Preparation of κ-carra-Oligosaccharides with Microwave Assisted Acid Hydrolysis Method
LI Guangsheng, ZHAO Xia, LV Youjing, LI Miaomiao, and YU Guangli,
1)Key Laboratory of Marine Drugs,Ministry of Education,Qingdao266003,P. R. China
2)Shandong Provincial Key Laboratory of Glycoscience and Glycotechnology,School of Medicine and Pharmacy,Ocean University of China,Qingdao266003,P. R. China
Preparation of κ-carra-Oligosaccharides with Microwave Assisted Acid Hydrolysis Method
LI Guangsheng1),2), ZHAO Xia1),2), LV Youjing1),2), LI Miaomiao1),2), and YU Guangli1),2),*
1)Key Laboratory of Marine Drugs,Ministry of Education,Qingdao266003,P. R. China
2)Shandong Provincial Key Laboratory of Glycoscience and Glycotechnology,School of Medicine and Pharmacy,Ocean University of China,Qingdao266003,P. R. China
A rapid method of microwave assisted acid hydrolysis was established to prepare κ-carra-oligosaccharides. The optimal hydrolysis condition was determined by an orthogonal test. The degree of polymerization (DP) of oligosaccharides was detected by high performance thin layer chromatography (HPTLC) and polyacrylamide gel electrophoresis (PAGE). Considering the results of HPTLC and PAGE, the optimum condition of microwave assisted acid hydrolysis was determined. The concentration of κ-carrageenan was 5 mg mL-1; the reaction solution was adjusted to pH 3 with diluted hydrochloric acid; the solution was hydrolyzed under microwave irradiation at 100 ℃ for 15min. Oligosaccharides were separated by a Superdex 30 column (2.6 cm × 90 cm) using AKTA Purifier UPC100 and detected with an online refractive index detector. Each fraction was characterized by electrospray ionization mass spectrometry (ESI-MS). The data showed that odd-numbered κ-carra-oligosaccharides with DP ranging from 3 to 21 could be obtained with this method, and the structures of the oligosaccharides were consistent with those obtained by traditional mild acid hydrolysis. The new method was more convenient, efficient and environment-friendly than traditional mild acid hydrolysis. Our results provided a useful reference for the preparation of oligosaccharides from other polysaccharides.
κ-carrageenan; oligosaccharides; microwave degradation; acid hydrolysis
1 Introduction
Carrageenans are highly sulfated galactans isolated from marine red algae. They are consisted of linearly repeated modules of alternating 3-linked β-D-galactopyranose (β-Gal, unit G) and 4-linked α-D-galactopyranose (α-Gal, unit D) with unit D frequently occurred as the 3,6-anhydro form (anGal, unit A) (Yanget al., 2009). The main types of carrageenan are kappa (κ-), iota (ι-) and lambda (λ-) depending on the number and position of sulfate groups (Abadet al., 2009).
Because of the superior gelling and high viscosity properties of the native carrageenan, their oligomers are often used. Many evidences have demonstrated that the molecular mass of sulfated polysaccharides was related to their biological activities. Oligomers of carrageenan have showed their promises as anti-herpetic and anti-oxidant (Carlucciet al., 1997; Sunet al., 2010; Yuanet al., 2005), anti-human immunodeficiency virus (HIV) (Yamadaet al., 2000), anti-virus (Wanget al., 2011; Yuet al., 2012) and anti-tumors (Huet al., 2006; Yuanet al., 2006).
Oligomers of carrageenan can be obtained either byhydrolyzing carrageenan or synthesizing from monosaccharide. The first process is considered as the most competitive because a wide variety of oligomers can be obtained from one polymer; while the second is complicated and expensive, and only a low degree of polymerization (DP) oligomers can be produced (Courtois, 2009). Many depolymerization methods of carrageenan have been reported, including acid hydrolysis (Karlsson and Singh, 1999; Myslabodskiet al., 1996), enzymatic hydrolysis (Wu, 2012; Collenet al., 2009; Mouet al., 2003), hydrogen peroxide degradation (Sunet al., 2010) and irradiation degradation with gamma rays (Abadet al., 2009; Relleveet al., 2005). Recently, application of microwave irradiation techniques is attracting more and more attentions due to its effectiveness. It has been used in many fields of carbohydrate research, such as extraction (Rodriguez-Jassoet al., 2011), methylation (Singhet al., 2003), degradation (Singhet al., 2006; Yuet al., 1996) of polysaccharides, and desulfation of sulfated polysaccharides (Navarroet al., 2007). Huet al. (2013) has reported the preparation of guluronic acid oligosaccharides by using microwave irradiation. This method is not only convenient, less time consuming, but also environment-friendly. However, the preparation of κ-carra-oligosaccharides using microwave has not been reported.
The purpose of this study is to establish an efficientand convenient method of preparing a series of different κ-carra-oligosaccharides and determine their structures by electrospray mass spectrometry (ESI-MS).
2 Experiments
2.1 Materials
κ-carraheptaose was prepared by the Shandong Provincial Key Laboratory of Glycoscience and Glycotechnology. Galactose (Gal) was purchased from Sigma (USA).
2.2 Optimization of Microwave-Assisted Acid Hydrolysis of κ-carrageenan
κ-carrageenan was purified by the KCl classification method (Smith and Cook, 1953). The purified κ-carrageenan was dissolved in water and adjusted to pH 2 to 4 with diluted hydrochloric acid. Then an orthogonal L9(3)4test design was used to optimize the hydrolysis condition based on the results of the single-factor experiment. Nine groups of different experiments were carried out under the specific experimental conditions (Table 1) for the depolymerization of κ-carrageenan, and the reaction volume was 2 mL. The parameters of the microwave oven (MARS, CEM Corporation, USA) were listed in Table 2.
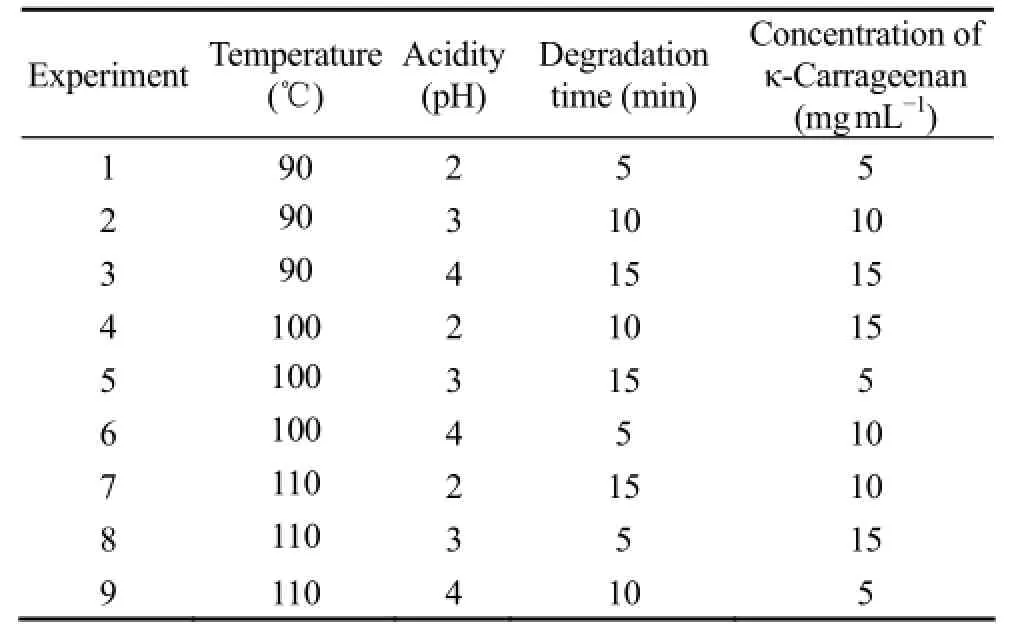
Table 1 Orthogonal experiments of degradation of κ-carrageenan
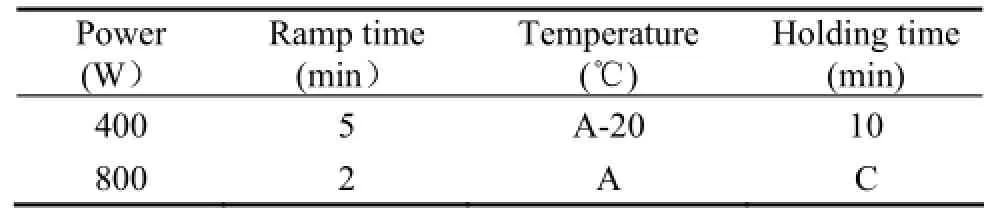
Table 2 The setting parameters of the microwave oven
2.3 High Performance Thin Layer Chromatography (HPTLC) Analysis
The degraded κ-carrageenan oligosaccharides (KCO) were checked on a silica gel HPTLC plate (Merck corporation, Germany) (Zhanget al., 2006), developed in a solvent system of n-butanol/formic acid/water (4:6:1) and Gal was used as a standard. The developed plate was stained by dipping in a reagent containing 1 mL of 37.5% HCl, 2 mL of aniline, 10 mL of 85% H3PO4, 200 mL of acetone and 2 g diphenylamine, and then heated at 100℃ for 5 min.
2.4 Polyacrylamide Gel Electrophoresis (PAGE) Analysis
PAGE was used to analyze the DP of KCO (DP 〉 7), which could not be well detected by HPTLC. An equal volume of each sample (5 µL) was combined with one volume of 50% sucrose, and the mixture was loaded onto a stacking gel of 5% (total acrylamide) and fractionated on a 22% resolving gel. Electrophoresis was performed on the model 3000Xi computer controlled electrophoresis (Bio-Rad, USA) at 100 V for 10 min for stacking gel and 200 V for 120 min for 22% resolving gel. The gel was fixed and stained with Alcian Blue in 2% acetic acid. The gel was scanned, digitized, and analyzed by the software Quantity One 4.6.2 software (Bio-Rad, USA).
2.5 Separation of κ-carrageenan Oligosaccharides
KCO (0.5 g) prepared by microwave-assisted acid hydrolysis under the optimum condition was dissolved in 10 mL of 0.1 mol L-1NH4HCO3and applied on a Superdex 30 column (2.6 × 90 cm) using an AKTA Purifier UPC100 (GE, USA) equipped with an online refractive index detector (Shodex R101, Japan). The column was eluted with 0.1 mol L-1NH4HCO3at a flow rate of 0.5 mL min-1, and each fraction (3 mL per tube) was collected using a fraction collector.
2.6 ESI-MS Analysis
Negative-ionization mode electrospray mass spectroscopy on Micromass Q-Tof Ultima instruments (Waters, Manchester, UK) was performed for the molecular mass analysis of each oligosaccharide. The sample was dissolved in 50% acetonitrile and injected in a 5 μL loop and delivered to the electrospray source using a syringe pump at a flow rate of 5 μL min-1. The nitrogen gas was used as nebulizing and desolvation gas at 4.2 kg cm-2and kept at 250℃.
3 Results and Discussion
3.1 HPTLC Analysis of the Orthogonal Test
In order to obtain the optimal condition of microwave-assisted acid hydrolysis κ-carrageenan, an orthogonal L9(3)4test was used. The KCO obtained under different hydrolysis conditions were analyzed by HPTLC (Fig.1). Gal (lane G) and κ-carraheptaose (lane 10) were used as standards. Lanes 1-9 were the different orthogonal experiments, respectively. Nearly no oligosaccharide (DP 〈 7) was prepared under the condition of experiments 2 and 3, and no oligosaccharide larger than DP 7 was prepared in experiments 4, 7 and 8. Lanes 1, 5, 6 and 9 showed that a series of oligosaccharides can be obtained in experiments 1, 5, 6 and 9, respectively. However, only the oligosaccharides with DP less than 7 can be well separated on the plate due to the low resolution of TLC. The higher resolution of κ-carrageenanoligosaccharides with DP 〉 7 need PAGE technique.
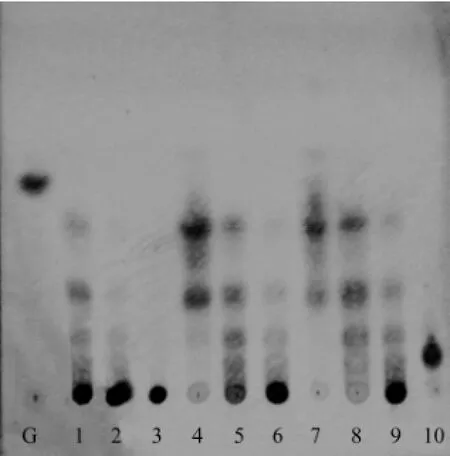
Fig.1 HPTLC analysis of the orthogonal test. Lane G, Gal; Lanes 1-9, samples from the nine orthogonal L9(3)4experiments; Lane 10, κ-carraheptaose.
3.2 PAGE Analysis of the Orthogonal Test
Because of the low resolution of TLC, PAGE was used subsequently to detect oligosaccharides with DP 〉 5 (Fig.2). A κ-carraheptaose (lane 10) was used as standard. Lanes 1-9 were bands for samples from the different orthogonal test, respectively. The result was basically in accordance with that in HPTLC. Nearly no band was observed in experiments 4, 7 and 8, because the oligosaccharides obtained in these experiments were too small to stain with Alcian Blue. The KCO in experiments 1 and 5 between DP 5 and 21 (Fig.3, the peaks were marked as K5 to K21) was showed up. The gel was scanned and analyzed using Quantity One 4.6.2 software, it showed that the mount of oligosaccharides obtained in experiment 5 was larger than that in experiment 1 (Fig.3). Oligosaccharides could also be prepared under the condition in experiments 2, 3, 6 and 9, however, a lot of polysaccharides were left. It means the yield of oligosaccharides in these experiments was lower than that in experiments 1 and 5.
Considering the results of HPTLC and PAGE, the optimum condition of microwave assisted acid hydrolysis of κ-carrageenan was in test 1 (90℃, pH 2, 5 min, 5 mg mL-1) or test 5 (100℃, pH 3, 15 min, 5 mg mL-1). The condition in test 5 (100℃, pH 3, 15 min, 5 mg mL-1) was chosen as the optimum one, because less salt was produced in the hydrolysis procedure for the relative higher pH value and more oligosaccharides could be obtained.

Fig.2 PAGE analysis result of the orthogonal test. Lanes 1-9, samples from the nine orthogonal L9(3)4experiments; Lane 10, κ-carraheptaose.

Fig.3 Grey integral analysis of experiments 1 and 5 in PAGE by Quantity One 4.6.2 software.
3.3 Separation of KCO
The KCO mixture was separated by size exclusion chromatography on a preparative Superdex 30 column. The 0.1 mol L-1NH4HCO3was used as elution solvent due to its good volatility, and it was easy to remove from KCO by rotary evaporation and lyophilization. The preparative Superdex 30 column gave a satisfactory resolution of KCO from DP 3 to 21. Ten major oligosaccharide fractions were marked as K3 to K21 (Fig.4) based on refractive index detector.
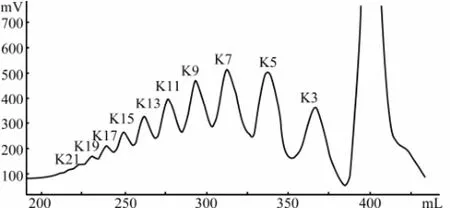
Fig.4 Separation graph of KCO on a Superdex 30 column (2.6 cm × 90 cm). Elution was performed with 0.1 mol L-1NH4HCO3at a flow rate of 0.5 mL min-1and detected by an online refractive index detector. K3-K21 indicated the fractions with degree of polymerization from 3 to 21.
3.4 ESI-MS Analysis of KCO
The main ions observed in the ESI-MS spectra of K3-K13 were listed in Table 3, and the DPs of KCO were determined by the molecular mass of each fraction. The ESI-MS spectra of K9 and K11 were shown in Fig.5. Taking K9 as an example, the ions at m/z 359.84 and 455.55 were corresponding to the five charged ion [M-5H]5-and the four charged ion [M-5H+Na]4-, respectively. So the molecular mass of K9 was calculated as 1804.20 Da (DP = 9). These results indicated that the oligosaccharides obtained by this method were oddnumbered, which was consistent with the oligosaccharides produced with the mild acid hydrolysis method (Yanget al., 2009; Yuet al., 2002; Yuet al., 2006).
In this study, a rapid degradation method of κ-carrageenan was established by microwave assisted acid hydrolysis. The optimum hydrolysis condition was obtained by an orthogonal test, and the results were analyzed by HPTLC, PAGE and ESI-MS. The optimum hydrolysis condition was 100℃ 15 min, pH 3 and 5 mg mL-1ofκ-carrageenan. Odd-numbered κ-carra-oligosaccharides of DP from 3 to 21 were obtained by using this method, and the structures of the oligosaccharides were consistent with the oligosaccharides obtained by acid hydrolysis. This method was more convenient and rapid, and the reaction time was only 15 min, which was significantly shorter than that of traditional mild acid hydrolysis (90 min) (Yanget al., 2009). Besides, the pH value (3) of the reaction solution was much higher than that in the mild acid hydrolysis (0.1 mol L-1H2SO4), producing less salt during the hydrolysis procedure, and this would be very convenient for the further research of carrageenan.
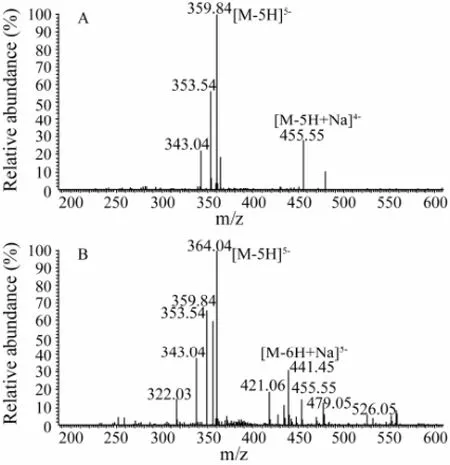
Fig.5 Negative-ion ESI-MS spectra of κ-carra-oligosaccharides. A, K9; B, K11.
4 Conclusions
κ-carra-oligosaccharides can be prepared from κ-carrageenan with a microwave assisted acid hydrolysis method. The optimum hydrolysis condition was 5 mg mL-1of κ-carrageenan, pH 3 and 100℃ for 15 min. Odd-numbered κ-carra-oligosaccharides with degree of polymerization (DP) from 3 to 21 could be obtained with this method, and the structures of the oligosaccharides were consistent with those obtained with traditional mild acid hydrolysis.
Acknowledgements
This research was supported by the Special Fund for Marine Scientific Research in the Public Interest (201005024), NSFC-Shandong Joint Fund for Marine Science Research Centers (U1406402), Qingdao Science & Technology Project (11-2-2-1-hy), and National Science & Technology Support Program of China (2013BAB 01B02).
Abad, L. V., Kudo, H., Saiki, S., Nagasawa, N., Tamada, M., Katsumura, Y., Aranilla, C. T., Relleve, L. S., and De La Rosa, A. M., 2009. Radiation degradation studies of carrageenans.Carbohydrate Polymers, 78 (1): 100-106.
Carlucci, M. J., Pujol, C. A., Ciancia, M., Noseda, M. D., Matulewicz, M. C., Damonte, E. B., and Cerezo, A. S., 1997. Antiherpetic and anticoagulant properties of carrageenans from the red seaweedGigartina skottsbergiiand their cyclized derivatives: Correlation between structure and biological activity.International Journal of Biological Macromolecules, 20 (2): 97-105.
Collen, P. N., Lemoine, M., Daniellou, R., Guegan, J. P., Paoletti, S., and Helbert, W., 2009. Enzymatic degradation of kappa-carrageenan in aqueous solution.Biomacromolecules, 10 (7): 1757-1767.
Courtois, J., 2009. Oligosaccharides from land plants and algae:Production and applications in therapeutics and biotechnology.Current Opinion in Microbiology, 12 (3): 261-273.
Hu, T., Li, C., Zhao, X., Li, G., Yu, G., and Guan, H., 2013. Preparation and characterization of guluronic acid oligosaccharides degraded by a rapid microwave irradiation method.Carbohydrate Research, 373: 53-58.
Hu, X., Jiang, X., Aubree, E., Boulenguer, P., and Critchley, A. T., 2006. Preparation andin vivoantitumor activity of κ-carrageenan oligosaccharides.Pharmaceutical Biology, 44 (9):646-650.
Karlsson, A., and Singh, S. K., 1999. Acid hydrolysis of sulphated polysaccharides. Desulphation and the effect on molecular mass.Carbohydrate Polymers, 38 (1): 7-15.
Mou, H. J., Jiang, X. L., and Guan, H. S., 2003. A κ-carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity.Journal of Applied Phycology, 15 (4): 297-303.
Myslabodski, D. E., Stancioff, D., and Heckert, R. A., 1996. Effect of acid hydrolysis on the molecular weight of kappa carrageenan by GPC-LS.Carbohydrate Polymers, 31 (1-2):83-92.
Navarro, D. A., Flores, M. L., and Stortz, C. A., 2007. Microwave-assisted desulfation of sulfated polysaccharides.Carbohydrate Polymers, 69 (4): 742-747.
Relleve, L., Nagasawa, N., Luan, L. Q., Yagi, T., Aranilla, C., Abad, L., Kume, T., Yoshii, F., and Dela Rosa, A., 2005. Degradation of carrageenan by radiation.Polymer Degrada-tion and Stability, 87 (3): 403-410.
Rodriguez-Jasso, R. M., Mussatto, S. I., Pastrana, L., Aguilar, C. N., and Teixeira, J. A., 2011. Microwave-assisted extraction of sulfated polysaccharides (fucoidan) from brown seaweed.Carbohydrate Polymers, 86 (3): 1137-1144.
Singh, V., Tiwari, A., Kumari, P., and Tiwari, S., 2006. Microwave-promoted hydrolysis of plant seed gums on alumina support.Carbohydrate Research, 341 (13): 2270-2274.
Singh, V., Tiwari, A., Tripathi, D. N., and Malviya, T., 2003. Microwave promoted methylation of plant polysaccharides.Tetrahedron Letters, 44 (39): 7295-7297.
Smith, D. B., and Cook, W. H., 1953. Fractionation of carrageenin.Archives of Biochemistry and Biophysics, 45 (1): 232-233.
Sun, T., Tao, H., Xie, J., Zhang, S., and Xu, X., 2010. Degradation and antioxidant activity of k-carrageenans.Journal of Applied Polymer Science, 117 (1): 194-199.
Wang, W., Zhang, P., Hao, C., Zhang, X. E., Cui, Z. Q., and Guan, H. S., 2011.In vitroinhibitory effect of carrageenan oligosaccharide on influenza A H1N1 virus.Antiviral Research, 92 (2): 237-246.
Wu, S., 2012. Degradation of κ-carrageenan by hydrolysis with commercial α-amylase.Carbohydrate Polymers, 89 (2): 394-396.
Yamada, T., Ogamo, A., Saito, T., Uchiyama, H., and Nakagawa, Y., 2000. Preparation of O-acylated low-molecularweight carrageenans with potent anti-HIV activity and low anticoagulant effect.Carbohydrate Polymers, 41 (2): 115-120.
Yang, B., Yu, G., Zhao, X., Jiao, G., Ren, S., and Chai, W., 2009. Mechanism of mild acid hydrolysis of galactan polysaccharides with highly ordered disaccharide repeats leading to a complete series of exclusively odd-numbered oligosaccharides.FEBS Journal, 276 (7): 2125-2137.
Yu, G., Guan, H., Ioanoviciu, A. S., Sikkander, S. A., Thanawiroon, C., Tobacman, J. K., Toida, T., and Linhardt, R. J., 2002. Structural studies on kappa-carrageenan derived oligosaccharides.Carbohydrate Research, 337 (5): 433-440.
Yu, G., Li, M., Wang, W., Liu, X., Zhao, X., Lv, Y., Li, G., Jiao, G., and Zhao, X., 2012. Structure and anti-influenza A (H1N1) virus activity of three polysaccharides fromEucheuma denticulatum.Journal of Ocean University of China, 11 (4): 527-532.
Yu, G., Zhao, X., Yang, B., Ren, S., Guan, H., Zhang, Y., Lawson, A. M., and Chai, W., 2006. Sequence determination of sulfated carrageenan-derived oligosaccharides by high-sensitivity negative-ion electrospray tandem mass spectrometry.Analytical Chemistry, 78 (24): 8499-8505.
Yu, H., Chen, S., Suree, P., Nuansri, R., and Wang, K., 1996. Effect of microwave irradiation on acid-catalyzed hydrolysis of starch.The Journal of Organic Chemistry, 61 (26): 9608-9609.
Yuan, H., Song, J., Li, X., Li, N., and Dai, J., 2006. Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides.Cancer Letters, 243 (2): 228-234.
Yuan, H., Zhang, W., Li, X., Lu, X., Li, N., Gao, X., and Song, J., 2005. Preparation andin vitroantioxidant activity of kappa-carrageenan oligosaccharides and their oversulfated, acetylated, and phosphorylated derivatives.Carbohydrate Research, 340 (4): 685-692.
Zhang, Z., Yu, G., Zhao, X., Liu, H., Guan, H., Lawson, A. M., and Chai, W., 2006. Sequence analysis of alginate-derived oligosaccharides by negative-ion electrospray tandem mass spectrometry.Journal of the American Society for Mass Spectrometry, 17 (4): 621-630.
(Edited by Qiu Yantao)
(Received May 29, 2013; revised August 9, 2013; accepted December 23, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
* Corresponding author. Tel: 0086-532-82031609 E-mail: glyu@ouc.edu.cn
 Journal of Ocean University of China2015年2期
Journal of Ocean University of China2015年2期
- Journal of Ocean University of China的其它文章
- Effect of Dietary Lipid on the Growth,Fatty Acid Composition and Δ5 Fads Expression of Abalone(Haliotis discus hannai Ino)Hepatopancreas
- Species Composition and Diversity of Macrobenthos in the Intertidal Zone of Xiangshan Bay, China
- Evaluation of Cytotoxicity and Genotoxicity of Insecticide Carbaryl to Flounder Gill Cells and Its Teratogenicity to Zebrafish Embryos
- Purification of a Diatom and Its Identification to Cylindrotheca closterium
- Mechanical Stress Induces Neuroendocrine and Immune Responses of Sea Cucumber (Apostichopus japonicus)
- Development of a Fast ELISA for the Specific Detection of both Leucomalachite Green and Malachite Green
