Dietary Protein Requirement of Juvenile Turbot (Scophthalmus maximus Linnaeus)
LIU Xingwang, MAI Kangsen LIUFU Zhiguo and AI Qinghui
1)Key Laboratory of Nutrition and Feed of Department of Agriculture of China,Ocean University of China,Qingdao266003,P. R. China
2)Guangdong Evergreen Feed Industrial Co.,Ltd.,Zhanjiang524094,P. R. China
Dietary Protein Requirement of Juvenile Turbot (Scophthalmus maximus Linnaeus)
LIU Xingwang1),2), MAI Kangsen1), LIUFU Zhiguo1), and AI Qinghui1),*
1)Key Laboratory of Nutrition and Feed of Department of Agriculture of China,Ocean University of China,Qingdao266003,P. R. China
2)Guangdong Evergreen Feed Industrial Co.,Ltd.,Zhanjiang524094,P. R. China
The dietary protein requirement of juvenile turbot (initial average weight, 38.2 g ± 0.1 g) reared indoor in aerated aquaria was determined in this study. Five energy equal experimental diets were formulated with fish meal as protein source, which contained different concentrations of protein (47.2%, 51.0%, 54.6%, 59.3% and 63.6% of dry diet). Three groups of fish with 18 individuals in each, were cultured in 300-L tanks and fed twice a day for 8 weeks. During culture, temperature was controlled between 15.0 and 18.0℃, salinity was controlled between 28.5 and 32.0, acidity was controlled between pH7.8 and pH8.5, and ammonia nitrogen was maintained below 0.03 mg L-1and dissolved oxygen was maintained about 7 mg L-1. Results showed that the growth of fish was significantly affected by dietary protein content (P〈 0.05). Specific growth rate (SGR) of turbot increased when dietary protein content varied between 47.2% and 51.0% (P〈 0.05), and then kept stable when dietary protein content was higher than 51.0%. Fish which were fed the diet containing 63.6% protein showed the highestSGRwhile those fed the diet containing 59.3% protein showed the highest feed efficiency rate. No significant difference of feed intake and protein efficiency ratio was found among experimental diets (P〉 0.05). Broken-line regression analysis ofSGRshowed that the optimal dietary protein requirement of turbot was about 57.0%.
turbot;Scophthalmus maximus; protein requirement; feed nutrition
1 Introduction
An optimal dietary protein content is important for fish growth and farming environment maintenance (Guoet al., 2012). Accurate information on the protein requirement of fish is critical for initiating a new aquaculture species as dietary protein usually represents the highest proportion of a commercial feed cost (Nget al., 2008). It is important to determine the optimum dietary protein content for meeting success in fish culture.
Due to its fast growth and high consuming demand, turbot,Scophthalmus maximus, has been widely cultured indoor intensively Northern China. A series of studies on protein nutrition of turbot have been carried out. The documented protein requirement ranged from 35% to 69.8% (Adronet al., 1976; Caceres-Martinezet al., 1984; Leeet al., 2003). The optimum dietary protein content for the most cost-effective turbot culture is not fixed. The variation of the optimum reported may be due to the difference in fish size and age, dietary protein quality and energy richness, and protein energy balance (Wilson,2002). Most in use commercial diets of turbot in China contained 11%-12% of crude lipid, which is the maximum that can be achieved with extruders in China currently. The objective of this study was to optimize the protein requirement of turbot with practical ingredients containing 11.5% lipid. The results may provide more practical value to Chinese turbot feed industry.
2 Materials and Methods
2.1 Diets
Five energy equal (about 20.5 MJ kg-1) testing diets were formulated to contain different concentrations of protein (47.2%, 51.0%, 54.6%, 59.3% and 63.6% of dry diet) (Table 1). Fish meal was used as the dietary protein source. Wheat flour and wheat-middlings were used to replace dietary protein. Ingredients were ground into a fine powder through a 320 μm mesh. All the ingredients were thoroughly mixed in a V-type blender (Shanghai Tianxiang & Chentai Pharmaceutical Machinery Co., Ltd., China) and 30% water was added to produce stiff dough. The dough was then extruded through a 3-mm die using an extruder (260, Shandong Weihai Youyi Machinery Co., Ltd., China). The moist pellets were dried in an oven set at 60℃ for about 10 h, and stored at -20℃.
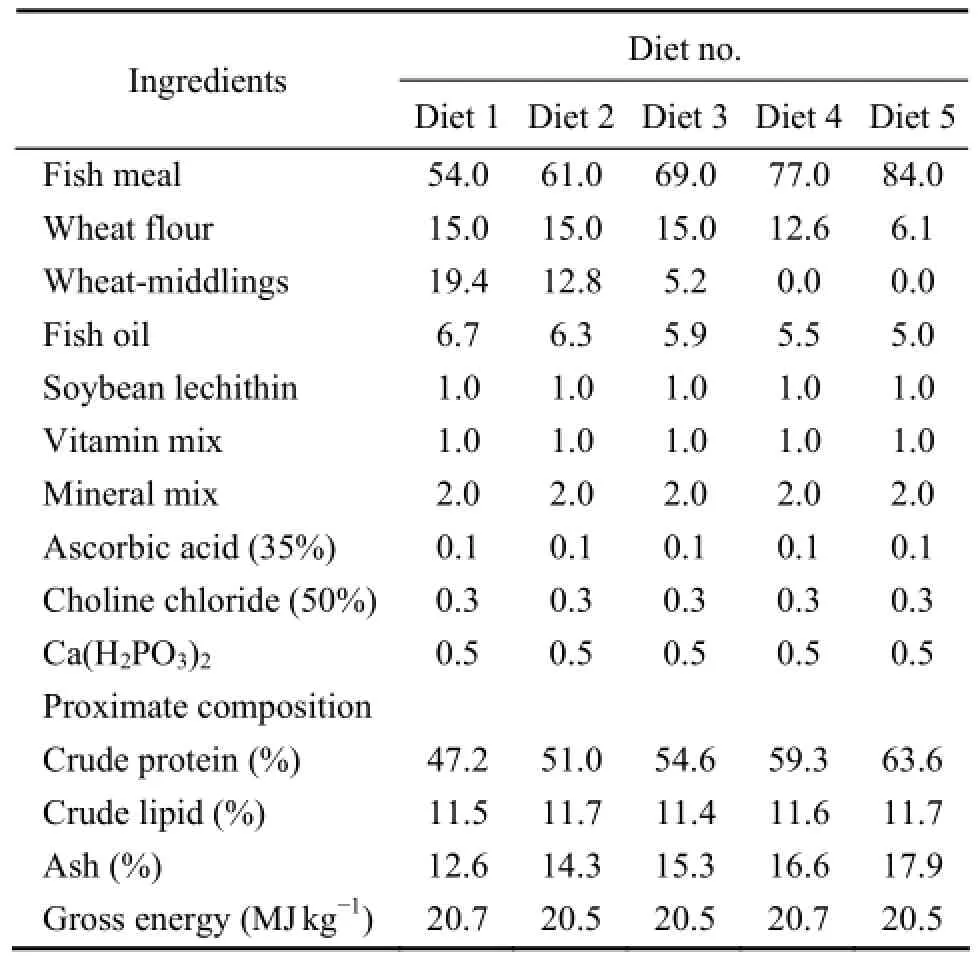
Table 1 Formulation and proximate composition (% dry weight) of five experimental diets fed to juvenile turbot
2.2 Growth Evaluation
Juvenile turbot were obtained from Haiyang Yellow Sea Aquatic Product Co., Ltd., Yantai, China. Prior to experiment, the fish were acclimated to a commercial diet (Qihao Bio-tech Co., Ltd., China) for 2 weeks and were fed twice a day to apparent satiation. At the beginning of experiment, fish were starved for 24 h, and those in similar size (initial weight 38.2 g ± 0.1 g) were distributed into 15 300-L fiberglass tanks, 18 each. Each testing diet was randomly assigned to triplicate tanks. Juvenile turbot were fed to visual satiety twice a day for 8 weeks. Each tank was provided a continuous flow of sand-filtered seawater (3 L min-1) with continuous aeration. Water quality was monitored daily. During experiment, temperature was controlled at 15.0-18.0℃, salinity was controlled at 28.5-32.0, acidity was controlled between pH7.8 and pH8.5, ammonia nitrogen was maintained lower than 0.03 mg L-1and dissolved oxygen was maintained about 7 mg L-1.
2.3 Sampling and Chemical Analysis
Fish were fasted for 24 h before harvesting. The total number and mean body weight of fish each tank were measured to calculate specific growth rate, feed efficiency ratio, protein efficiency ratio and survival. Six fish at beginning and six at the end each tank were randomly collected and frozen (-70℃) for determining proximate analysis. Analysis of dry matter (105℃, 24 h), crude protein (Kjeldahl nitrogen × 6.25), crude lipid (ether extraction by Soxhlet method) and ash (550℃, 18 h) in diets and whole-body were performed following standard laboratory procedures (AOAC, 1990). Gross energy in diet was determined by an adiabatic bomb calorimeter (Parr 1281, USA).
2.4 Calculations and Statistical Analysis
The following variables were calculated:
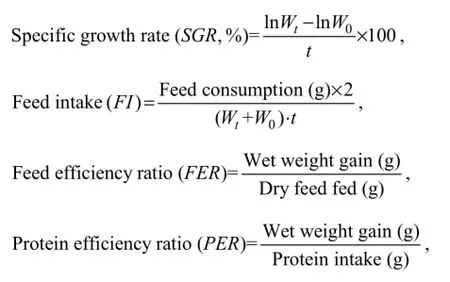
whereWtis final body weight,W0is initial body weight,tis experimental duration in days.
All data obtained for the test diets were subjected to one-way ANOVA to determine if there was significant effect due to dietary crude protein. When significant (P〈0.05) differences were detected, Duncan’s multiple range was used to compare mean values among testing diets. Optimal dietary protein level for juvenile turbot was determined by the broken-line model usingSGRdata. All analyses were conducted using SPSS (version 11.0, Chicago, IL, USA).
3 Results
In 8 weeks feeding trial, mean survival ranged from 90.7% to 100%, which was not related to dietary protein content (Table 2).FIandPERwere not significantly different among testing diets (P〉 0.05). However,SGRandFERwere significantly affected by dietary protein content (P〈 0.05).SGRandFERof turbot increased when dietary protein was increased from 47.2% to 51.0% (P〈 0.05). When the protein concentration was higher than 51.0%, no significant difference was found. Fish fed the diet with 63.6% protein had the highestSGRwhile those fed the diet with 59.3% protein obtained the highestFER. Based onSGRresponse using the broken-line model (Fig.1), optimum dietary protein content for the growth of juvenile turbot was estimated to be 57.0% (Y= 1.54-0.05(57.00-X) (R2= 0.69)).
Carcass moisture, protein, lipid and ash contents were not significantly affected by protein content of diets (P〉0.05) (Table 3). Carcass moisture was fairly consistent as it ranged from 76.7% to 77.5%. Carcass protein rangedfrom 15.2% to 15.9%. Carcass lipid ranged from 3.8% to 4.1% and carcass ash ranged from 3.2% to 3.5%.
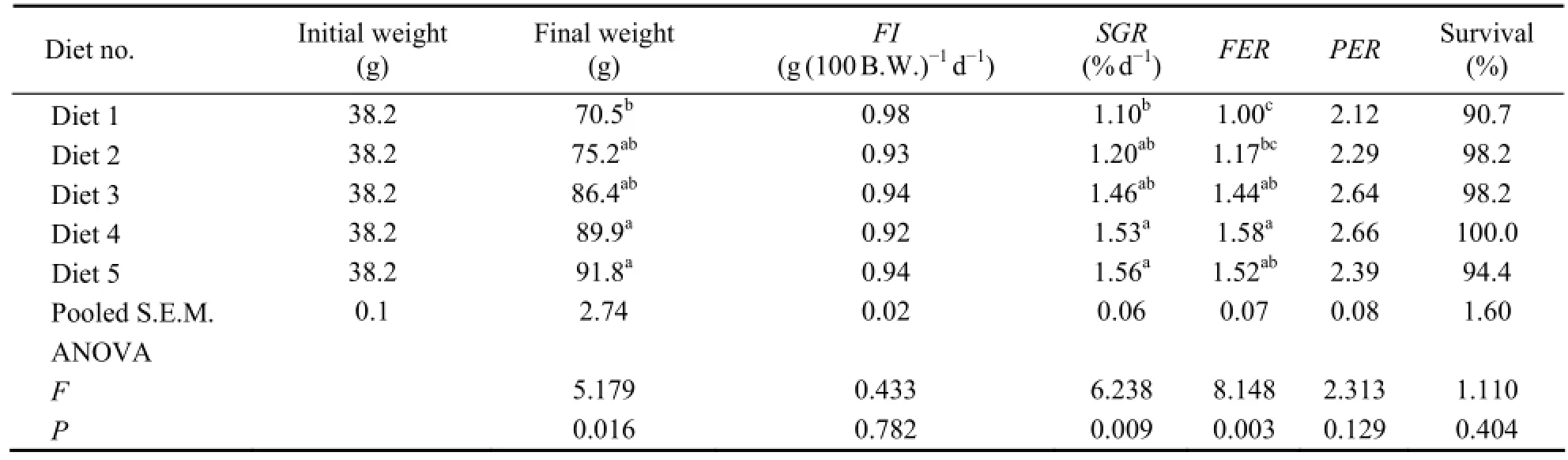
Table 2 Growth performances of turbot fed diets containing various protein levels

Fig.1 Optimal dietary protein requirement of turbot juveniles, based on the broken-line regression model ofSGRversus dietary protein level.
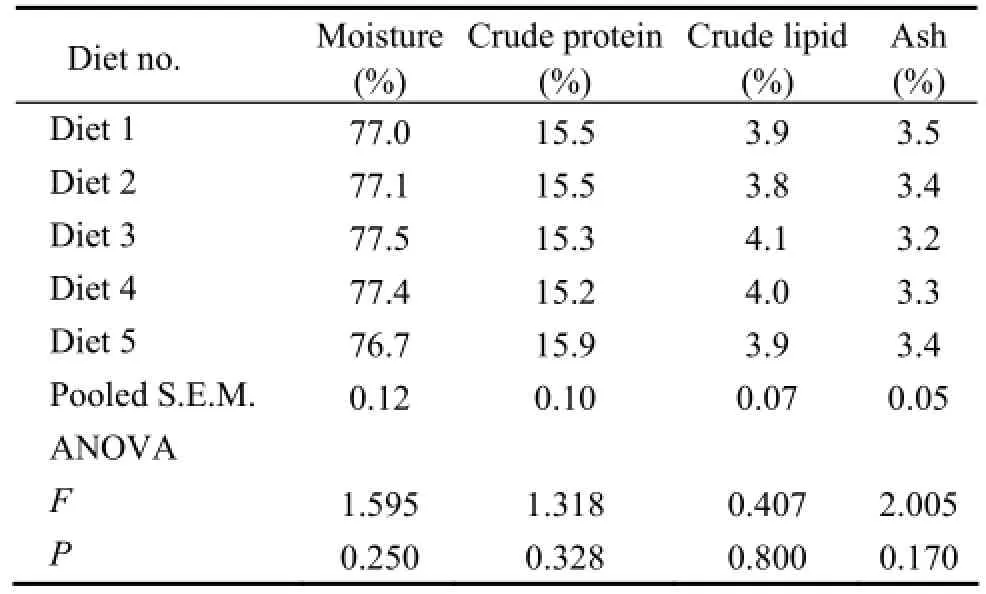
Table 3 Proximate composition (% wet weight) of the whole body of turbot fed diets containing various protein levels
4 Discussion
Results of the present study showed thatSGRandFERof turbot increased when dietary protein content increased from 47.2% to 51.0%, and then became stable when in the dietary protein content increased higher. The best growth was observed in fish fed 63.6% of protein. A broken-line model equation on basis of regression analysis ofSGRagainst dietary protein content indicated that the optimal dietary protein for the maximum growth was 57.0%. This requirement value is higher than that reported in other flatfish including plaice, Japanese flounder, winter flounder, southern flounder and starry flounder (50.0%-50.8% crude protein) (Coweyet al., 1972; Leeet al., 2000; Alamet al., 2004; Hebbet al., 2003; Gonzalezaet al., 2005; Leeet al., 2006). For turbot, a series of studies on protein nutrition have been carried out. Adronet al. (1976) reported that the growth of 10 g turbot fed 35% protein containing diet was almost the same as those fed 50% protein containing diet while Caceres-Martinezet al. (1984) observed the best growth when the diet contained 69.8% of protein. For 89-155 g turbot, Leeet al. (2003) concluded that the optimal dietary protein content was 49.4% with white fishmeal as protein source. The difference among these experiments may be due to different fish sizes, energy levels or ingredients. In this study, practical formulations were designed and high quality fishmeal was used as the only protein source. And the lipid level designed in this research was about 11.5%, which was the maximum of fat that could be achieved by extruder now used in China. So the result obtained from this study would be more valuable for Chinese turbot farming industry.
Normally, protein efficiency ratio (PER) tends to decrease with dietary protein content increase (Denget al., 2011; Coutinhoet al., 2012). It is generally known that fish cannot utilize excess dietary protein for protein synthesis but can utilize it for energy generation (Kim and Lee, 2009). In this study, there was no significant difference ofPERamong different testing diets (P〉 0.05). The maximumPERwas observed at 59.3% dietary protein content, which was similar to the optimal obtained by broken-line model. Similar result has also been reported by Leeet al. (2003). These results suggested that the protein requirement of turbot was relatively higher than that of other flat fish.
In this study, dietary protein content did not significantly affect final whole-body content of turbot (Table 3). This agreed well with the results obtained from turbot by other researchers (Adronet al., 1976; Caceres-Martinezet al., 1984; Leeet al., 2003). It was reported that the protein and moisture content of whole body of fish generally tended to increase as dietary protein content increased while body lipid content showed an inverse relationship with body protein and moisture (Martinez-Palacioset al., 1996; Allanet al., 2001; Luoet al., 2004; Craiget al., 2006). However, other results showed that proximate composition of the whole body of fish was not significantly affected by dietary protein content (Mooreet al., 1988; Nget al., 2001). Shearer (1994) pointed out that the body proximate composition of fish was influenced by both endogenous factors such as fish size and sex and exogenous factors such as diet composition and culture environment. This may partly explain the different conclusions of the experiments.
The results of the present study indicated that turbot juvenile require about 57.0% of protein in their diets to obtain maximum growth, which was in the range of reported for other carnivorous fish. Further studies will be conducted to investigate the essential amino acid requirements and the apparent digestibility coefficiency of protein.
Acknowledgements
The study was supported by the earmarked fund for Modern Agro-Industry Technology Research System with Grant No. CARS 50-G-08. We thank Drs. Y. J. Zhang, W. Xu, W. B. Zhang, H. H. Zhou, X. N. Li, L. Zhu, X.W. Yi, M. Peng and D. Xu for their helps in the study.
Adron, J. W., Blair, A., Cowey, C. B., and Shanks, A. M., 1976. Effects of dietary energy level and dietary energy source on growth, feed conversion and body composition of turbot (Scophthalmus maximusL.).Aquaculture, 7: 125-132.
Alam, M. S., Teshima, S. I., Koshio, S., Uyan, O., and Ishikawa, M., 2004. Effects of dietary protein and lipid levels on growth and body composition of juvenile Japanese flounder,Paralichthys olivaceus, fed diets containing intact proteins or crystalline amino acids.Journal of Applied Aquaculture, 14: 115-131.
Allan, G. L., Johnson, R. J., Booth, M. A., and Stone, D. J., 2001. Estimating digestible protein requirements of silver perch,Bidyanus bidyanusMitchell.Aquaculture Research, 32:337-347.
Association of Official Analytical Chemists (AOAC), 1990.Official Methods of Analysis. 15th edition. Association of Official Analytical Chemists, Arlington, VA, 1298pp.
Caceres-Martinez, C., Cadena-Roa, M., and Metailler, R., 1984. Nutritional requirements of turbot (Scophthalmus maximus): I. A preliminary study of protein and lipid utilization.Journal of the World Marine Society, 15: 191-202.
Coutinho, F., Peres, H., Guerreiro, I., Pousao-Ferreira, P., and Oliva-Teles, A., 2012. Dietary protein requirement of sharpsnout sea bream (Diplodus puntazzo, Cetti 1777) juveniles.Aquaculture, 356-357: 391-397.
Cowey, C. B., Pope, J. A., Asron, J. W., and Blair, A., 1972. Studies on the nutrition of marine flatfish. The protein requirement of plaice (Pleuronectes platessa).The BritishJournal of Nutrition, 28: 447-456.
Craig, S. R., Schwarz, M. H., and McLean, E., 2006. Juvenile cobia (Rachycentron canadum) can utilize a wide range of protein and lipid levels without impacts on production characteristics.Aquaculture, 261: 384-391.
Deng, D., Ju, Z. Y., Dominy, W., Murashige, R., and Wilson, R. P., 2011. Optimal dietary protein levels for juvenile Pacific threadfin (Polydactylus sexfilis) fed diets with two levels of lipid.Aquaculture, 316: 25-30.
Gonzaleza, S. R., Craig, E., McLean, M. H., Schwarz, M. H., and Flick, G. J., 2005. Dietary protein requirement of southern flounder,Paralichthys lethostigma.Journal of Applied Aquaculture, 17: 37-50.
Guo, Z. Q., Zhu, X. M., Liu, J. S., Han, D., Yang, Y. X., Lan, Z. Q., and Xie, S. Q., 2012. Effects of dietary protein level on growth performance, nitrogen and energy budget of juvenile hybrid sturgeon,Acipenser baerii ♀×A. gueldenstaedtii ♂.Aquaculture, 338-341: 89-95.
Hebb, C. D., Castell, J. D., Anderson, D. M., and Batt, J., 2003. Growth and feed conversion of juvenile winter flounder (Pleuronectes americanus) in relation to different protein-to-lipid levels in isocaloric diets.Aquaculture, 221: 439-449.
Kim, S. S., and Lee, K. J., 2009. Dietary protein requirement of juvenile tiger puffer (Takifugu rubripes).Aquaculture, 287:219-222.
Lee, J. K., Cho, S. H., Park, S. U., Kim, K. D., and Lee, S. M., 2003. Dietary protein requirement for young turbot (Scophthalmus maximusL.).Aquaculture Nutrition, 9: 283-286.
Lee, S. M., Cho, S. H., and Kim, K. D., 2000. Effects of dietary protein and energy levels on growth and body composition of juvenile flounder (Paralichthys olivaceus).Journal of World Aquaculture Society, 31: 306-315.
Lee, S. M., Lee, J. H., Kim, K. D., Kim, K. D., and Lee, S. M., 2006. Optimum dietary protein for growth of juvenile starry flounder,Platichthys stellatus.Journal of the World Aquaculture Society, 37: 200-203.
Luo, Z., Liu, Y., Mai, K., Tian, L., Liu, D., and Tan, X., 2004. Optimal dietary protein requirement of grouperEpinephelus coioidesjuveniles fed isoenergetic diets in floating net cages.Aquaculture Nutrition, 10: 247-252.
Martinez-Palacios, C. A., Harfush-Melendez, M., Chavez-Sanchez, C., and Ross, L. G., 1996. The optimum dietary protein level for the Mexican cichlidCichlasoma urophthalmus(Gunther): A comparison of estimates derived from experiments using fixed-rate feeding and satiation feeding.Aquaculture Nutrition, 2: 11-20.
Moore, B. J., Hung, S. S. O., and Medrano, J. F., 1988. Protein requirement of hatchery-produced juvenile white sturgeon (Acipenser transmontanus).Aquaculture, 71: 235-245.
Ng, W. K., Abdullah, N., and De Silva, S. S., 2008. The dietary protein requirement of the Malaysian mahseer,Tor tambroides(Bleeker), and the lack of protein-sparing action by dietary lipid.Aquaculture, 284: 201-206.
Ng, W. K., Soon, S. C., and Hashim, R., 2001. The dietary protein requirement of a bagrid catfish,Mystus nemurus(Cuvier & Valenciennes), determined using semipurified diets of varying protein level.Aquaculture Nutrition, 7: 45-51.
Shearer, K. D., 1994. Factors affecting the proximate composition of cultured fishes with emphasis on salmonids.Aquaculture, 119: 63-88.
Wilson, R. P., 2002. Amino acids and proteins. In:Fish Nutrition. 3rd edition. Halver, J. E., and Hardy, R. W., eds., Academic Press, San Diego, CA, 144-175.
(Edited by Qiu Yantao)
(Received April 3, 2013; revised May 7, 2013; accepted November 3, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
* Corresponding author. Tel: 0086-532-82031943 E-mail: qhai@ouc.edu.cn
 Journal of Ocean University of China2015年2期
Journal of Ocean University of China2015年2期
- Journal of Ocean University of China的其它文章
- Impacts of the Two Types of El Niño on Pacific Tropical Cyclone Activity
- Numerical Simulation of Typhoon Muifa (2011) Using a Coupled Ocean-Atmosphere-Wave-Sediment Transport (COAWST) Modeling System
- Estimating the Turbulence Characteristics in the Bottom Boundary Layer of Monterey Canyon
- Composition and Origin of Ferromanganese Crusts from Equatorial Western Pacific Seamounts
- Hydroelastic Analysis of a Very Large Floating Structure Edged with a Pair of Submerged Horizontal Plates
- A Storm Surge Intensity Classification Based on Extreme Water Level and Concomitant Wave Height
