Distribution and Ecological Risk Assessment of HCHs and DDTs in Surface Seawater and Sediment of the Mariculture Area of Jincheng Bay, China
HU Yanbing, SUN Shan SONG Xiukai MA Jianxin and RU Shaoguo
1)College of Marine Life Sciences,Ocean University of China,Qingdao266003,P. R. China
2)Provincial Key Laboratory of Restoration for Marine Ecology of Shandong,Shandong Marine Resource and Environment Research Institute,Yantai264006,P. R. China
3)Research Center for the Environment Administration and Development Strategy,Third Institute of Oceanography State Oceanic Administration,Xiamen361005,P. R. China
Distribution and Ecological Risk Assessment of HCHs and DDTs in Surface Seawater and Sediment of the Mariculture Area of Jincheng Bay, China
HU Yanbing1),2),3), SUN Shan2), SONG Xiukai2), MA Jianxin2), and RU Shaoguo1),*
1)College of Marine Life Sciences,Ocean University of China,Qingdao266003,P. R. China
2)Provincial Key Laboratory of Restoration for Marine Ecology of Shandong,Shandong Marine Resource and Environment Research Institute,Yantai264006,P. R. China
3)Research Center for the Environment Administration and Development Strategy,Third Institute of Oceanography State Oceanic Administration,Xiamen361005,P. R. China
The distribution of hexachlorocyclohexanes (HCHs) and dichlorodiphenyltrichloroethanes (DDTs) in the surface seawater and sediment of Jincheng Bay mariculture area were investigated in the present study. The concentration of total HCHs and DDTs ranged from 2.98 to 14.87 ng L-1and were 〈 0.032 ng L-1, respectively, in surface seawater, and ranged from 5.52 to 9.43 and from 4.11 to 6.72 ng g-1, respectively, in surface sediment. It was deduced from the composition profile of HCH isomers and DDT congeners that HCH residues derived from a mixture of technical-grade HCH and lindane whereas the DDT residues derived from technical-grade DDT and dicofol. Moreover, both HCH and DDT residues may mainly originate from historical inputs. The hazard quotient of α-HCH, β-HCH, γ-HCH and δ-HCH to marine species was 0.030, 0.157, 3.008 and 0.008, respectively. It was estimated that the overall probability of adverse biological effect from HCHs was less than 5%, indicating that its risk to seawater column species was low. The threshold effect concentration exceeding frequency of γ-HCH,p,p’-DDD,p,p’-DDE andp,p’-DDT in sediment ranged from 8.3% to 100%, and the relative concentration of the HCH and DDT mixture exceeded their probable effect level in sediment. These findings indicated that the risk to marine benthos was high and potentially detrimental to the safety of aquatic products,e.g., sea cucumber and benthic shellfish.
DDT; ecological risk assessment; HCH; mariculture area; organochlorine pesticide; sediment quality guideline
1 Introduction
Hexachlorocyclohexanes (HCHs) and dichlorodiphenyltrichloroethanes (DDTs) are representative efficacious organochlorine pesticides that were used extensively all over the world between 1950s and early 1980s. Due to their semi-volatility and high persistence, HCHs and DDTs can be transported over long distancesviaatmospheric circulation and ocean currents (Guglielmoet al., 2009; Li and Macdonald, 2005). Accordingly, these chemicals are ubiquitous throughout the world including polar regions. From 1950s to 1980s, 4.9 million tons of technical-grade HCH and 0.4 million tons of technical-grade DDT were produced in China, accounting for 33% and 20% of the world total, respectively. After their prohibition in agriculture in 1983, lindane containing 〉99% of γ-HCH and dicofol containing 3%-7% of DDT gradually took their place in China (Qiuet al., 2005;Zhanget al., 2011). As a consequence, HCHs and DDTs were remained in some regions of China, especially in estuaries and coastal waters (Gonget al., 2007). As reported by Zhaoet al. (2010), the concentration of total HCHs (∑HCH) and total DDTs (∑DDT) ranged from 1.9 to 1620 and from bellow detection limit to 155 ng g-1, respectively, in surface sediment of tidal reach of Haihe River, with the highest higher than the maximum allowable, 1500 and 100 ng g-1set as Grade III of theMarine Sediment Quality of China(GB 18668-2002). Linet al., (2009) reported that the concentration of ∑HCH and∑DDT ranged from bellow detection limit to 12 and from 9 to 7350 ng g-1in nine fishing harbours of China, respectively, and the highest of ∑DDT was 70-fold higher than the maximum allowable set as Grade III of GB 18668-2002.
HCHs and DDTs can be absorbed by organisms through multiple routes and may lead to diverse pathologies (Bernardet al., 2007; Ssebugereet al., 2010). Phippset al. (1995) reported that trace amount ofp,p’-DDT decreased the survival of amphipodHyalella azteca, with a median lethal concentration (LC50) of 0.07 μg L-1. Sumithet al. (2009) demonstrated that the residue of γ-HCH may affect phenoloxidase enzyme activity in the postlarvae ofPenaeus monodonFabricius inhabiting Chantaburi River estuary, Thailand, and the maximum in sediment was as high as 58.1 ng g-1. In addition, both HCHs and DDTs can accumulate in aquatic organisms gradually due to their high hydrophobicity. These findings may allow us to believe that exposure to HCHs and DDTs, even at low concentration, is detrimental.
Jincheng Bay south Bohai Sea is an important mariculture area North China, whereArgopecten irradias,Apostichopus japonicusare cultured and diverse wild shellfish such asRuditapes philippinarumandRapana venosainhabit. The territorial region surrounding the bay is used to culturing cotton and cereals. Due to intensive application of HCHs and DDTs there (Li, 1999; Liet al., 1998), a considerable amount of HCHs and DDTs residues were received by Jincheng Bay and its adjacent areas. By bioconcentration and biomagnifications, HCHs and DDTs were largely accumulated in competent species and diverse normal species. The study on the distributions of HCHs and DDTs and their risk to marine species and aquatic products should be highly appreciated.
The widely adopted methods of ecological risk assessment (ERA) include the hazard quotient (HQ) and probabilistic approaches. The HQ approach, a simple point estimating approach based on less data, is applicable to pollutant identification and control, but not the calculation of the probability of adverse biological effects as it highly depends on assessment factors (AFs) (Suter, 2007). Accordingly, HQ is frequently used in tentative risk assessment. In contrast, the probabilistic approaches, working on species sensitivity distributions (SSDs), are suitable for high risk assessment as they avoid the uncertainty of exposure and toxicity (Suter, 2007). In the present study, both approaches were adopted for ERA of seawater and sediment HCHs and DDTs to marine species inhabiting Jincheng Bay mariculture area (JBMA), aiming to provide a basis for protecting marine biodiversity and ensuring the security of fishery products.
2 Materials and Methods
2.1 Collection of Samples and Their Analysis
Followingthe Specification for Marine Monitoring(GB 17387-2007) (SPC, 2007), samples were collected from 15 grid arranged stations (3 km × 3 km) in JBMA (Fig.1) during four cruises in 2009. In total, 60 water and 60 sediment samples were collected. The water samples were filtered and extracted with n-hexane. The extract was purified with sulphuric acid, and concentrated with nitrogen ahead of analysis. The sediment samples were Soxhlet extracted with n-hexane/acetone (1:1). The extract was purified with florisil and concentrated with nitrogen. HCHs and DDTs were profiled on an Agilent 6890N gas chromatograph equipped with a 63Ni electron capture detector. The components were identified according to their retention time and quantified according to their the external peak area with the six-point calibration curve of corresponding standards.

Fig.1 Distribution of sampling stations in Jincheng Bay mariculture area.
2.2 Ecological Risk Assessment
The ecological risk of HCHs to water species was assessed using HQ and probabilistic approaches (Solomonet al., 2000) whereas that of HCHs and DDTs to benthos was assessed with sediment quality guidelines (SQGs) (MacDonaldet al., 1996). The risk is potential ifHQ〉 1 and the risk is acceptable ifHQ〈 1. For a specific region such as the JBMA, the 90thpercentile concentration is recommended as the exposure value and the predicted no effect concentration (PNEC), the lowest toxicity value (LTV)/ appropriate AF, is treated as the effect value (EC, 2003). The LTV can be either LC50, or median effect concentration (EC50), or no observed effect concentration (NOEC) available in ECOTOX database (http://www.epa. gov/ecotox). Two probabilistic approaches, namely joint probability curves (JPCs) and Monte Carlo analysis (MCA) (Solomonet al., 2000; Zolezziet al., 2005), were used to calculate the probability of adverse biological effects. For a JPC, the area under the curve indicates the overall risk probability (ORP) of the adverse effects expected to occur, which is calculated as

whereEXP(x) is the probability of exposure associated with 100x% of the species expected to be adversely affected (Wanget al., 2008). In the MCA approach, multiple random HQs were generated by re-sampling the exposure and toxicity data, with the frequency ofHQ〉 1 as the description of the probability of adverse biological effects.
In probabilistic approaches, log-normal models were employed to fit the distributions of exposure and toxicity data, followed by the quantile-quantile plots and Kolmogorov-Smirnov test, respectively. The toxicity data were retrieved from the ECOTOX database and screened following the criteria described by Zwart (2002). If multiple toxicity values were available for an individual species, the geometric mean of the data was used as a surrogate. Because chronic toxicity data, such as NOEC, available for HCHs were scarce, the acute toxicity data (Table 1) were converted to chronic data under homogeneous test conditions using the acute to chronic ratios approach (Raimondoet al., 2007).

Table 1 Statistical summary of the acute toxicity (EC50/LC50) for HCHs to aquatic species
The ecological risk to benthos was assessed by comparing the sediment concentrations with two different levels of SQGs, a threshold effect concentration (TEC) and a probable effect concentration (PEC). Adverse effects were rarely observed when exposure concentrations were less than the TEC but frequently observed when exposure concentrations were higher than the PEC (MacDonaldet al., 1996).
Because HCHs and DDTs have a similar toxic mode of action (Raimondoet al., 2007), their joint risk was based on concentration addition (Gómez-Gutiérrezet al., 2007). In this approach, one compound of the mixture is chosen as a reference, with the exposure concentrations of the others being converted to relative concentrations with an effect equivalent to that of the reference. The joint risk of the mixture is based on the relative concentrations of the mixture, which is calculated as

for sediment exposure, whereCiandCr,irepresent the absolute and relative concentration of compoundi, respectively, andTCiandTCrare the toxicity value/environmental quality criteria of compoundiand the reference, respectively, with the SQGs adopted in priority (Suter, 2007). Considering the probabilistic distribution of the multiple toxicity date for water exposure, relative concentrations were based on a non-linear dependence equation of

which is derived as follows:
Both the distributions of compound i and the reference are hypothesized to follow lognormal models given by

whereTiandTrrepresent their respective logarithms of toxicity data. IfTiandTrare normalised to the standard normal distribution, Eq. (4) becomes
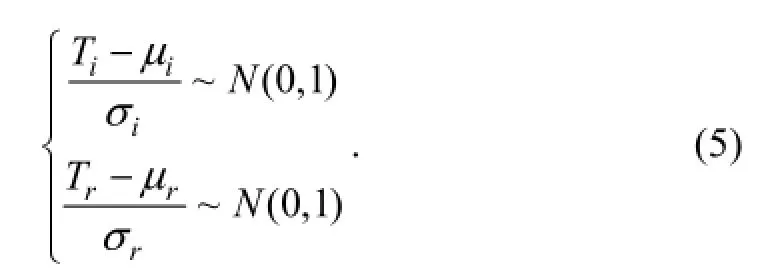
GivesP=φ(x) being the standard normal cumulative distribution function, then

Accordingly, Eq. (5) becomes

wherePiandPrrepresent potentially affected fraction for compoundiand the reference, respectively. While the toxicity of compoundiequals that of the reference,Pi=Pr, then

Therefore,

TiandTrare given by

whereCiandCrare the toxicity concentrations for compoundiand the reference, respectively. Accordingly, the compoundiat the concentration ofCiis expected to lead to an equivalent effect to that of the reference at the concentration ofCr, with the equation of
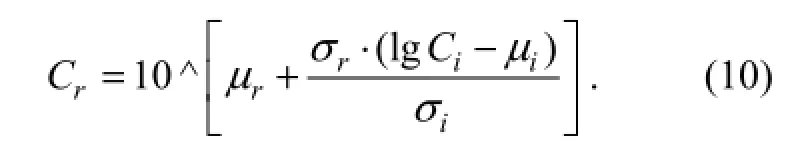
And conclusively, the relative concentration of the mixture can be calculated in Eq. (3).
3 Results
3.1 Distribution of HCHs and DDTs
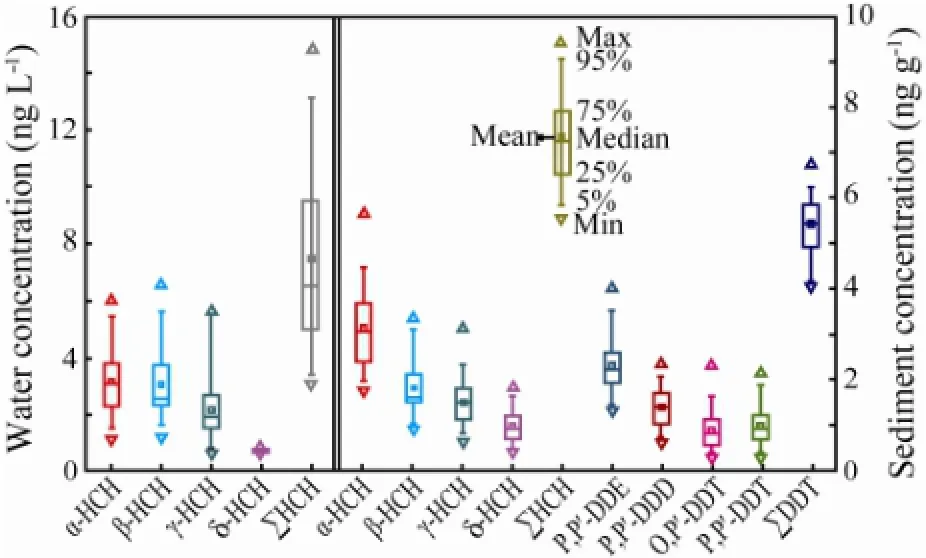
Fig.2 Statistics of HCHs and DDTs in the surface water and sediment of Jincheng Bay mariculture area.
The statistics of HCHs and DDTs concentrations in the surface seawater and sediment of JBMA was presented in Fig.2. The concentration of ∑HCH ranged from 2.98 to14.87 ng L-1, with a mean of 7.45 ng L-1, whereas DDTs were not detected (〈0.032 ng L-1), in the surface seawater. The sediment concentration of ∑HCH and ∑DDT ranged from 5.52 to 9.43 and from 4.11 to 6.72 ng g-1, respectively, with relative means of 6.62 and 5.36 ng g-1, respectively.
Both the water and sediment concentrations of ∑HCH in August and October were significantly higher than those in May and December (ttest,P〈 0.01) (Table 2). The coefficient of variability (CVs) of the water concentration of ∑HCH was 0.38, 0.33, 0.27 and 0.30 for the four seasons, respectively. Comparatively, the sediment concentration of ∑HCH was more spatially constant, with the relative CV ranged from 0.08 to 0.11. Therefore, the sediment distribution reflected the sources of contamination. As shown in Fig.3, the sediment concentration of∑HCH generally decreased from the close to far offshore, suggesting that the HCH residues in the JBMA originated mainly from land. Compared with those of ∑HCH, the concentration of ∑DDT was monthly similar, except for those between October and May (Table 2), and spatially even, suggesting that territorial source around the bay was the dominant and antifouling painting for fishing boats was the secondary.

Table 2 Thet-test of the seasonal difference of total HCHs (∑HCH) and total DDTs (∑DDT) in surface seawater and sediment

Fig.3 Horizontal distributions of ∑HCH (A-D) and ∑DDT (E-H) in surface sediment of Jincheng Bay mariculture area.
3.2 Ecological Risk of HCHs and DDTs
The HQs were less than 1 for α-HCH, β-HCH and δ-HCH and greater than 1 for γ-HCH, suggesting that the risk posed by α-HCH, β-HCH and δ-HCH was acceptable, while the risk posed by γ-HCH needs more concern (Table 3).
The JPCs of HCHs are shown in Fig.4, with the ORPs ranked in the following order: δ-HCH 〈 α-HCH 〈β-HCH 〈γ-HCH. The ORPs for the HCH mixture were slightly higher than those for γ-HCH, which were 5.80 × 10-4, 3.77 × 10-3and 3.55 × 10-2on non-conservative (AF= 1), conservative (AF= 5) and very conservative (AF= 50) estimation bases, respectively, and all of these values were less than 5%, which represents a critical level for management control. In terms of protecting the most sensitive species, the probability that γ-HCH presents the highest individual risk adversely affecting 5% of the most sensitive species was 7.04 × 10-11, 1.08 × 10-5and 1.26 × 10-1under non-conservative, conservative and highly conservative estimates, respectively. Consequently, a low ecological risk, even to sensitive species, was presented by HCHs in the seawater of the studied area.
The MCA-based HQs are summarized in Table 4. In terms of the different levels of conservativeness adopted in making the estimates (AF= 1,AF= 5 andAF= 50), the 95thpercentile for a single HCH isomer and the HCH mixture was all less than 1. In addition, the frequency ofHQ〉 1 were all less than 5%, which is in line with the ORP values derived from the JPC presenting identical ecotoxicological behaviour. Therefore, to protect 95% of the species and thereby maintain the ecosystem functioning, the potential risk posed by HCHs in the seawater of this area was acceptable.
The comparison with the available SQGs (Table 5) showed that the sediment concentration of γ-HCH exceeded the TEC and PEC by a frequency of 100% and 85%, respectively, suggesting that high risk was posed byγ-HCH to marine benthos in the JBMA. In contrast, no exceeding of PEC was observed forp,p’-DDD,p,p’-DDE orp,p’-DDT, and their exceeding frequencies of TEC were low, suggesting that these toxicants presented a generally moderate individual risk to benthos. Based on Eq. (3), the relative sediment concentration of the mixture in the JBMA was estimated to range from 1.90 to 4.92 ng g-1, with a mean of 2.79 ng g-1, approximately 2-folds of the level of γ-HCH and much higher than the corresponding probable effect level (PEL) of 0.99 ng g-1. Therefore, compared with a single HCH isomer or DDT congener, the mixture presented a much higher potential risk to marine benthos in the JBMA. With regard to the contributions of the different toxicants to the relative concentrations of the mixture, γ-HCH played a dominant role, with the average relative percentage of 52.7%, which is comparable to or slightly higher than the total
percentage of that of the other seven toxicants (Fig.5). This result indicated that a generally high risk was posed to marine benthos exposed to the HCH and DDT mixture in the sediment of this area, where γ-HCH contributed the most.

Table 3 HQs and associated parameters for HCHs in seawater

Fig.4 Joint probability curves for the HCHs to marine species.
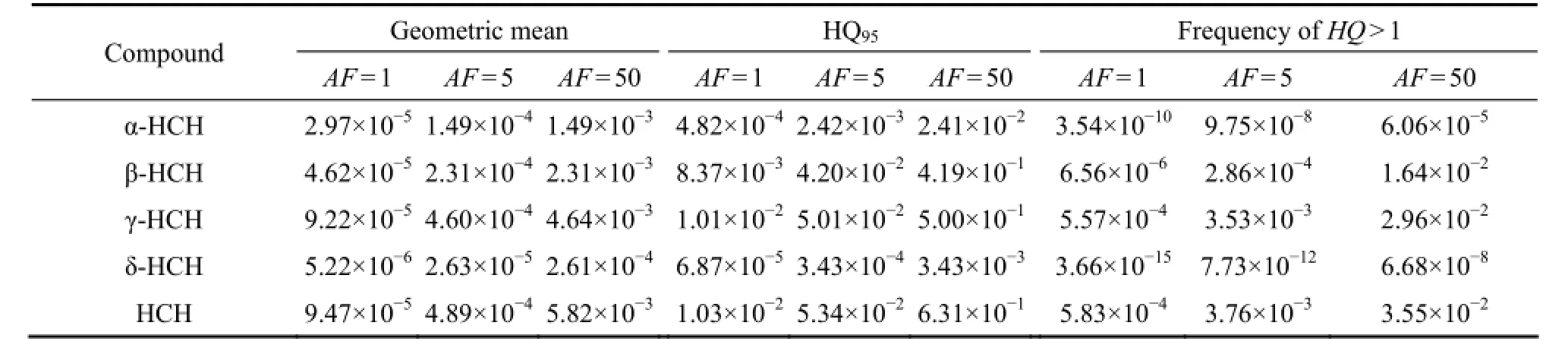
Table 4 Geometric mean, 95thpercentile of HQs (HQ95) and frequency ofHQ〉 1 based on 106-steps Monte Carlo simulation
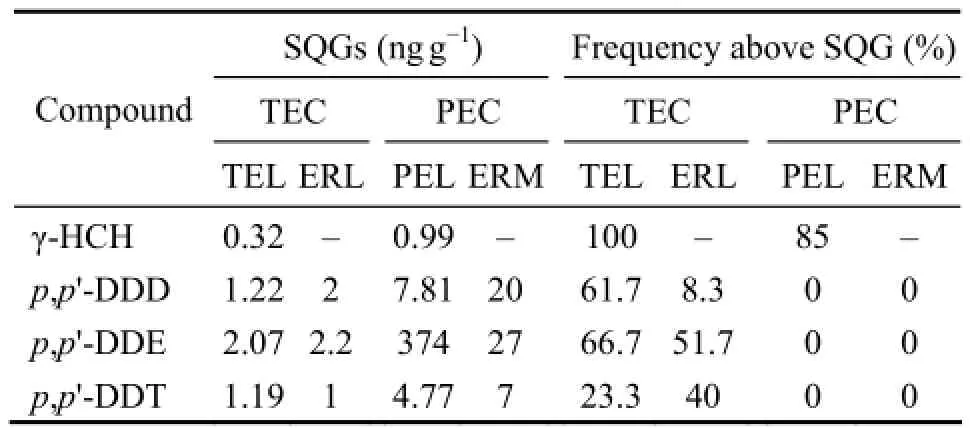
Table 5 Comparison of the sediment concentrations of HCHsand DDTs with the SQGs

Fig.5 Cumulative frequency of relative concentrations of the HCH and DDT mixture. The circles (○) represent the relative concentrations of the mixture, with the corresponding confidence limits delineated by the error bars. The asterisks (*) represent the absolute concentrations of γ-HCH. The pluses (+) represent the relative concentrations of the mixture excluding γ-HCH.
4 Discussion
The environmental distributions of HCHs and DDTs have been extensively documented for coastal waters throughout the world. Their sediment concentration ranged from bellow detection limits to tens of ng L-1as were observed in Deep Bay of China, Bengal Bay of India and Sea Lots of Trinidad (Binelliet al., 2008; Mohammedet al., 2011; Qiuet al., 2009). In comparison, the sediment concentrations of HCHs and DDTs in the JBMA are similar to or higher than most coastal waters in China and other regions, but lower than those reported for typical agricultural watersheds of East China, such as Daliao River, Haihe River and Qiantang River (Sunet al., 2010).
Two formulations of HCH were used as pesticides in China,i.e., technical-grade HCH and lindane. The former is composed of 60%-70% α-HCH, 5%-12% β-HCH, 10%-15% γ-HCH and 6%-10% δ-HCH, while the latter is nearly pure γ-HCH (Huet al., 2009). HCHs are stable under normal conditions with a stability order: β-HCH 〉α-HCH 〉 γ-HCH. γ-HCH may be readily photo-converted to α-HCH, and both α-HCH and γ-HCH can be gradually converted to β-HCH. Thus, β-HCH would be predominant in the environment without fresh inputs of technical-grade HCH or lindane (Yanget al., 2010). The percentages of α-HCH, β-HCH, γ-HCH and δ-HCH were 40.4%, 39.3%, 18.1% and 2.2%, respectively, in the surface seawater and 42.9%, 24.8%, 20.4% and 11.9%, respectively, in the surface sediment of the JBMA. The value of α-HCH/γ-HCH was 2.2 in the surface seawater and 2.1 in the surface sediment of the JBMA, both were less than that of 3-7 for technical-grade HCH, suggesting that HCH residues in this area were derived from the contamination of both technical-grade HCH and lindane. In addition, α-HCH was predominant in both the surface seawater and sediment of the JBMA, as in many other coastal waters, such as Laizhou Bay, Jiaozhou Bay and Pearl River Estuary (Xuet al., 2007; Yuet al., 2008), implied a recent release of fresh HCHs around the area. This phenomenon was different from that observed in the waters far offshore, such as Bohai Sea and East China Sea, which are dominated by β-HCH and/or δ-HCH with a longer half-life (Huet al., 2009; Yanget al., 2010). However, compared with the technical-grade formulations, the species observed in this study underwent great changes, as indicated by the composition profiles, from which the historical usage of HCH could be concluded to have contributed much to the HCH residues in this area.
The percentage ofp,p’-DDD,p,p’-DDE,o,p’-DDT andp,p’-DDT was 25.4%, 43.5%, 14.3% and 16.8%, respectively. Compared with those in technical-grade DDT, which is composed of approximately 75%p,p’-DDT, 15%o,p’-DDT, 5%p,p’-DDE and a few other components (Yanget al., 2010), the percentage ofp,p’-DDT in the JBMA was significantly low, while the percentage ofp,p’-DDD andp,p’-DDE was significantly high. As reported by Ssebugereet al. (2010), DDT could be gradually biodegraded to DDD under anaerobic condition and to DDE under aerobic condition, and both DDD and DDE could be further biodegraded to DDA. Therefore, the values of (DDD+DDE)/DDT in the environment may have gradually increased after the prohibition of technical-grade DDT for agricultural use. Generally, a value of (DDD+DDE)/DDT greater than 1 suggests that there is no novel release of technical-grade DDT (Ssebugereet al., 2010). This value in the sediment of the JBMA ranged from 1.3 to 16.1 with a mean of 4.1, suggesting that the DDT residues in the area mainly originated from historical inputs. Historically, technical-grade DDT was one of the most dominant pesticides in China, while dicofol containing a certain fraction of DDT impurities began to be extensively used in agriculture after the prohibition of technical-grade DDT. Becausep,p’-DDT is more stable thano,p’-DDT, the degeneration process will not increase the value ofo,p’-DDT/p,p’-DDT, which was 0.2-0.3 for technical-grade DDT and 1.3-9.3 (mean 7.0) for dicofol manufactured in China (Qiuet al., 2005). The mean value ofo,p’-DDT/p,p’-DDT in the surface sediment of JBMA was 0.85, suggesting that DDT residues were derived from the contamination of both technical-grade DDT and dicofol. Additionally, there are many fishery villages around the bay, such as Haibeizui and Shihuzui (Fig.1), where the exfoliation of antifouling paint with a certain proportion of DDT as a biocide agent from boat hulls was releasedin situduring the routine maintenance of fishing boats (Xinet al., 2011). Therefore, the usage of DDT-containing antifouling paint also played an important role in the relatively high levels of DDT residues detected in this area. The composition profiles of the DDT congeners in the JBMA are analogous to those in most coastal waters around China, with a dominance ofp,p’-DDD andp,p’-DDE, which could be attributed mostly to the historical input of DDT-containing pesticides (Huet al., 2009; Xuet al., 2007; Yanget al., 2010).
To assess the adverse biological effect of these chemicals, an comprehensive ERA were performed using multiple methodologies, which were extensively used in the ERA of persistent organic pollutants (POPs) and heavy metals in edaphic and aquatic environments (Huet al., 2014; Randet al., 2010; Zolezziet al., 2005), and in the development of the environmental criteria (Posthumaet al., 2002). With regard to the SQGs being a point estimation category, this approach is merely another form of HQ and serves as a dominant powerful tool in the pollutant screening and ERA of sediment POPs. Based on the HQ approach, the ecological risk for a single HCH isomer in the seawater of the JBMA was acceptable, with the exception of γ-HCH. In contrast, with respect to the ERA of the protection of 95% of marine species, the ecological risk of HCHs was acceptable, both singly and jointly, and the frequencies of adversely affecting 5% of marine species were also relatively low. The HQ approach aims toprotect all species in the environment, for which a fixed value of the lowest toxicity and a single value of exposure concentrations (usually the 90thpercentile or maximum) are used, whereas the variations in toxicity and exposure distributions are not taken into consideration. Furthermore, the application of a conservative AF to estimate PNEC tends to cause overprotection in most cases. Hence, the actual risk is often overestimated through this approach, and a HQ that is greater 1 does not always indicates an inevitable risk (Zolezziet al., 2005). Comparatively, aiming to protect most (typically 95%) rather than all species, probabilistic approaches make full use of the distributions of exposure and toxicity data and depend less on AFs. However, the most sensitive species may sometimes be ignored, and if they are keystone species, this assessment is unacceptable. Therefore, the HQ and probabilistic approaches could complement each other, and based on the combination of the two types of approaches, the total ecological risk of HCHs in the seawater of the study area could be concluded to be acceptable. Compared with the SQGs, the relative concentrations of the HCH and DDT mixture were higher than the corresponding PEL for all samples, which presented a high risk to marine benthos, where γ-HCH posed an equivalent or slightly higher risk than all of the other toxicants. In conclusion, the risk posed by HCHs and DDTs from the sediment was much higher than that from the seawater in this area, which should be attributed to their high hydrophobicity. The logkowvalues of the HCHs and DDTs were 3.7-3.9 and 5.5-6.6, respectively (Zhaoet al., 2009), and accordingly, the species tended to accumulate in the sediment gradually. The experimental results of this study indicated that the concentrations of∑HCH and ∑DDT were significantly higher in the surface seawater than those in the surface sediment by a factor of 985 and more than 1409, respectively.
The present study revealed that the ecological risk posed by HCHs and DDTs to the marine species inhabiting the middle-layer and upper-layer seawater column was considerably low; thereby, little detrimental effect on the bay scallop raft cultured in this area was expected. In contrast, a high risk was posed to lower-layer marine species, particularly the benthos, such as wild shellfishes, suggesting a potential detrimental effect on the safety of aquatic products. This study could serve as a basis for risk monitoring and controlling pesticide contamination for aquaculture in the JBMA. However, uncertainties were inherently inevitable in the ERA, which were due mainly to the availability of raw data rather than the methods or models themselves (Domet al., 2012). Thus, investigating biological responses together with the residues of HCHs and DDTs in the benthos is necessary to provide efficient data for the refinement of the ERA and the effects on aquatic products in the studied area.
5 Conclusion
The distributions of HCHs and DDTs in the surface seawater and sediment of the JBMA were determined and an ERA of the risks posed by HCHs and DDTs to marine species was performed in the present study. The sediment concentrations of ∑HCH and ∑DDT in the JBMA were relatively high compared with those of coastal waters elsewhere, both in and outside of China. The HCH residues in this area were derived from the mixture of technical-grade HCH and lindane, whereas the mixture profiles of DDT were derived from technical-grade DDT and dicofol. The residues of both HCHs and DDTs originated in large part from historical inputs. The potential risks posed by an individual HCH isomer and the HCH mixture to seawater column species were acceptable, while the risk posed by the HCH and DDT mixture to marine benthos was considerably high, suggesting that the safety of specific kinds of aquatic products from this sea area may be adversely affected. The results of this study could serve as a general basis for risk monitoring and controlling pesticide contamination for aquaculture in the JBMA. The biological residues and the joint toxicity of HCHs and DDTs in this area should be attached importance to for further study.
Acknowledgements
This work was supported by the Marine Special Scientific Fund for the Non-profit Public Industry of China (200805031), Fund of Key Laboratory of Fishery Ecology and Environment, Guangdong Province (LFE-2014-4), and Scientific Research Foundation for the Third Institute of Oceanography, State Oceanic Administration (No. 2013031).
Bernard, L., Martinat, N., Lécureuil, C., Crépieux, P., Reiter, E., Tilloy-Ellul, A., Chevalier, S., and Guillou, F., 2007. Dichlorodiphenyltrichloroethane impairs follicle-stimulating hormone receptor-mediated signaling in rat Sertoli cells.Reproductive Toxicology, 23 (2): 158-164.
Binelli, A., Sarkar, S., Chatterjee, M., Riva, C., Parolini, M., Bhattacharya, B., Bhattacharya, A., and Satpathy, K., 2008. A comparison of sediment quality guidelines for toxicity assessment in the Sunderban wetlands (Bay of Bengal, India).Chemosphere, 73 (7): 1129-1137.
Dom, N., Knapen, D., and Blust, R., 2012. Assessment of aquatic experimental versus predicted and extrapolated chronic toxicity data of four structural analogues.Chemosphere, 86 (1):56-64.
European Communities (EC), 2003.Technical Guidance Document on Risk Assessment. Office for official publications of the European Communities, Luxembourg, 149-150.
Gómez-Gutiérrez, A., Garnacho, E., Bayona, J. M., and Albaigés, J., 2007. Screening ecological risk assessment of persistent organic pollutants in Mediterranean sea sediments.Environment International, 33 (7): 867-876.
Gong, X., Qi, S., Wang, Y., Julia, E. B., and Lv, C., 2007. Historical contamination and sources of organochlorine pesticides in sediment cores from Quanzhou Bay, Southeast China.Marine Pollution Bulletin, 54 (9): 1434-1440.
Guglielmo, F., Lammel, G., and Maier-Reimer, E., 2009. Global environmental cycling of γ-HCH and DDT in the 1980s-Astudy using a coupled atmosphere and ocean general circulation model.Chemosphere, 76 (11): 1509-1517.
Hu, J., Zhang, Z., Wei, Q., Zhen, H., Zhao, Y., Peng, H., Wan, Y., Giesy, J. P., Li, L., and Zhang, B., 2009. Malformations of the endangered Chinese sturgeon,Acipenser sinensis, and its causal agent.Proceedings of the National Academy of Sciences of the United States of America, 106 (23): 9339-9344.
Hu, Y., Song, X., Gong, X., Xu, Y., Liu, H., Deng, X., and Ru, S., 2014. Risk assessment of butyltins based on a fugacitybased food web bioaccumulation model in the Jincheng Bay mariculture area: II. Risk assessment.Environmental Science: Processes & Impacts, 16 (8): 2002-2006.
Li, Y., 1999. Global gridded technical-grade hexachlorocyclohexane usage inventory using a global cropland as a surrogate.Journal of Geophysical Research, 104 (D19): 23785-23797.
Li, Y., and Macdonald, R. W., 2005. Sources and pathways of selected organochlorine pesticides to the Arctic and the effect of pathway divergence on HCH trends in biota: A review.Science of the Total Environment, 342 (1-3): 87-106.
Li, Y. F., Cai, D. J., and Singh, A., 1998. Historical DDT use trend in China and usage data gridding with 1/4° by 1/6° longitude/latitude resolution.Advances in Environmental Research, 2 (4): 497-506.
Lin, T., Hu, Z., Zhang, G., Li, X., Xu, W., Tang, J., and Li, J., 2009. Levels and mass burden of DDTs in sediments from fishing harbors: the importance of DDT-containing antifouling paint to the coastal environment of China.Environmental Science and Technology, 43 (21): 8033-8038.
MacDonald, D. D., Carr, R. S., Calder, F. D., Long, E. R., and Ingersoll, C. G., 1996. Development and evaluation of sediment quality guidelines for Florida coastal water.Ecotoxicology, 5:253-278.
Mohammed, A., Peterman, P., Echols, K., Feltz, K., Tegerdine, G., Manoo, A., Maraj, D., Agard, J., and Orazio, C., 2011. Polychlorinated biphenyls (PCBs) and organochlorine pesticides (OCPs) in harbor sediments from Sea Lots, Port-of-Spain, Trinidad and Tobago.Marine Pollution Bulletin, 62 (6): 1324-1332.
Phipps, G. L., Mattson, V. R., and Ankley, G. T., 1995. Relative sensitivity of three freshwater benthic macroinvertebrates to ten contaminants.Archives of Environmental Contamination and Toxicology, 28 (3): 281-286.
Posthuma, L., Suter, G. W., and Traas, T., 2002.Species Sensitivity Distributions in Ecotoxicology. Lewis Publishers, Boca Raton, Florida, 616pp.
Qiu, X., Zhu, T., Yao, B., Hu, J., and Hu, S., 2005. Contribution of dicofol to the current DDT pollution in China.Environmental Science and Technology, 39 (12): 4385-4390.
Qiu, Y., Zhang, G., Guo, L., Cheng, H., Wang, W., Li, X., and Wai, O. W. H., 2009. Current status and historical trends of organochlorine pesticides in the ecosystem of Deep Bay, South China.Estuarine, Coastal and Shelf Science, 85 (2):265-272.
Raimondo, S., Montague, B. J., and Barron, M. G., 2007. Determinants of variability in acute to chronic toxicity ratios for aquatic invertebrates and fish.Environmental Toxicology and Chemistry, 26 (9): 2019-2023.
Rand, G. M., Carriger, J. F., Gardinali, P. R., and Castro, J., 2010. Endosulfan and its metabolite, endosulfan sulfate, in freshwater ecosystems of South Florida: A probabilistic aquatic ecological risk assessment.Ecotoxicology, 19 (5): 879-900.
Solomon, K. R., Giesy, J., and Jones, P., 2000. Probabilistic risk assessment of agrochemicals in the environment.Crop Protection, 19: 649-655.
Standards Press of China (SPC), 2007. The Specification for Marine Monitoring (GB17387-2007), Beijing.
Ssebugere, P., Wasswa, J., Mbabazi, J., Nyanzi, S. A., Kiremire, B. T., and Marco, J. A. M., 2010. Organochlorine pesticides in soils from south-western Uganda.Chemosphere, 78 (10):1250-1255.
Sumith, J. A., Parkpian, P., and Leadprathom, N., 2009. Dredging influenced sediment toxicity of endosulfan and lindane on black tiger shrimp (Penaeus monodon Fabricius) in Chantaburi River estuary in Thailand.International Journal of Sediment Research, 24 (4): 455-464.
Sun, J., Feng, J., Liu, Q., and Li, Q. L., 2010. Distribution and sources of organochlorine pesticides (OCPs) in sediments from upper reach of Huaihe River, East China.Journal of Hazardous Materials, 184 (1-3): 141-146.
Suter, G. W., 2007.Ecological risk assessment, 2nd edition. Lewis Publishers, Boca Raton, Florida, 680pp.
Xin, J., Liu, X., Liu, W., Jiang, L., Wang, J., and Niu, J., 2011. Production and use of DDT containing antifouling paint resulted in high DDTs residue in three paint factory sites and two shipyard sites, China.Chemosphere, 84 (3): 342-347.
Wang, B., Yu, G., Huang, J., Yu, Y., Hu, H., and Wang, L., 2008. Tiered aquatic ecological risk assessment of organochlorine pesticides and their mixture in Jiangsu reach of Huaihe River, China.Environmental Monitoring and Assessment, 157 (1-4): 29-42.
Xu, X., Yang, H., Li, Q., Yang, B., Wang, X., and Lee, F., 2007. Residues of organochlorine pesticides in near shore waters of LaiZhou Bay and JiaoZhou Bay, Shandong Peninsula, China.Chemosphere, 68 (1): 126-139.
Yang, H., Xue, B., Yu, P., Zhou, S., and Liu, W., 2010. Residues and enantiomeric profiling of organochlorine pesticides in sediments from Yueqing Bay and Sanmen Bay, East China Sea.Chemosphere, 80 (6): 652-659.
Yu, M., Luo, X., Chen, S., Mai, B., and Zeng, E. Y., 2008. Organochlorine pesticides in the surface water and sediments of the Pearl River Estuary, South China.Environmental Toxicology and Chemistry, 27 (1): 10-17.
Zhang, J., Qi, S., Xing, X., Tan, L., Gong, X., Zhang, Y., and Zhang, J., 2011. Organochlorine pesticides (OCPs) in soils and sediments, southeast China: A case study in Xinghua Bay.Marine Pollution Bulletin, 62 (6): 1270-1275.
Zhao, L., Hou, H., Zhou, Y., Xue, N., Li, H., and Li, F., 2010. Distribution and ecological risk of polychlorinated biphenyls and organochlorine pesticides in surficial sediments from Haihe River and Haihe Estuary Area, China.Chemosphere, 78 (10): 1285-1293.
Zhao, Z., Zhang, L., Wu, J., and Fan, C., 2009. Distribution and bioaccumulation of organochlorine pesticides in surface sediments and benthic organisms from Taihu Lake, China.Chemosphere, 77 (9): 1191-1198.
Zolezzi, M., Cattaneo, C., and Tarazona, J. V., 2005. Probabilistic ecological risk assessment of 1,2,4-trichlorobenzene at a former industrial contaminated site.Environmental Science and Technology, 39 (9): 2920-2926.
Zwart, D., 2002. Observed regularities in species sensitivity distributions for aquatic species. In:Species Sensitivity Distributions in Ecotoxicology. Posthuma, L.,et al., eds., Lewis Publishers, Boca Raton, Florida, 133-154.
(Edited by Qiu Yantao)
(Received February 20, 2013; revised May 10, 2013; accepted June 20, 2014)
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
* Corresponding author. Tel: 0086-532-82031962 E-mail: rusg@ouc.edu.cn
 Journal of Ocean University of China2015年2期
Journal of Ocean University of China2015年2期
- Journal of Ocean University of China的其它文章
- Effect of Dietary Lipid on the Growth,Fatty Acid Composition and Δ5 Fads Expression of Abalone(Haliotis discus hannai Ino)Hepatopancreas
- Species Composition and Diversity of Macrobenthos in the Intertidal Zone of Xiangshan Bay, China
- Evaluation of Cytotoxicity and Genotoxicity of Insecticide Carbaryl to Flounder Gill Cells and Its Teratogenicity to Zebrafish Embryos
- Purification of a Diatom and Its Identification to Cylindrotheca closterium
- Mechanical Stress Induces Neuroendocrine and Immune Responses of Sea Cucumber (Apostichopus japonicus)
- Preparation of κ-carra-Oligosaccharides with Microwave Assisted Acid Hydrolysis Method
