Development of a Fast ELISA for the Specific Detection of both Leucomalachite Green and Malachite Green
JIANG Yousheng, CHEN Li HU Kun, YU Wenjuan, YANG Xianle, and LU Liqun
College of Fisheries and Life Science,Shanghai Ocean University,Shanghai201306,P. R. China
Development of a Fast ELISA for the Specific Detection of both Leucomalachite Green and Malachite Green
JIANG Yousheng#,*, CHEN Li#, HU Kun, YU Wenjuan, YANG Xianle, and LU Liqun
College of Fisheries and Life Science,Shanghai Ocean University,Shanghai201306,P. R. China
Malachite green (MG), a dye, is an antifungal agent that has been used to treat and prevent fish diseases. It is metabolized into reduced leucomalachite green forms (LMG) that may reside in fish muscles for a long period, thus being harmful to human health. The aim of this study was to develop a competitive and direct enzyme-linked immunosorbent assay (ELISA) to detect MG and LMG specifically. The monoclonal antibody (mAb) to LMG was generated using a hybridoma technique. The obtained mAb showed good cross-reactivity (CR) to malachite green (MG), but not to crystal violet (CV) and Brilliant Green (BG). The mAb was used to develop a fast detecting ELISA of MG and LMG in fish. By introducing the conjugation LMG-HRP, the detection capability was 0.37 ng mL-1for MG and LMG. The mean recovery from spiked grass carp tissues ranged from 76.2% to 82.9% and the coefficients of variation varied between 1.8% and 7.5%. The stable and efficient monoclonal cell line obtained is a sustainable source of sensitive and specific antibody to MG and LMG.
monoclonal antibody; Malachite Green; ELISA; fish
1 Introduction
Malachite Green (MG), a dye, has been extensively and illegally used as a therapeutic agent in aquaculture due to its low production cost, easy availability and high efficacy of treating and preventing fungal and parasitic infections in fish. During the treatment, the dye is released directly into aquatic environments where it is extensively absorbed and metabolized to the reduced form, Leucomalachite Green (LMG) which may reside in fish tissues for a long time (Mitrowskaet al., 2007). It was demonstrated in many animal species and cell lines that LMG residues in the consumed animal tissues may have carcinogenic, genotoxic, mutagenic and teratogenic risks (Hajeeet al., 1995). No safe level of the residue has been established; however, the uplimit required for the detection of total MG and LMG has been set at 2 ng g-1by the Commission Decision 2004/25/EC. MG-related fishery product involved incidents often occur and have been a serious concern to human health. Therefore, the careful monitoring of LMG residues in fishery products for human consumption with sensitive and specific methods is crucial for food safety and human health. The conventional methods of quantifying triphenylmethane dyes andtheir leucometabolites mainly include liquid chromatography with visible or fluorescence detection (Mitrowskaet al., 2005; Longet al., 2009), liquid chromatographymass spectrometry (Dowlinget al., 2007; Tarbinet al., 1998; Taoet al., 2011) and capillary electrophoresis (Luoet al., 2010; Tsaiet al., 2007). These methods usually require professional operators and expensive equipments.
Enzyme-linked immunosorbent assay (ELISA) is a simple, sensitive, economical and high throughput procedure for the specific detection of antigen. Several trials have met successful attempts in producing polyclonal and monoclonal antibodies against triphenylmethanes (Yanget al., 2007; Xinget al., 2009; Shenet al., 2011; Oplatowskaaet al., 2011), and there are ELISA kits available on market,e.g., MaxSignalTM MG/LMG ELISA Test Kit; Malachite Green ELISA Kit and Leucomalachite Green ELISA Kit (GlycoNex Inc., Taiwan); Malachite Green Plate Kit (Abraxis, USA); and Malachite Green EIA (EuroProxima, The Netherlands). Recently, a RNA-aptamer based assay has been reported (Steadet al., 2010).
The aim of this study was to develop a more fast and specific ELISA for the detection of MG and LMG in fish. LMG was conjugated with protein carriers, yielding diverse hapten carrier complexes which were used to immunizing mice, hybridoma cell lines producing high affinity anti-LMG mAb were established. The procedures were used for the preparation of fishery products and mAb-based direct competitive ELISA, with which the LMG and MG residues were detected in the fisheryproducts sensitively and specifically.
2 Materials and Methods
2.1 Materials
Leucomalachite green (purity ≥ 98.5%) was purchased from Zhejiang Guobang Pharmaceutical Co.; while bovine serum albumin (BSA), ovalbumin (OVA) and peroxidase horseradish (HRP) were obtained from Dingguo Biotechnology. The 1-Ethyl-3-(dimethylaminopropyl)-carbodiimide (EDC, purity ≥ 99.3%) was from Shanghai Yanchang Biochemical Technology, and 3,3’,5,5’-tetramethylbenzidine (TMB), HRP-conjugated goat anti-rabbit IgG, RPMI 1640, hypoxanthine aminopterin thymidine (HAT) medium, calf serum clarified, incomplete Freund’s adjuvant (IFA) and complete Freund’s adjuvant (CFA) were purchased from Sigma Chemical (St. Louis, MO, USA). Chemical reagents, such as sodium chloride, potassium chloride and sulfuric acid, were from Shanghai Guoyao. The mouse monoclonal antibody isotyping reagents were purchased from Invitrogen (Carlsbad, CA, USA).
2.2 Preparation of Immunogens and Coating Antigens
The conjugates of LMG-BSA and LMG-OVA were prepared using a modified Carbodiimide method described previously (Huanget al., 2008), and characterized by spectrophotometer absorption, electrophoresis and mass spectra. Firstly, the LMG was deoxidized to NH2-LMG according to the method described by Zhaoet al. (2009). Then, a total of 8 mg BSA, 20 mg NH2-LMG and 240 mg EDC were mixed in 4 mL PBS (pH 5.0, 0.01 mol L-1) at 28℃ for 2 h. The mixture was dialyzed against 200 volumes of PBS (pH 7.2, 0.01 mol L-1) for two days. The conjugates were lyophilized and stored at -80℃. Similarly, the conjugate of CPFX-OVA was generated.
2.3 Generation of mAbs Against LMG
The mAbs were generated by a similar procedure described previously (Huet al., 2010). Briefly, individual Balb/c mice were immunized with 200 μg of LMG-BSA (dissolved in 0.01 mol L-1PBS, pH 7.4) in 50% CFA and boosted with the same amount of antigen in 50% IFA three times. Subsequently, blood samples were obtained from individual mice for measuring the LMG-specific antibodies. After the antibodies titers reached 1:60000, the mice were sacrificed and their spleen cells were fused with SP2/0 myeloma cells, followed by screening the hybridomas using the indirect ELISA. Furthermore, the hybridoma cells were injected into Balb/c mice for the induction of ascites, and mAb in the ascites was purified using a 5 mL protein G sepharose-4B fast flow column according to the manufacturer’s instruction (Beijing Search Biotech, Beijing). In addition, the subclass of the mAb was determined using the mouse monoclonal antibody isotyping reagents with its affinity measured as described previously (Zhouet al., 2009). The cross-reactivity of anti-LMG mAb toward MG and other competitors were assessed according to the method (Holtzappleet al., 1997).
2.4 Indirect ELISA for Fusion Screening
Firstly, 100 μL of LMG-OVA (10 μg mL-1) was added into a 96-well microtitre plate and incubated at 37℃ for 1 h. After being washed three times with washing buffer (0.01 mol L-1PBS, pH 7.4, containing 0.5% Tween 20, PBST), the wells were blocked with 3% BSA in PBS at 4℃overnight. The blocking buffer was discarded and the plate was washed three times with PBST. 100 μL of diluted hybridoma supernatant was added to each well, two wells containing OVA only were used as control. After incubation at 37℃ for 1 h on a plate shaker, the plate was washed three times. 100 μL of anti-mouse HRP-IgG (1:2000) was added to each well and the plate was further incubated for 1 h. After washing three times with PBST, 100 μL of TMB substrate solution (1 mL TMB, 1.5 mg), 5 mL of H2O2(0.03%), 4 mL of 0.2 mol L-1NaAc, pH 5.3) was added to each well. The enzymatic reaction was stopped with 50 μL of 2.0 mol L-1sulphuric acid after 5-10 min, depending on the intensity of the color. The absorbance was measured at 450 nm on a plate reader.
2.5 Preparation of the HRP Conjugates
The LMG haptens were conjugated to HRP in N,N-dicyclohexylcarbodiimide/N-hydroxysuccinimide (DCC/ NHS) reaction according to the method described by Hanet al(2007). LMG-HRP and MG-HRP conjugates were prepared and purified by gel filtration using PD-10 desalting column. All conjugates were stored at -20℃.
2.6 Sample Preparation and Extraction
The muscles of grass carp (Ctenopharyngodon idella) were mixed with the extraction solution (30 mL) of 1 mol L-1HCl-acetonitrile (4/500). After being vortexed for 5 min and centrifuged at 495 g and 4℃ for 15 min, the supernatants were collected and the residue was re-extracted with the same procedures. Subsequently, the supernatants were harvested and loaded on a separate funnel, and then mixed with n-hexane (25 mL). After shaking vigorously for 5 min and settling for 5 min, the aqueous layer was removed and the organic layer was evaporated using a vortex evaporator. The resulting residues were dissolved in 1.0 mL of extraction solution and filtered through a 0.45 μm disposable syringe filter equipped with cellulose acetate membranes.
2.7 Direct Competitive ELISA (Assay Protocol)
Firstly, 100 μL of monoclonal antibody diluted in PBS buffer (pH 7.4, 0.01 mol L-1, 1:2000) were added into each well of the ELISA plates (Dingguo Biotechnology, Shanghai, China) and incubated at 37℃ for 1 h. Then the unbound antibody was tapped out and the wells were blocked with 3% BSA in PBS at 4℃ overnight. After three washes with PBST, 100 μL of sample in PBS buffer (pH 7.4) was added and incubated at room temperaturefor 5 min. And then 100 μL of LMG-HRP conjugate in PBS buffer (1:10000) was added to each well and incubated at 37℃ for 1 h. The plate was washed three times with PBST. Then 100 μL of TMB substrate solution was added to each well and the plate was incubated at room temperature for 10 min. Finally, 50 μL 2.0 mol L-1sulphuric acid was added to stop the reaction. The results were read on a plate reader at 450 nm. Standard curves were obtained by plotting absorbance against the logarithm of LMG concentrations, which were fitted to a fourparameter logistic equation:
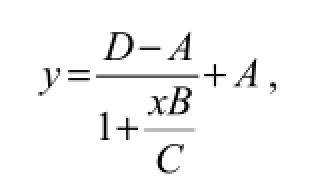
whereAis the minimum absorbance at infinite concentration,Bis the curve slope at the inflection point,Cis the concentration of IC50, andDis the maximum absorbance in the absence of LMG. The limit of detection (LOD) was calculated following the method described by Wanget al. (2007). The LMG residues in the prepared samples were also determined by HPLC using the procedure of Yanget al. (2010).
3 Results
To generate mAbs against LMG, the NH2-LMG was conjugated with BSA by a modified carbodiimide method with a higher coupling efficiency. The conjugation rate of LMG-BSA reached 7:1. The conjugates were characterized by spectrophotometric absorption, electrophoresis, mass spectra, and UV-vis spectra. The UV scanning spectra (220-380 nm) of NH2-LMG, BSA and NH2-LMGBSA conjugates were shown in Fig.1. The characteristic peaks in the region of maximum absorbance of BSA, NH2-LMG and NH2-LMG-BSA conjugates were at 280, 262 and 314 nm, respectively. The success of coupling reaction between LMG and BSA could be determined accordingly.
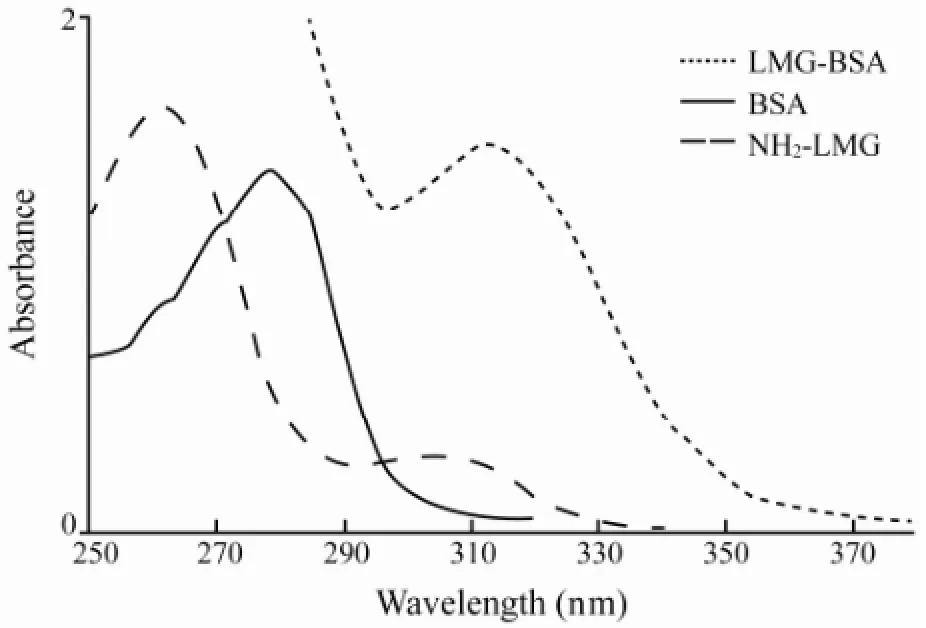
Fig.1 UV scanning spectra of conjugates.
The conjugates were injected into Balb/c mice. The blood samples were collected from individual mice after each boosting and the titers of anti-LMG sera were determined by ELISA. Following boosted for three times, the anti-LMG sera in most mice reached a titer of 1:62500. The antibodies appeared to be specifically for LMG. The indirect ELISA test indicated that the antibodies only reacted with the LMG-OVA, but not OVA. The high titer of anti-LMG specific sera in the mice provided the basis for the generation of mAbs against LMG. Following fusion of SP 2/0 myeloma cells with the splenic cells from the immunized mice with high titer of anti-LMG antibodies, hybridoma cell lines were screened for antibodies against LMG and subjected to cloning by the limited dilution. One of the hybridoma clones, D5, produced high concentrations of mAb and grew stablyin vitrofor at least 3 months. To generate large amounts of mAb, the D5 clone cells were injected into Balb/c mice for producing ascites and the mAb D5 was obtained from ascites. Characterization of D5 mAb revealed that the D5 mAb belonged to IgG1 subclass with an affinity of 4.92 × 109mol L-1.
Next, the direct competitive ELISA was used to prepare calibration curves for LMG-HRP conjugates with a dilution of 1:10000. Different concentrations of LMG resulted in a standard curve (Fig.2). The four-parameter logistic equation was
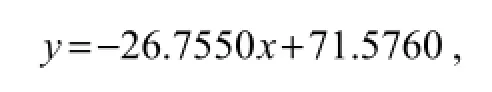
where the value ofR2was 0.9837. The LOD detected by direct competitive ELISA was 0.37 ng mL-1and the linear range was between 50 and 6250 ng mL-1. The specificity of the mAb to other similar triphenylmethane dyes was assayed by the direct competitive ELISA (data not showed). The assay showed 100% cross-reactivity with MG. In contrast, it did not detect crystal violet (CV) and Brilliant Green (BG), because there was no more than 0.01% cross-reactivity to any of other triphenylmethane dyes.
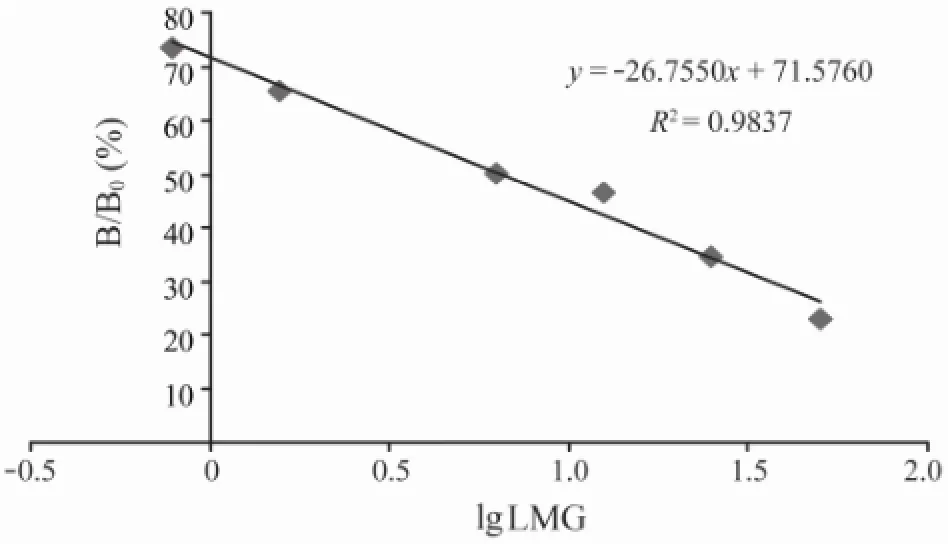
Fig.2 Standard curve established for the detection of LMG with direct ELISA. The assay was used for the detection of different concentrations (0-50 ng mL-1) of LMG and the resulting absorbance values were calculated as the value of individual concentrations of LMG (B)/the value of control without LMG (B0), respectively. The standard curve was established by different concentrations of LMG versus the values of B/B0. Individual value represents the mean ± SD of the concentration of LMG from three independent experiments.
To test the potential application of direct ELISA, fish-ery samples were mixed with different concentrations of LMG, and the recovery rates of LMG were determined by direct competitive ELISA (Table 1). The recovery rates and RSD of LMG from these fish samples ranged from 76.2% to 82.9% and from 1.8% to 7.5%, respectively. Furthermore, the recovery rates and RSD of LMG from different fish samples ranged from 70.5% to 81.3% and from 4.7% to 5.3%, respectively, as were determined with HPLC. The recovery rates and RDS values of LMG by direct ELISA were similar to those by HPLC (P>0.05), demonstrating the high sensitivity and accuracy of direct competitive ELISA for detecting LMG in fishery products.
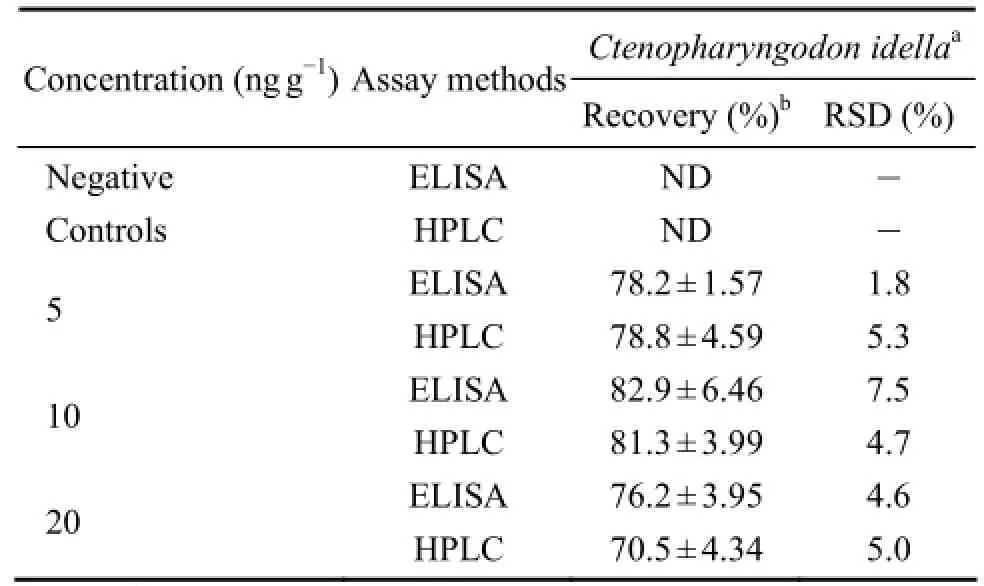
Table 1 Recoveries of ELISA Method to assay LMG residues in muscles of grass carpCtenopharyngodon idella†
4 Discussion
It is found that 80 ng mL-1of LMG-OVA for coating antigen and 0.22 µg of mAb led to a specific detection of 0.37 ng mL-1LMG under optimized conditions. Interestingly, the mAb used in the direct competitive ELISA showed 100% cross-reactivity with MG, a compound with highly structural similarity to LMG (Fig.3), which was similar to the results of Shenet al. (2008) and Gonget al. (2008). Importantly, the direct competitive ELISA did not detect the other triphenylmethane dyes. In addition, the direct competitive ELISA detected LMG with recovery rates and RSD of 76.2%-82.9% and 1.8%-7.5%, respectively, which were similar to those determined with traditional HPLC. Moreover, the sensitivity of ELISA for detection of LMG was similar to that reported with mAb (Zhaoet al., 2009; Wanget al2009) and higher than that using polyclonal antibodies (Xinget al., 2010). So, the mAb produced in this study is highly sensitive and specific, and can be used for the detection of LMG and MG in fishery products by direct competitive ELISA.
ELISA has been demonstrated to be highly sensitive, specific, and suitable for simultaneous analysis of many samples. Furthermore, ELISA usually is relatively cheaper than other common methods and easily performable in this study, we detected small amount of LMG within 2 h. The developed method allowed the detection of both MG and LMG simultaneously, rather than separately as in immunoassays developed by Yanget al. (2007), while the sensitivity of the assay for the analysis of fish was similar to these procedures that claimed by a number of ELISA test kits available on market. In summary, we developed a rapid and sensitive ELISA for the detection of LMG and MG with a sensitivity of 0.37 ng mL-1. The LOD was below current MRL established by the EU and China.

Fig.3 Molecular structure of MG and LMG.
The configuration of assays reported is indirect competitive type: MG/LMG-antigen is immobilized on the well surface, sample/standard and anti-MG/LMG antibody are added and incubated for a defined time and after the washing step an anti-species antibody labeled with HRP is added. This procedure has been applied by Yanget al. (2007), Xinget al. (2009), and some MG and LMG ELISA kits. The ELISA assay developed in the present study was also based on the approach. However, the microtitre plate was precoated with anti-LMG antibody. The subsequent incubation steps with sample/standard and LMG-HRP were kept. This allowed the development of a rapid and sensitive immunoassay for the routine screening of fish samples for MG/LMG contamination.
In conclusion, we developed a rapid and sensitive monoclonal antibody-based ELISA for the detection of LMG and MG with a sensitivity of 0.37 ng mL-1. Therefore, the method can be used as a convenient tool for the rapid detection of LMG and MG in fish tissues.
Acknowledgements
This study was supported by the National High Technology Research and Development Program of China (Granted no. 2011AA10A216), Special Fund for Agroscientific Research in the Public Interest (Granted no. 201203085). The authors were supported by the knowledge service platform of Fish and Fishery Sciences, Shanghai Municipal Education Commission.
Dowling, G., Mulder, P. P. J., Duffy, C., Regan, L., and Smyth, M. R., 2007. Confirmatory analysis of malachite green, leucomalachite green, crystal violet and leucocrystal violet in salmon by liquid chromatography-tandem mass spectrometry.Analytica Chimica Acta, 586 (1-2): 411-419.
Gong, P. F., Zhou, M. H., and Zheng, Z. Q., 2008. Used for testing the whole antigens of malachite green synthesis and identification.Veterinary Pharmaceuticals & Feed Additives, 23 (6): 1-4.
Hajee, C. A. J., and Haagsma, N., 1995. Simultaneous determination of malachite green and its metabolite leucomalachite green in eel plasma using post-column oxidation.Journal of Chromatography B, 669 (2): 219-227.
Han, D., Yu, M., Knopp, D., Niessner, R., Wu, M., and Deng, A., 2007. Development of a highly sensitive and specific enzyme-linked immunosorbent assay for detection of Sudan I in food samples.Journal of Agricultural and Food Chemistry, 55 (16): 6424-6430.
Holtzapple, C. K., Buckley, S. A., and Stanker, L. H., 1997. Production and characterization of monoclonal antibodies against sarafloxacin and cross-reactivity studies of related fluoroquinolones.Journal of Agricultural and Food Chemistry, 45: 1984-1990.
Hu, K., Huang, X. Y., Jiang, Y. S., Fang, W., and Yang, X. L., 2010. Monoclonal antibody based enzyme-linked immunosorbent assay for the specific detection of ciprofloxacin and enrofloxacin residues in fishery products.Aquaculture, 310:8-12.
Huang, X. Y., Hu, K., Fang, W., Jin, Y., and Yang, X. L., 2008. Preparation of ciprofloxacin-carrier protein conjugation and identification of its products.Journal of Shanghai Fishery University, 17 (5): 585-590.
Long, C. Y., Mai, Z. B., Yang, Y. F., Zhu, B. H., Xu, X. M., Lu, L., and Zou, X. Y., 2009. Determination of multi-residue for malachite green, gentian violet and their metabolites in aquatic products by high-performance liquid chromatography coupled with molecularly imprinted solid-phase extraction.Journal of Chromatography A, 1216 (12): 2275-2281.
Luo, X. B., Jiang, X., Tu, X. M., Luo, S. L., Yan, L. S., and Chen, B., 2010. Determination of malachite green in fish water samples by cloud-point extraction coupled to cation-selective exhaustive injection and sweeping-MEKC.Electrophoresis, 31 (4): 688-694.
Mitrowska, K., Posyniak, A., and Zmudzki, J., 2007. The effects of cooking on residues of malachite green and leucomalachite green in carp muscles.Journal of Analytical chemistry, 586 (1-2): 420-425.
Mitrowska, K., Posyniak, A., and Zmudzki, J., 2005. Determination of malachite green and leucomalachite green in carp muscle by liquid chromatography with visible and fluorescence detection.Journal of Chromatography A, 1089 (1-2):187-192.
Oplatowskaa, M., Connollya, L., Stevensonb, P., Steadc, S., and Elliott, C. T., 2011. Development and validation of a fast monoclonal based disequilibrium enzyme-linked immunosorbent assay for the detection of triphenylmethane dyes and their metabolites in fish.Analytica Chimica Acta, 698:51-60.
Shen, Y. D., Deng, X. F., Xu, Z. L., Wang, Y., Lei, H. T., Wang, H., Yang, J. Y., Xiao, Z. L., and Sun, Y. M., 2011. Simultaneous determination of malachite green, brilliant green and crystal violet in grass carp tissues by a broad-specificity indirect competitive enzyme-linked immunosorbent assay.Analytica Chimica Acta, 707: 48-152.
Shen, Y. D., Wang, Y., Sun, Y. M., Lei, H. T., Wang, H., and Xiao, Z. L., 2008. Design, synthesis and identification of leucomalachite green haptens and complete antigens.Food Science, 29 (7): 263-266.
Stead, S. L., Ashwin, H., Johnston, B., Dallas, A., Kazakov, S. A., Tarbin, J. A., Sharman, M., Kay, J., and Keely, B. J., 2010. An RNA-aptamer-based assay for the detection and analysis of malachite green and leucomalachite green residues in fish tissue.Analytical Chemistry, 82 (7): 2652-2660.
Tarbin, J. A., Barnes, K. A., Bygrave, J., and Farrington, W. H., 1998. Screening and confirmation of triphenylmethane dyes and their leuco metabolites in trout muscle using HPLC-Vis and ESP-LC-MS.Analyst, 123 (12): 2567-2571.
Tao, Y. F., Chen, D. M., Chao, X. Q., Yu, H., Pan, Y. H., Liu, Z. L., Huang, L. L., Wang, Y. L., and Yuan, Z. H., 2011. Simultaneous determination of malachite green, gentian violet and their leuco-metabolites in shrimp and salmon by liquid chromatography-tandem mass spectrometry with accelerated solvent extraction and auto solid-phase clean-up.Food Control, 22 (8): 246-1252.
Tsai, C. H., Lin, J. D., and Lin, C. H., 2007. Optimization of the separation of malachite green in water by capillary electrophoresis Raman spectroscopy (CE-RS) based on the stacking and sweeping modes.Talanta, 72 (2): 368-372.
Wang, Z. H., Zhu, Y., Ding, S. Y., He, F. Y., Beier, R. C., Li, J. C., Jiang, H. Y., Feng, C. W., Wan, Y. P., Zhang, S. X., Kai, Z. P., Yang, X. L., and Shen, J. Z., 2007. Development of a monoclonal antibody-based broad-specificity ELISA for fluoroquinolone antibiotics in foods and molecular modeling studies of cross-reactive compounds.Analytical Chemistry, 79: 4471-4483.
Wang, M., Wang, Q., Cai, X. L., Wang, X. C., and Wu, J. J., 2009. Preparation and identification of monoclonal antibody of leuco malachite green.Animal Husbandry and Feed Science, 30 (3): 1-3.
Xing, W., He, L., Yang, H., Sun, C., Li, D., Yang, X., Li, Y., and Deng, A., 2009. Development of a sensitive and group-specific polyclonal antibody-based enzyme-linked immunosorbent assay (ELISA) for detection of malachite green and leucomalachite green in water and fish samples.Journal of the Science of Food and Agriculture, 89 (13): 2165-2173.
Xing, W. W., Wang, R. M., Wang, J. Q., Yang, H., He, L., and Den, A. P., 2010. Sensitive enzyme-linked immunosorbent assay for determination of malachite green and leucomalachite green in fish and fishpond water samples.Chemical Research and Application, 1: 2-46.
Yang, M. C., Fang, J. M., Kuo, T. F., Wang, D. M., Huang, Y. L., Liu, L. Y., Chen, P. H., and Chang, T. H., 2007. Production of antibodies for selective detection of malachite green and the related triphenylmethane dyes in fish and fishpond water.Journal of Agricultural and Food Chemistry, 55 (22): 8851-8856.
Yang, J. L., Chen, P. J., Li, Z. G., Zhao, D. H., and Zhou, Q., 2010. Optimization of HPLC determination of malachite green residue in aquatic products.South China Fisheries Science, 6 (4): 44-48.
Zhao, C. C., Liu, Y. J., Xu, B. X., Yang, T. T., Wu, J., Shen, W. J., Zhang, L. S., Sun, X. L., and Zhao, X. L., 2009. Preparation and identification of anti-leucomalachite green antibodies.Chinese Journal of Health Laboratory Technology, 19 (1): 39-41.
Zhou, Y., Li, Y. S., Pan, F. G., Zhang, Y. Y., Lu, S. Y., Ren, H. L., Li, Z. H., Liu, Z. H., and Zhang, J. H., 2009. Development of a new monoclonal antibody based direct competitive enzyme-linked immunosorbent assay for detection of brevetoxins in food samples.Food Chemistry, 118 (2): 467-471.
(Edited by Qiu Yantao)
(Received May 28, 2013; revised August 5, 2013; accepted July 9, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
# These authors contributed equally to this study.
* Corresponding author. Tel: 0086-21-61900453 E-mail: ysjiang@shou.edu.cn
 Journal of Ocean University of China2015年2期
Journal of Ocean University of China2015年2期
- Journal of Ocean University of China的其它文章
- Impacts of the Two Types of El Niño on Pacific Tropical Cyclone Activity
- Numerical Simulation of Typhoon Muifa (2011) Using a Coupled Ocean-Atmosphere-Wave-Sediment Transport (COAWST) Modeling System
- Estimating the Turbulence Characteristics in the Bottom Boundary Layer of Monterey Canyon
- Composition and Origin of Ferromanganese Crusts from Equatorial Western Pacific Seamounts
- Hydroelastic Analysis of a Very Large Floating Structure Edged with a Pair of Submerged Horizontal Plates
- A Storm Surge Intensity Classification Based on Extreme Water Level and Concomitant Wave Height
