tocher, D. R., Dickson, C. A., Bell, J. G., and Teale, A. J., 2005a. Highly unsaturated fatty acid synthesis in vertebrates: New insights with the cloning and characterization of a Δ6 desaturase of Atlantic salmon.Lipids, 40: 13-24.
Zheng, X., Torstensen, B. E., Tocher, D. R., Dick, J. R., Henderson, R. J., and Bell, J. G., 2005b. Environmental and dietary influences on highly unsaturated fatty acid biosynthesis and expression of fatty acyl desaturase and elongase genes in liver of Atlantic salmon (Salmo salar).Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids, 1734: 13-24.
(Edited by Qiu Yantao)
(Received March 30, 2013; revised May 23, 2013; accepted October 20, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
* Corresponding author. Tel: 0086-532-82032038 E-mail: kmai@ouc.edu.cn
 Journal of Ocean University of China2015年2期
Journal of Ocean University of China2015年2期
- Journal of Ocean University of China的其它文章
- Effect of Dietary Lipid on the Growth,Fatty Acid Composition and Δ5 Fads Expression of Abalone(Haliotis discus hannai Ino)Hepatopancreas
- Species Composition and Diversity of Macrobenthos in the Intertidal Zone of Xiangshan Bay, China
- Evaluation of Cytotoxicity and Genotoxicity of Insecticide Carbaryl to Flounder Gill Cells and Its Teratogenicity to Zebrafish Embryos
- Purification of a Diatom and Its Identification to Cylindrotheca closterium
- Mechanical Stress Induces Neuroendocrine and Immune Responses of Sea Cucumber (Apostichopus japonicus)
- Preparation of κ-carra-Oligosaccharides with Microwave Assisted Acid Hydrolysis Method
