Effect of Replacing Fish Meal with Extruded Soybean Meal on Growth, Feed Utilization and Apparent Nutrient Digestibility of Juvenile White Shrimp (Litopenaeus vannamei)
YANG Qihui, TAN Beiping, DONG Xiaohui, CHI Shuyan, and LIU Hongyu
Laboratory of Aquatic Animal Nutrition and Feed, College of Fisheries, Guangdong Ocean University, Zhanjiang 524025, P. R. China
Effect of Replacing Fish Meal with Extruded Soybean Meal on Growth, Feed Utilization and Apparent Nutrient Digestibility of Juvenile White Shrimp (Litopenaeus vannamei)
YANG Qihui, TAN Beiping*, DONG Xiaohui, CHI Shuyan, and LIU Hongyu
Laboratory of Aquatic Animal Nutrition and Feed, College of Fisheries, Guangdong Ocean University, Zhanjiang 524025, P. R. China
© Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
Extruded soybean meal (ESBM) was evaluated as a protein source for partial replacement of fish meal (FM) in diets of juvenile Litopenaeus vannamei. In the control diet (Diet 1), FM protein was replaced with increasing dietary levels of ESBM (4.28%, 8.40%, 12.62%, 16.82%, and 25.26%) at 10%, 20%, 30%, 40%, and 60% levels (Diets 2 to 6, respectively). An eight-week feeding trial was conducted on 720 juvenile shrimp (0.67 g ± 0.01 g mean initial weight), and nutrient digestibility of the six diets was determined. ESBM could replace 20% of FM without causing a significant reduction in growth of shrimp, but other dietary treatments strongly affected whole body composition. Crude protein content of the whole body fed Diet 6 was significantly lower than that fed Diet 2 (P < 0.05), while crude lipid content of the whole body fed Diet 5 or 6 was significantly higher than that fed Diet 2 (P < 0.05). Protein digestibilities of Diets 5 and 6 were significantly lower than that of Diet 1 (P < 0.05). Digestibility of lipids ranged from 96.97% in Diet 6 to 98.34% in Diet 3, whereas dry matter digestibility decreased with increasing replacement level. This study indicates that 20% FM replacement with ESBM in the basic diet containing 40% protein and 30% FM is optimal for juvenile L. vannamei.
Litopenaeus vannamei; fish meal; extruded soybean meal; growth performance; feed utilization; nutrient digestibility
1 Introduction
White shrimp, Litopenaeus vannamei, is one of the most widely cultured shrimp species in the world. It is a good choice for extensive and intensive grow-out production because of rapid growth, good survival in high-density culture, and strong disease tolerance (Williams et al., 1996; Ponce-Palafox and Ross, 1997). To support the growing market for cultured shrimp, the demand for improved feeds has created a need for high quality protein sources. An increasing use of fish meal (FM) for fish and shrimp culture has directed attention toward the assessment of alternative ingredients as a replacement of FM protein (Bharadwaj et al., 2002; Amin et al., 2012; Gamboa-Delgado et al., 2013; César Molina-Poveda et al., 2013). Thus, FM replacement with less expensive and abundant protein sources in commercial diets should be considered to maintain the continued growth and intensification of aquaculture production.
Among plant protein sources, soybean products have received considerable attention in replacing FM in aquatic animal feeds, mainly because of their well-balancedamino acid profiles, consistent compositions, worldwide availability, and low prices (Tacon, 2000; Divakaran et al., 2000; Samocha et al., 2004; Sookying and Davis, 2011; William et al., 2012; Mahbuba et al., 2013). At presently, soybean ingredients are widely used in diets for many cultured species as a cost-efficient alternative protein source to FM (Gatlin III et al., 2007; Crisantema et al., 2008). Compared with ordinary soybean meal, extruded soybean meal (ESBM) is the addition of a puffing technology in soybean crushing. The main purpose is to improve not only oil extraction but also the quality of soybean meal (low urinary enzyme activity) and its palatability.
ESBM has been used as a substitute for FM in diets of different shrimp species, including Penaeus monodon (Sudaryono et al., 1996), L. vannamei (Davis and Arnold, 2000; Mendoza-Alfaro et al., 2001; Daranee and Davis, 2011), and L. schmitti (Alvarez et al., 2007). In the present study, we determined the growth performance and feed utilization in juvenile L. vannamei fed diets in which FM was gradually replaced with ESBM. The effect of ESBM inclusion on juvenile shrimp was evaluated in terms of whole body compositions, apparent nutrient digestibility, and amino acid availability. The results may aid to identifying an optimal level of FM replacementwith ESBM in diets for white shrimp.
2 Materials and Methods
2.1 Experimental Animals and Culture Conditions
The feeding trial was conducted in an indoor flowthrough aquarium system manufactured by the Guangdong Evergreen Group, Zhanjiang, China. Litopenaeus vannamei juveniles were obtained from a local commercial shrimp farm and acclimated to laboratory conditions by feeding the control diet for 10 d. At the beginning of the experiment, 720 acclimated shrimp (0.67 g ± 0.01 g initial mean weight) were randomly distributed into eighteen 500-L cylindrical fiber-glass tanks, 40 shrimps each. Tanks were supplied with flowing filtered seawater (about 1.0 L min–1) and adequate aeration.
Triplicate groups of shrimp were fed the reference and test diets by hand until visual satiety four times a day at 07:00, 11:30, 17:00, and 21:30, respectively. Total feed provided was 8% to 10% of body weight per day. Half an hour after feeding, the rearing tanks and collection column were brushed out to remove uneaten feed and fecal residues by siphoning (Lin et al., 2004; Yang et al., 2009). The daily ration was adjusted according to feed consumption.
Seawater temperature and salinity were monitored twice daily between 09:00 and 15:00. During the experimental period, water conditions were as follows: temperature 28.0–30.5℃, salinity 26.5–28.0 g L–1, dissolved oxygen content 6.5–7.0 mg L–1, and total ammonia nitrogen content 0.3–0.5 mg L–1.
2.2 Diet Preparations
Six isonitrogenous and isoenergetic experimental diets were formulated (Table 1). FM protein in the control diet (Diet 1) was replaced with increasing dietary levels of ESBM (4.28%, 8.40%, 12.62%, 16.82%, and 25.26%) at 10%, 20%, 30%, 40%, and 60% levels (Diets 2 to 6, respectively). Chromium oxide (Cr2O3) was chosen as an indicator. All diet ingredients were ground through a 60-mesh size sieve and thoroughly homogenized in a Hobart mixer (The Science and Technology Industrial General Factory of South China University of Science and Technology, Guangzhou, Guangdong, China). Micro components, such as mineral and vitamin premixes, were mixed using the progressive enlargement method (Yang et al., 2009). Fish oil, soybean oil and water were added to the premixed dry ingredients and thoroughly mixed to form a homogenous mixture. Pellets (1.0 and 1.5 mm diameter) were wet-extruded for shrimp of different growth stages using an F-26 Extruder (South China University of Science and Technology, Guagnzhou, China). Feed pellets were air-dried to approximately 10% moisture, sealed in vacuum-packed plastic bags, and stored at -20℃ until further use.

Table 1 Formulation and proximate composition of experimental diets (% dry-weight basis) fed to juvenile Litopenaeus vannamei
2.3 Sample Collection
At the beginning of the experiment, 20 shrimp were sampled and stored at -20℃ before proximate analysis of whole body composition. Upon termination of the 8-week feeding trial, shrimp in each tank were individually weighed and sampled for tissue analysis 24 h after the last feeding. Five to nine shrimp were randomly selected each tank and used for proximate composition analysis of the whole body.
Fecal samples were collected from the settling column in each tank three times a day (08:30, 13:00, and 18:30). After collection, feces were immediately filtered on the moderate speed qualitative filter paper (120 mm in diameter) for 60 min at 4℃ and then stored at –18℃ before chemical analyses. Daily fecal samples from each tank were pooled together through the course of the experiment until a sufficient sample (about 10 g per pool) was obtained for chemical analysis.
2.4 Analytical Methods
Proximate compositions of diets, whole body samples, and feces were analyzed using standard methods (AOAC 1995). Crude protein (CP, N × 6.25) was determined using the Kjeldahl method after acid digestion on an Auto Kjeldahl System (1030-Auto-analyzer, Tecator, Sweden). Crude lipid (CL) was determined using the ether extraction method with a Soxtec System HT (Soxtec System HT6, Tecator, Sweden). Moisture was determined by weighing after 24 h of oven-drying at 105℃. Gross energy (GE) was determined using an adiabatic bomb calorimeter.
Chromic oxide and phosphorus contents of diets and feces were determined using inductively coupled plasma atomic emission spectrophotometry (IRIS Advantage (HR), Thermo Jarrell Ash, Woburm, USA). Amino acid concentrations in diets and fecal materials were determined using an automatic amino acid analyzer (Hitachi Model 835-50, Tokyo, Japan) equipped with a column for physiological fluid analysis (2.6–150 mm, Hitachi custom ion-exchange resin No. 2619).
Growth parameters were calculated as follows:
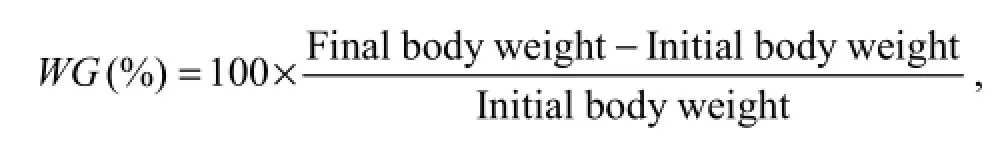
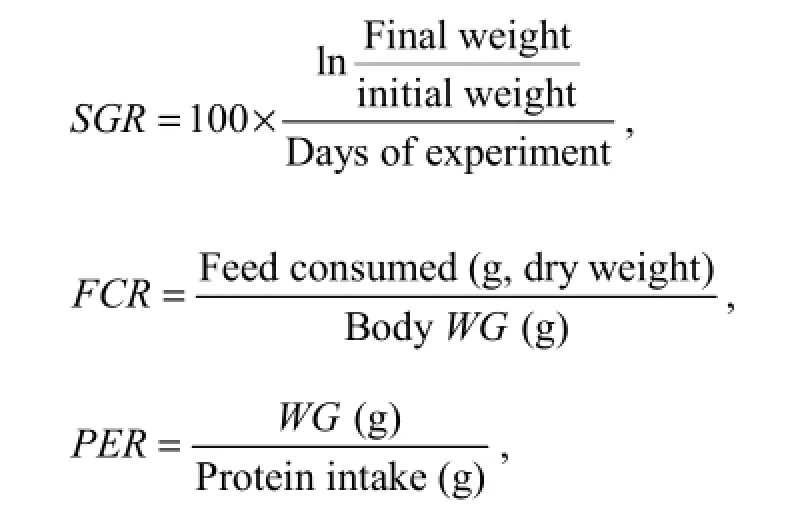
where WG is weight gain, SGR is specific growth ratio, FCR is feed conversion ratio, and PER is protein efficiency ratio.
Apparent digestibility coefficients (ADCs) of nutrients and energy for the reference and test diets were calculated using the indicator method (Pond et al., 1995):
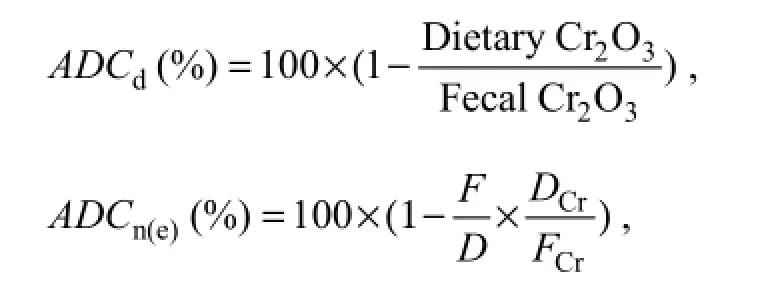
where ADCdis ADC of dry matter, ADCn(e)is ADC of nutrients or energy, F is the percentage of nutrient or energy in feces, D is the percentage of nutrient or energy in diet, DCris the percentage of chromic oxide in diet, and FCris the percentage of chromic oxide in feces.
2.5 Statistical Analyses
Data were analyzed using SPSS® version 13.0 (SPSS Inc., Chicago, IL, USA). One-way ANOVA was performed, and Duncan’s multiple range test was used to identify significant difference between dietary treatments. P < 0.05 was considered statistically significant.
3 Results
The effects of dietary treatments on growth performance and feed utilization in juvenile shrimp are shown in Table 2. Significant differences were observed in growth performance, feed utilization, and survival between dietary treatments (P < 0.05). WG and PER significantly decreased while FCR increased as FM replacement level increased from 0% to 30% (P < 0.05).
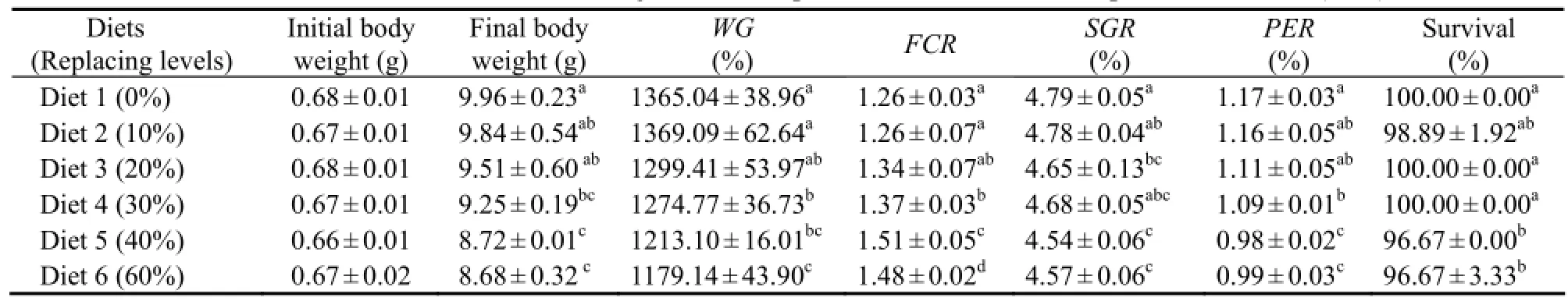
Table 2 WG, FCR, SGR and PER of juvenile Litopenaeus vannamei fed experimental diets (n=3)
Whole body proximate composition of shrimp fed different diets is presented in Table 3. No significant differences were observed in moisture and ash contents of the whole body between any two of the six treatments (P >0.05). CP content of the whole body fed Diet 2 was significantly higher than that fed Diet 6 (P < 0.05), while CL content of the whole body fed Diet 5 or 6 was significantly higher than that fed Diet 2 (P < 0.05).
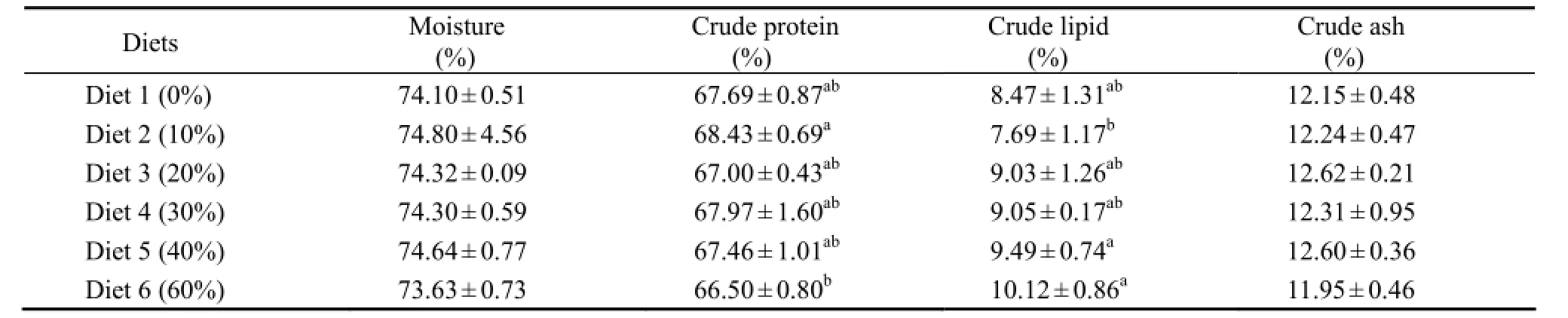
Table 3 The whole body composition (dry matter basis) of juvenile Litopenaeus vannamei fed experimental diets
The effects of dietary treatments on nutrient digestibility are shown in Tables 4 and 5. The ADCs of dry matter, protein, energy, and amino acids were significantly affected by FM replacement with ESBM (P < 0.05). However, no significant difference was observed in the apparent digestibility of crude lipids (ADCLs) of lipid which ranged from 96.97% to 98.34%. The ADCs of dry matter and CP significantly decreased as FM replacement level increased from 40% (Diet 5) to 60% (Diet 6) (P < 0.05), but no significant differences were observed between the other four diets. The apparent digestibility of energy (ADE) in shrimp fed Diet 1 or 2 were significantly higher than those fed Diet 5 or 6 (70.22% or 70.08% vs. 64.03% or 65.23%, respectively).

Table 4 Apparent digestibility of nutrients and energy of juvenile Litopenaeus vannamei fed experimental diets

Table 5 Apparent digestibility of individual amino acids of juvenile Litopenaeus vannamei fed experimental diets
A positive correlation was observed between amino acid availability and apparent digestibility of crude pro-tein (ADCP). Most amino acid availability values of Diet 1 were significantly greater than those of the other diets. The digestibility of individual amino acids (except aspartic acid and serine) significantly varied between different dietary treatments (P < 0.05), ranging from 60.99% to 90.62%. The ADCs of essential amino acids were generally high, especially for arginine. Except tyrosine, serine, and proline, most amino acids exhibited the highest digestibility in Diet 1 (P < 0.05) and the lowest digestibility in Diet 5 or 6. With respect to amino acid availability for all diets, there was a general trend toward reduced growth in shrimp fed diets with > 30% FM replacement levels.
4 Discussion
In this study, juvenile L. vannamei fed diets with >30% FM replacement had significantly lower WG and SGR than those fed the other experimental diets, including the control (Table 2). These results indicated that the juvenile shrimp demonstrated better tolerance to dietary ESBM and suffered no significant reduction in growth performance, as long as dietary FM protein was replaced with ≤30% soybean meal protein. However, supplementation of lysine and methionine was still required (Table 1).
ESBM is a good substitute for FM in diets of shrimp (Pascual et al., 1990; Akiyama et al., 1992; Davis and Arnold, 2000; Mendoza-Alfaro et al., 2001; Alvarez et al., 2007). A study reported that there was no significant difference in WG, survival, and feed efficiency of L. vannamei when FM was completely replaced with co-extruded soybean poultry by-product meal containing egg supplement (Samocha et al., 2004). Similarly, Amaya et al. (2007) demonstrated that FM in practical shrimp feeds could be completely replaced with alternative vegetable protein sources without compromising production and economic performance of L. vannamei reared in ponds.
Growth of juvenile L. vannamei fed the control diet was similar to those obtained in this species elsewhere under laboratory conditions for experimental evaluation of the effects of replacing FM with other protein sources (Davis and Arnold, 2000; Cheng et al., 2002; Samocha et al., 2004; Tan et al., 2005; Goytortúa-Bores et al., 2006; Amaya et al., 2007). When ESBM was included in the control diet to replace 10% to 30% of FM protein, WG became significantly higher than data of the control (Table 2). Given that all the test diets had a fixed ESBM level (12.62%), up to 30% of dietary protein could be provided by FM in shrimp diets (Table 2). However, >30% FM protein replacement with ESBM posed adverse affects on shrimp performance. WG, FCR, SGR, and PER were all significantly affected by increasing replacement levels of FM protein (30% to 60%) in the experimental diets (Table 2). The reduction in growth performance of shrimp might be due to any anti-nutrition factor or lysine and methionine limitation in soybean meal compared with FM (Yang et al., 2009).
High dietary concentrations of soybean products negatively affect palatability in a few shrimp species (Alvarez et al., 2007). Lim and Dominy (1990) reported a significant decline in feed intake of L. vannamei fed diets with dietary soybean meal concentration higher than 42%. However, in our trial, FCR of the experimental diets became significantly higher with increasing replacement levels of FM protein (30% to 60%), to the contrary of WG.
With respect to the same facilities and similar feed composition, the production results of juvenile L. vannamei reported in the present study were similar to those reported by Venero (2006) and Amaya et al. (2007), and better than those obtained by Zelaya (2005) and Garza et al. (2004). Replacement of marine protein sources in practical diets for L. vannamei has been less successful. Using the same marine protein mix, Lim and Dominy (1990) reported that 40% of the marine protein mix could be replaced with solvent-extracted soybean meal, but higher levels of replacement resulted in reduced growth of L. vannamei compared with the control. Lim (1996) demonstrated that solvent-extracted cottonseed meal could replace 40% of a marine protein mix (53% menhaden FM, 34% shrimp waste meal, and 13% squid meal) in a 32% CP practical diet, containing 45% of the marine protein mix.
In the present study, moisture and crude ash contents of the whole body were not significantly affected in juvenile L. vannamei by dietary replacement of FM with ESBM. However, significant differences were observed in CP and CL contents of the whole body between several treatments (Table 3). Cowey et al. (1985) emphasized the importance of determining the effects of diet on the biochemical composition of animals. Actual growth in crustaceans was achieved by increasing the contents of protein, lipid, carbohydrate, and ash (Louis et al., 1997). It was possible that replacement of FM with ESBM at a suitable level promoted protein deposition and reduced lipid content in white shrimp. In the present study, changes in the apparent digestibility of dry matter (ADDM) indicated that inclusion of ESBM in the experimental diets significantly affected feed utilization. ADDM of the control diet (72.92%) was significantly higher than those of Diets 5 and 6 (63.61% and 61.56%, respectively) (Table 4).
Brunson et al. (1997) found that apparent digestibility of CP (ADCP) in menhaden FM did not differ significantly from that in any plant products (P > 0.05), other than wheat gluten and soybean meal. Results of the present study indicated that ADCP and apparent digestibility of energy (ADE) were unaffected by the inclusion of dietary ESBM with FM replacement levels of 10% to 30%. The ADCPs obtained in the present study were similar to those reported by other investigators who used crustacean meals in shrimp diets (Sudaryono et al., 1996; Lee and Lawrence, 1997). The ADCPs obtained in diets containing ESBM with < 40% FM replacement levels were not significantly higher than those obtained in the control diet. The ADCP coefficients of all the diets were as high as 75.43% to 78.51%. Akiyama (1988) reported that Penaeus vannamei could digest protein of soybean meal with ADCP coefficients as high as 92% and 90%, comparable to those reported for P. monodon (90%) and P. japonicus(90%).
The results of amino acid availability (Table 5) reflected ADCP of the ingredients tested. The ADCs of individual amino acids were high. Arginine showed the highest variability among the amino acids. Most amino acid availability values of Diet 1 were significantly greater than those of other diets. The digestibility of individual amino acids all significantly differed between dietary treatments (P < 0.05), ranging from 60.99% to 90.62%. Previous studies reported that the ADCs of individual amino acids within a feed ingredient were variable (Zhou et al., 2004). A better amino acid profile in soybean meal than in tuna meal possibly improved the use of proteins (PER from growth assay) and enhanced the digestibility of dietary protein (Ezquerra et al., 1997).
In the present study, the ADE of the control diet (70.22%) was significantly higher than those of Diet 5 and 6 (64.03% and 65.23%, respectively). This result was similar to previous findings in red swamp crayfish, Procambarus clarkii (Akiyama et al., 1989; Brown et al., 1986; Reigh et al., 1990). The ADE values obtained in the present study also coincided with those from a study by Law et al. (1990), who reported that giant river prawn, Macrobrachium rosenbergii, could digest energy with high ADCs of energy. The ADCL (from 96.97% to 98.34%) of the diets was not significantly affected by the inclusion of ESBM, even though FM replacement level increased from 0% to 60%. Little information on ADCL exists, but lipids are known to be highly digestible (85% to 95%) in shrimp (Cuzon et al., 1994). Many factors strongly affect lipid digestibility (Lee and Lawrence, 1997), including heat treatment. ESBM is an excellent source of triglycerides and phospholipids (Pierce et al., 1969) that can be more efficiently digested than free fatty acids (Merican and Shim, 1995). Although the quantitative level of lipids in the experimental diets was similar, the relative proportions of different fatty acids and their availability possibly varied with the inclusion level of ESBM, which could partially explain the growth enhancement observed in shrimp. Fatty acid content of the ingredients and diets was not determined in this study. Further research is necessary to elucidate the nutritional value of dietary fatty acids from ESBM for shrimp.
In commercially manufactured feeds, FM can be partially removed from the formulation with alternative vegetable protein sources (e.g., ESBM) without reducing the growth performance and feed utilization in shrimp. According to the effects of FM protein replacement with ESBM on growth, feed utilization, and apparent nutrient digestibility, 20% FM replacement level in the basic diet containing 40% protein and 30% FM is optimal for juvenile L. vannamei.
Acknowledgements
This study was financially supported by the Special Fund for Agro-scientific Research in the Public Interest of China (201003020), the Guangdong University Innovation Talents Cultivating Project of China (1009324), the Guangdong Natural Science Foundation of China (S2012 040007863), and by the Guangdong Province Universities and College Pearl River Scholar Funded Scheme (GD UPS-2011).
Akiyama, D. M., 1988. Soybean meal utilization by marine shrimp. AOCS World Congress on Vegetable Protein Utilization in Human Food and Animal Feedstuffs, Singapore. American Soybean Association, Singapore.
Akiyama, D. M., Dominy, W. G., and Lawrence, A. L., 1991. Penaeid shrimp nutrition for the commercial feed industry revised. In: Proceedings of the Aquaculture Feed Processing and Nutrition Workshop, Thailand and Indonesia September 19–25, 1991. American Soybean Association, Singapore, 80-98.
Alvarez, J. S., Alfredo, H. L., Galindo, J., Fraga, I., Garcia, T., and Villarreal, H., 2007. Substitution of fish meal with soybean meal in practical diets for juvenile white shrimp Litopenaeus schmitti (Pérez-Farfante & Kensley 1997). Aquaculture Research, 38: 689-695.
Amaya, E. A., Davis, D. A., and Rouse, D. B., 2007. Replacement of fish meal in practical diets for the Pacific white shrimp (Litopenaeus vannamei) reared under pond conditions. Aquaculture, 262: 393-401.
Amin, O., Jafar, S., Abdolmohammad, A. K., and Masoud, R., 2012. Growth and apparent digestibility of nutrients, fatty acids and amino acids in Pacific white shrimp, Litopenaeus vannamei, fed diets with rice protein concentrate as total and partial replacement of fish meal. Aquaculture, 342-343: 56-61.
Association of Official Analytical Chemists (AOAC), 1995. Official Methods of Analysis of AOAC. 16th edition. Helrich, K., ed., Association of Official Analytical Chemists, Inc., Arlington, VA, USA, 1-12.
Bharadwaj, A. S., Brignon, W. R., Gould, N. L., Brown, P. B., and Wu, Y. V., 2002. Evaluation of meat and bone meal in practical diets fed to juvenile hybrid striped bass Morone chrysops×M. saxatilis. Journal of the World Aquaculture Society, 33: 448-457.
Brown, P. B., Tazik, M. L. H., and Blithe, W. G., 1990. Consumption and apparent dry matter digestibility of aquatic macrophytes by male and female crayfish Orconectes virirlis. Aquaculture, 86: 345-349.
Brunson, J. F., Romaire, R. P., and Reigh, R. C., 1997. Apparent digestibility of selected ingredients in diets for white shrimp Penaeus setiferus L. Aquaculture Nutrition, 3: 9-16.
Carver, L. A., Akiyama, D. M., and Dominy, W. G., 1989. Processing of wet shrimp heads and squid viscera with soy meal by a dry extrusion process. American Soybean Association Technical Bulletin, 16: 89-94.
César, M. P., Mariela, L., and Miguel, J., 2013. Evaluation of the potential of Andean lupin meal (Lupinus mutabilis Sweet) as an alternative to fish meal in juvenile Litopenaeus vannamei diets. Aquaculture, 410-411: 148-156.
Cheng, Z. J., Behnke, K. C., and Dominy, W. G., 2002. Effects of poultry byproduct meal as a substitute for fish meal in diets on growth and body composition of juvenile pacific white shrimp, Litopenaeus vannamei. Journal of Applied Aquaculture, 12: 71-83.
Colvin, L. V., and Brand, C. W., 1977. The protein requirement of penaeid shrimp at various life-cycle stages in controlledenvironment systems. Proceedings of the World Mariculture Society, 8: 821-840.
Cowey, C. B., Mackie, A. M., and Bell, J. G., 1985. Nutrition and Feeding in Fishes. Academic Press, London, 63-89.
Crisantema, H., Miguel, A. O. N., Karla, A. V., Blanca, G. R., and Isabel, A. P., 2008. Partial replacement of fish meal by porcine meat meal in practical diets for Pacific white shrimp (Litopenaeus vannamei). Aquaculture, 277: 244-250.
Cruz-Suárez, L. E., Nieto-López, M., Ricque-Marie, D., Guajardo-Barbosa, C., and Scholz, U., 2004. Uso de harina de subproductos avícolas en alimentos para L. vannamei. In: Avances en Nutricion Acuícola VII. Cruz Suárez, L. E., et al., eds., Memorias del VII Simposium Internacional de Nutrición Acuícola, 16-19.
D’Abramo, L., and Lovell, T., 1991. Aquaculture research needs for the year 2000: Fish and crustacean nutrition. Journal of the World Aquaculture Society, 22: 57-62.
Davis, D. A., and Arnold, C. R., 2000. Replacement of fish meal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 185: 291-298.
Divakaran, S., Velasco, M., Beyer, E., Forster, I., and Tacon, A. G. J., 2000. Soybean meal apparent digestibility for Litopenaeus vannamei, including a critique of methodology. In: Avances en Nutricion Acuicola V. Memorias del V Simposium Internacional de Nutricion Acuicola, 19-22.
El-Saidy, D. M. S. D., and Gaber, M. M. A., 2002. Complete replacement of fish meal by soybean meal with dietary L-lysine supplementation for the nile tilapia Oreochromis niloticus (L.) fingerlings. Journal of the World Aquaculture Society, 33: 297-306.
Ezquerra, J. M., García-Carreño, F. L., Civera, R., and Haard, N. F., 1997. pH-stat method to predict protein digestibility in white shrimp (Penaeus vannmei). Aquaculture, 157: 249-260.
FAO, 2005. FAO Fisheries Department, Fishery Information, Data and Statistics Unit. FISHSTAT Plus: Universal software for statistical time series, Version 2.3.
Garza de Yta, A., Rouse, D. B., and Davis, D. A., 2004. Influence of nursery period on the growth and survival of Litopenaeus vannamei. Journal of the World Aquaculture Society, 35: 357-365.
Gatlin III, D. M., Barrows, F. T., Brown, P., Dabrowski, K., Gaylord, T. G., Hardy, R. W., Herman, E., Hu, G. S., Krogdahl, A., Nelson, R., Overturf, K., Rust, M., Sealey, W., Skonberg, D., Souza, E. J ., Stone, D., Wilson, R., and Wurtele, E., 2007. Expanding the utilization of sustainable plant products in aquafeeds: A review. Aquaculture Research, 38: 551-579.
Goytortúa-Bores, E., Civera-Cerecedo, R., Rocha-Meza, S., and Green-Yee, A., 2006. Partial replacement of red crab (Pleuroncodes planipes) meal for fish meal in practical diets for the white shrimp Litopenaeus vannamei. Effects on growth and in vivo digestibility. Aquaculture, 256: 414-422.
Halver, J. E., and Hardy, R. W., 2002. Fish Nutrition. 3rd edition. Academic Press, New York, 735-824.
Hardy, R. W., 1999. Alternate protein sources. Feed Manage, 50: 25-28.
Hermosillo, S., Cuzon, G., Guillaume, J., and Cahu, C., 1994. Composition, preparation and utilization of feeds for Crustacea. Aquaculture, 124: 253-267.
Julián, G. D., Mónica, G. R. C., Martha, G. N. L., and Lucía, E. C., 2013. Simultaneous estimation of the nutritional contribution of fish meal, soy protein isolate and corn gluten to the growth of Pacific white shrimp (Litopenaeus vannamei) using dual stable isotope analysis. Aquaculture, 383: 33-40.
Law, A. T., Chin, K. S. S., Ang, K. J., and Kamamdin, M. S., 1990. Digestibility of low cost ingredients in pelleted feeds by Macrobrachium rosenbergii (De Man). In: The Second Asian Fisheries Forum. Hirano, R., and Hanyu, I., eds., Manila, 333-336.
Lee, P. G., and Lawrence, A. L., 1997. Digestibility. In: Crustacean Nutrition, Vol. 6. D’Abramo, L. R., et al., eds., World Aquaculture Society, 194-260.
Lim, C., and Dominy, W., 1990. Evaluation of soybean meal as a replacement for marine animal protein in diets for shrimp (Penaeus vannamei). Aquaculture, 87: 53-63.
Lim, C., 1996. Substitution of cottonseed meal for marine animal protein in diets for Penaeus vannamei. Journal of the World Aquaculture Society, 27: 402-409.
Lim, C., Beames, R. M., Eales, J. G., Prendergast, A. F., McLeese, J. M., Shearer, K. D., and Higgs, D. A., 1997. Nutritive values of low and high fiber canola meals for shrimp (Penaeus vannamei). Aquaculture Nutrition, 3: 269-279.
Lim, C., Klesius, P. H., and Dominy, W., 1998. Soybean products. International Aquaculture Feeds, 3: 17-23.
Dabramo, L. R., Conkin, D. E., and Akiyama, D. M., 1997. Crustacean Nutrition. World Aquaculture Society, 243-246.
Mahbuba, B., Shunsuke, K., Manabu, I., Saichiro, Y., and Abdul, K. R., 2013. Performance of kuruma shrimp, Marsupenaeus japonicus fed diets replacing fishmeal with a combination of plant protein meals. Aquaculture, 372: 45-51.
Mendoza-Alfaro, R., De Dios, A., VaŁ zquez, C., Cruz-SuaŁ rez, L. E., Ricque-Marie, D., Aguilera, C., and Montemayor, J., 2001. Fishmeal replacement with feather-enzymatic hydrolyzates co-extruded with soya-bean meal in practical diets for the Pacific white shrimp (Litopenaeus vannamei). Aquaculture Nutrition, 7: 143-151.
Merican, Z. O., and Shim, K. F., 1995. Apparent digestibility of lipid and fatty acids in residual lipids of meals by adult Penaeus monodon. Aquacultur, 133: 275-286.
National Research Council (NRC), 1993. Nutrient Requirements of Fish. National Academy Press, Washington, DC, 332-411.
Olvera-Novoa, M. A., and Olivera-Castillo, L., 2000. Potencialidad del uso de las leguminosas como fuente proteica en alimentos para peces. In: Avances en Nutrición Acuícola IV. Civera-Cerecedo, R., et al., eds., Memorias del IV Simposium Internacional de Nutrición Acuícola, México, 327-348.
Pascual, F., Cruz-SuaŁ rez, L. E., and Sumalangcay, A., 1990. Supplemental feeding of Penaeus monodon juveniles with diets containing various levels of defatted soybean meal. Aquaculture, 89: 183-191.
Pierce, R. W., Van der Veen, J., and Olcott, H. S., 1969. Proximate and lipid analyses of krill (Euphasia species) and red crab (Pleuroncodes planipes). Journal of Agricultural and Food Chemistry, 17: 367-369.
Ponce-Palafox, J., Martinez-Palacios, C., and Ross, L., 1997. The effects of salinity and temperature on the growth and survival rates of juvenile white shrimp, Penaeus vannamei, Boone, 1931. Aquaculture, 157: 107-115.
Reigh, R. C., Braden, S. L., and Craig, R. J., 1990. Apparent digestibility coefficients for common feedstuffs in formulated diets for red swamp crayfish Procambarus clarkii. Aquaculture, 84: 321-340.
Samocha, T., Davis, D. A., Saoud, I. P., and DeBault, K., 2004. Substitution of fish meal by co-extruded soybean poultry by-product meal in practical diets for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture, 231: 197-203.
Sookying, D., and Davis, D. A., 2011. Pond production of Pacific white shrimp (Litopenaeus vannamei) fed high levels ofsoybean meal in various combinations. Aquaculture, 319: 141-149.
Sudaryono, A., Tsventnenko, E., and Evans, L. H., 1996. Digestibility studies on fisheries by-product based diets for Penaeus monodon. Aquaculture, 143: 331-340.
Swick, R. A., Akiyama, D. M., Boonyaratpalin, M., and Creswell, D. C., 1995. Use of Soybean Meal and Synthetic Methionine in Shrimp Feed. Technical Bulletin, American Soybean Association, 43pp.
Tacon, A. G. J., and Forster, I. P., 2000. Trends and challenges to aquaculture and aquafeed development in the new millennium. In: Avances en Nutrición Acuícola V. Cruz-Suárez, L. E., et al., eds., World Aquaculture Society, Baton Rouge, 520-549.
Tan, B., Mai, K., Zheng, S., Zhou, Q., Liu, L., and Yu, Y., 2005. Replacement of fish meal by meat and bone meal in practical diets for the white shrimp Litopenaeus vannamei (Boone). Aquaculture Research, 26: 439-444.
Velasco, M., 2002. Nutrition de camaron. In: Curso Lance en Acuacultura, 13–17 Mayo 2002. Cruz-Sua Ł rez, L. E., et al., eds., Monterrey, NuevoLeon, Mexico, 122pp.
Venero, J., 2006. Optimization of dietary nutrient inputs for Pacific white shrimp Litopenaeus vannamei. Doctoral dissertation. Auburn University, Auburn, Alabama, USA.
Williams, A. S., Davis, D. A., and Arnold, C. R., 1996. Density-dependent growth and survival of Penaeus setiferus and Penaeus vannamei in a semi-closed recirculating system. Journal of the World Aquaculture Society, 27: 107-112.
William, B., Carlos, P. H., Marcelo, B. T., Wilson, W. J., and Luís, H. S. P., 2012. Substitution of fishmeal with microbial floc meal and soy protein concentrate in diets for the pacific white shrimp Litopenaeus vannamei. Aquaculture, 342-343: 112-116.
Yang, Q. H., Zhou, X. Q., Zhou, Q. C., Tan, B. P., Chi, S. Y., and Dong, X. H., 2009. Apparent digestibility of selected feed ingredients for white shrimp Litopenaeus vannamei, Boone. Aquaculture Research, 41: 78-86.
Yu, Y., 2004. Replacement of fishmeal with poultry by-product meal and meat and bone meal in shrimp, tilapia and trout diets. In: Avances en Nutricion Acuícola VII. Cruz Suárez, L. E., et al., eds., Memorias del VII Simposium Internacional de Nutrición Acuícola, 183-201.
Zaldivar, F. J., 2002. Las harinas y aceites de pescado en la alimentacion acuicola. In: Avances en Nutricio n Acuicola VI. Memorias delVI Simposio Internacional de Nutricion Acuicola, 516-526.
Zelaya, O., 2005. An evaluation of nursery techniques and feed management during culture of marine shrimp Litopenaeus vannamei. Doctoral dissertation. Auburn University, Auburn, Alabama, USA.
Zhou, Q. C., Tan, B. P., Mai, K. S., and Liu, Y. J., 2004. Apparent digestibility of selected feed ingredients for juvenile cobia, Rachycentron canadum. Aquaculture, 241: 441-451.
(Edited by Qiu Yantao)
(Received December 21, 2013; revised February 19, 2014; accepted May 25, 2015)
J. Ocean Univ. China (Oceanic and Coastal Sea Research)
DOI 10.1007/s11802-015-2572-6
ISSN 1672-5182, 2015 14 (5): 865-872
http://www.ouc.edu.cn/xbywb/
E-mail:xbywb@ouc.edu.cn
* Corresponding author. Tel: 0086-759-2383373
E-mail: bptan@126.com
 Journal of Ocean University of China2015年5期
Journal of Ocean University of China2015年5期
- Journal of Ocean University of China的其它文章
- Seasonal Dynamics of Turbidity Maximum in the Muthupet Estuary, India
- Decadal Variability of Global Ocean Significant Wave Height
- An Observational and Modeling Study of Extratropical Transition of Hurricane Sandy in 2012
- The Nonlinear Bifurcation and Chaos of Coupled Heave and Pitch Motions of a Truss Spar Platform
- Beach Morphology and Coastline Evolution in the Southern Bohai Strait
- Comparison of Meiofaunal Abundance in Two Mangrove Wetlands in Tong’an Bay, Xiamen, China
