Analysis of the Controllability of Drug Risk Based on AHP
YAN Quan, SHAO Rong
Analysis of the Controllability of Drug Risk Based on AHP
YAN Quan, SHAO Rong
()
Objective To evaluate drug risk controllability rationally. Methods AHP was used to analyze drug risk in drug lifecycle so as to formulate a reasonable evaluation index on the basis of questionnaires and experts interviews. Results and Conclusion Suggestions are put forward to reduce drug risk at stages of R&D, production, marketing and use and to allocate drug regulatory resources rationally after drug risk controllability index raised by the author being applied to analyzing the controllability of 20 types of drug risk factors.
drug risk controllability; AHP; drug injury event
Drug risk management and risk assessment are widely used in the United States, Japan and the European Union for a long time. However, the history of China’s risk management concept is short. There are many problems in the process of risk management practice. People used to attribute the ADR events to regulatory flaw. They think that more regulations can avoid drug safety risks and lead to “zero risk”. This concept ignores the effort made by drug regulatory agency. That is, most of the people in China have a deviation in understanding risk management. The lack of drug risk control evaluation criteria is another important reason as well.
1 The advantages of AHP analysis
There are many methods of risk analysis and evaluation and they can be applied to various scopes with certain conditions. On the whole, they can be roughly divided into three kinds, quantitative analysis method, qualitative analysis method and qualitative and quantitative analysis method. Two aspects should be taken into consideration when we make a research on drug risk control assessment standard. On the one hand, it needs to classify all kinds of risks. On the other hand, it needs to go through a lot of data to judge whether drug regulatory department can control risk factors. To determine the evaluation standard, traditional mathematical optimization method can’t build a model for many factors and the result is often divorced from reality. In addition, the solution obtained by experts relying on subjective judgment lacks of theoretical basis. AHP (Analytic Hierarchy Process) was founded in 1977 by Satty who proposed a practical multi-criteria decision making method[1]. It serves as a kind of comprehensive evaluation method for the risk assessment. It is widely used in the assessment of safety and environmental risks. This paper used AHP method to set up the control of drug risk evaluation system. It took the advantages of conventional mathematical optimization and expert advice and overcame their limitations at the same time. It solved the problems which consist of quantitative and qualitative factors systematically.
2 AHP comprehensive evaluation of drug risk controllability
The object can be classified and divided into three levels by constructing the hierarchy. The top layer for final target contains only one element. On the second layer elements are scheduled according to certain rules. The bottom is alternative management which can be further analyzed comprehensively. Figure 1 is the hierarchy model of 20 kinds of drug safety risk[2].
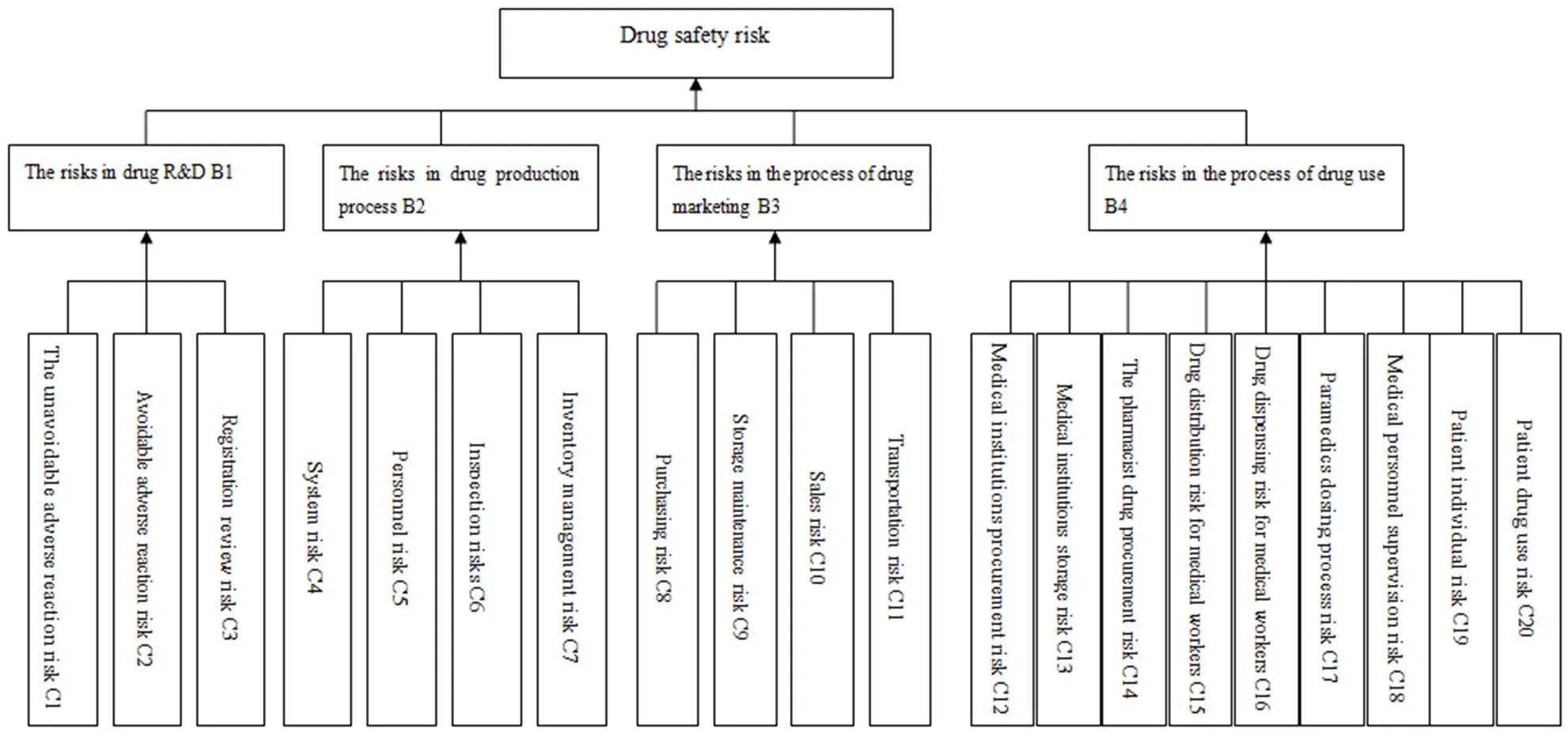
Figure 1 Three layers hierarchical structure risk
The author designed the questionnaire according to the 20 kinds of risk factors in drug research and development, production, circulation and use[3]. In the description of risk factors, drug factors and human factors must be distinguished so that some questions on controllability of the risk factors can be asked later on. At the same time, in the description of the drug use risks, pharmacists and medical staffs should be distinguished because drug supervision and administrative departments have limited inspection in medical institutions.
The questionnaire is divided into two parts. The first part is the basic information involving the participants’ offices, nature of work, position, work experiences and understanding of drug risk. The second part is the survey on risk factors and drug safety supervision in drug research and development, production, circulation and use. There are three questions on R&D, four questions on production, four on circulation and nine questions on drug use.
Participants of the questionnaire are divided into four categories: pharmaceutical companies, pharmaceutical supervisory and administrative departments and universities. Pharmaceutical enterprises mainly refer to the pharmaceutical producing enterprises and pharmaceutical trading enterprises. Pharmaceutical supervision and administration departments mainly include national and provincial food and drug supervision and administration departments. Universities include medicine and related professional lecturers and graduate students. In order to guarantee the quality of the questionnaire, the beginning of questionnaire explains the classification of the risks based on the source of the drug safety risk which includes drug internal risks and external risks. At the same time, each level from 1 to 5 is clarified to avoid misunderstanding for participants. Nearly 60% of the questionnaire is filled in on the spot, if the participants have any questions, they can put forward and get the answer immediately to guarantee the quality of the questionnaire.
The work experience and position of the participants are as follows: Most of them have 2 or 3 years of relevant pharmaceutical work experience and the proportion is 38.7%. 74.2% of the participants have working experience. As to position distribution, 16 people are managers (including first-line managers, middle managers and top managers). The proportion is 19.8% and the first-line managers’ accounts for the vast majority. Most graduate students are studying medicine or related pharmacy courses. As can be seen from the experience and position distribution, the questionnaire is filled in by experienced personnel who guarantee the quality of the questionnaire with reliable data.
81 questionnaires are valid in the total of 100. Participants surveyed are as follows:
Table 1 Participants information
PeopleNo.Proportion (%)ExperienceNo.Proportion (%)PositionNo.Proportion (%) Enterprise1316.1none2125.8Manager1619.8 SFDA1012.31-2 year1417.3Not-manager4656.4 Medical institution1822.22-3 year3138.4Master2125.8 Education Institutions4049.4>3 year1518.5 Total81100Total81100Total81100
Based on the upper level of elements, judgment matrix of the total risk, drug research risk, drug production risk and drug marketing risk can be formed respectively to calculate their importance and compatibility. After examination, five elements of judgments matrix were<0.1, therefore, the weights of all the calculated values were acceptable.
From Table 2, we calculated the comprehensive weight of risk.
Table 2 Comprehensive weight calculation
B1B2B3B4 0.15190.48960.28260.0759 C10.00950.0095 C20.04330.0433 C30.09910.0991 C40.09140.1810 C50.20230.1375 C60.14310.0972 C70.05280.0739 C80.10890.1089 C90.02460.0246 C100.04020.0402 C110.10890.1089 C120.00750.0075 C130.00390.0039 C140.01200.0120 C150.00680.0068 C160.00680.0068 C170.00510.0051 C180.00400.0040 C190.01400.0140 C200.01580.0158
3 Statistical analysis of the data
According toWvalues shown in Table 2, weight classification criteria is made after consulting related drug safety departments and relevant experts.
Such situation shows that drug regulatory department has the minimal controllability of these risk factors. At present, such risks are not controllable for drug regulatory department. They include: the unavoidable adverse reaction of C1 and medical institutions procurement risk C12, medical institutions and drug store procurement risk C14 and C13, risk of drug distribution of C15 for pharmacists and nurses, risks of drug dispensing of C16 for medical personnel, paramedics dosing process risk of C17, medical personnel supervision risk of C18, patient individual risk of C19 and patient drug use risk of C20.
Through the analysis we can conclude the following reasons for this type of risk control:
(1) It is still difficult to avoid the adverse drug reaction risk though we have taken a series of risk prevention measures based on drug reaction risk data collected. Due to the limitation of scientific and technological level in the process of research and development and the lack of adverse reaction monitoring means, risk of controllability belongs to the smallest class compared to other known risks[4].
(2) Besides C1 risk, the others belong to drug use risk factors, two groups of drug are medical staff of medical institutions and drug consumers.
A. As to medical institutions, drug is one part of the whole treatment. Prescribing, dispensing and nursing the patients are indispensable in the process of drug use. However, department of health administration has the right to control medical institutions in China. Department of drug administration should certificate and inspect drug preparations based on pharmaceutical administration law, it’s difficult to make actual regulation for other fields although there is thecorresponding legal basis, and punishment for illegal consequences is not enough.
B. Patients’ drug use risks are caused by individual different medication. These risk factors can be minimized by inquiring the patients' medical education and medical history, but basically they are independent risk factors. As patients’ rational drug use will be affected by personal physique[5]and basic medicine knowledge, it is hard for the department of drug regulatory to make patients have rational drug use.
Drug regulatory department is difficult to control the risk factors caused in the process of drug use.
This case shows that drug regulatory department has a moderate controllability of such risk factors. Certain results could be achieved by strengthening the regulation of these risk factors. Those risks include the adverse reaction of C2, registration review risk of C3, system risk of C4, inventory management risk of C7, store maintenance risk of C9 and sales risk of C10.
The reasons for controlling these moderate risks:
(1) Some predictable adverse drug reactions, such as the side effects caused by drug combination can be reduced through the choice of treatment though they belong to the inherent risk of drugs. If we strengthen the regulation of drug rational use, such risks can be reduced in theory. But in the process of drug use there are still some factors that affect the therapeutic choice, such as the price, unknown side effects which may result in new risks.
(2) In the rest of other five risk factors, C3 is research risk, C4, C7 are the production risks, risk of C9 and C10 belong to marketing processes.
First of all, according to the analysis of different stages, C3, C4, C7, C9 and C10 have higher controllability.
A. At drug registration stage, increasing the drug original data source and authenticity of censorship as well as strengthening the drug clinical trial data source and the authenticity of the regulation can control the quality of medicines and avoid drug risks caused by drug manufacturers forging data.
B. Departments of drug administration should strengthen the supervision of production enterprises’ rules at the stage of drug production. They should focus on whether the enterprises have established a relatively perfect quality management system, quality assurance system and quality risk management system. In addition, pharmaceutical supervisory and administrative department should have irregular inspection on the production enterprises, especially on the warehousing schedule and special storage conditions. Through the above method, the drug regulatory department can better control the risks in the process of drug production.
C. At the stage of drug marketing, departments of drug administration should have the inspection schedule for enterprises’ drug storage facilities and equipment status. Irregular frequent check should be increased and special storage conditions of drugs, such as storage conditions and methods should be focused on. If any problem arises, the corresponding guidance must be put forward timely; for retail pharmacies, on-the-spot investigation should be done, attention must be paid to drugs propaganda way and sales record. By doing so, the drug regulatory agency can effectively prevent the corresponding risk.
After the above analysis, it can be seen that drug regulatory department has high controllability of C3, C6, C7, C9 and C10. But the risk factors are caused by improper behaviors of the enterprises and the prevention and control are more dependent on pharmaceutical enterprise’s consciousness. The drug regulatory agency plays a guiding role in the supervision.
The mistake of drug safety in China is that we do not have a clear concept for the role that enterprise plays in drug safety. Enterprise is the “first responsible person” does not mean drug safety agency is not liable for the drug safety risk. It means the enterprise is the main body who should undertake the primary responsibility for drug safety by improving its safety control ability for the long-term development[5]. In 2010, the number of reports about adverse reaction accounted for only 6.7% in China’s drug enterprises, and in the United States the figure reached 93.80%[6].
Enterprises should have a responsible attitude to produce good products. Otherwise, they will take advantage of the vulnerabilities even if there is a reasonable regulatory system and the strict implementation of the rules.
In addition, at the stage of registration and production, the approval system and checking method restrict drug regulatory department to prevent and control such risks. There are also some problems at the stage of drug marketing, such as numerous retail pharmacies which make it difficult to guarantee the quality of sampling frequency and spot check. So drug regulatory department has more responsibilities to reduce the risks.
The situation shows that drug regulatory agency has high controllability of risk factors. Through strengthening the regulation, these risk factors could be prevented. Such risks include four parts: personnel risk of C5, inspection risk of C6, purchasing risk of C8 and transportation risk of C11.
(1) Risk of C5 and C6 has the biggest proportion at the stage of drug production and drug regulatory department has the ability to take the initiative to prevent and control them. If the production enterprise recruiting technical personnel do not have the corresponding qualification, the pharmaceutical supervisory and administrative department can find out the fact by strengthening the spot check of enterprise. Drug regulatory department occasionally send staff or invite experts to examine the process of drug production. At the same time, any problems arising in the process of sampling, inspection method and checking error must be solved with the guidance from the department of drug administration. And they have a certain relationship with enterprises misconducts. Theoretically, the regulation of drug regulatory department has a limit, but the enterprise personnel qualifications and working experience is an objective standard. So the department of drug administration should take the initiative to control such risks.
(2) At the stage of drug marketing, the weight of C8 and C11 both are 0.1089, which is as much as 0.89 higher than standard one. It shows they have high controllability compared with other risk factors. But compared with the range of C5, C6, there are still some uncontrolled factors. If enterprises do not audit supplier qualification, business license, the legitimacy of the imported drugs, related documents and purchasing records in the purching procedures, the pharmaceutical supervisory and administrative department can find it out by strengthening the routine inspection of enterprises. In the process of transportation, the drug regulatory department can reduce such risks by checking drug quality tracking record. The above two risks have high controllability because they involve many departments, methods and processes. But compared with other risks, the supplier’s qualification, licensing, scope of business, the legitimacy of the imported drugs, related documents and purchasing records belong to objective standards which can be inspected simply. At the same time, regular check on transportation made by staff or experts can guarantee the drug users to get safe medicines.
After analyzing three different risk factors, we can get the weights of B1 for R&D, B2 for drug production, B3 for drug marketing and B4 for drug use in Table 2. Their weights are 0.1519, 0.4896, 0.0759 and 0.2826 respectively.
As to the drug regulatory department, drug circulation and production have high risk controllability. By strengthening drug supervision, enterprises must comply with GMP and GSP requirements to produce qualified drugs and establish standard drug distribution channels which can reduce the risk of drugs.
Drug R&D and use have low controllability, but they should not be ignored[7]. Drug risk control of R&D includes preclinical safety evaluation and the safety evaluation during clinical trials. A new drug can be approved after a series of preclinical and clinical studies to obtain enough data on drug safety and effectiveness and the thorough analysis of benefit/risk. Drug safety risk control mainly depends on the review and registration from department of drug administration as well as the constraints of GCP and GLP quality management standard. Therefore, the law enforcement ability of the regulatory supervision for drug research risk control is of great significance. Drug quality starts from design and good drug development process can ensure drug safety. Drug supervision department also should pay attention to drug use risk for the irrational drug use results in the frequent ADR events, and the reasonable guide made by the medical staff to patients’ drug use can reduce the risk.
4 Conclusions
Different drug safety risks have different properties and characteristics; therefore, we should put the limited regulatory resources into drug safety risk control.
The stages of drug production and marketing have high risk controllability, and drug regulatory department should supervise and control the production and marketing of enterprises according to the existing regulatory system. This process involves three parties and all of them must work together to prevent the risk to the greatest extent. First party is the regulatory system. A good system can provide the basis for the correct way of supervision and the system must be based on the current situation of China. Second is the main body for law enforcement, the department of drug administration, who needs to strictly implement the corresponding supervision system. Finally, the most important main body is the enterprise who should make the high quality products and reduce the risk by all means.
This questionnaire is mainly for medical professionals, respondents including those people from pharmaceutical production, management and supervision department and scholars of drug risk prevention. Most of them think that the risk of drug manufacturing and marketing processes is directly related to regulatory supervision. However, they overlook the risk from drug R&D and use comparatively. In fact, the risk prevention from the department of drug administration for drug production and marketing is not enough. Enterprises’ consciousness of risk prevention and control must be awakened and strengthened. As to the stage of R&D, regulation measures should be perfect and effective to prevent the risk from the beginning; For the stage of drug use, the administrative department shall strengthen the medical and health regulatory responsibilities to ensure the safety of drug use.
[1] Satty T L.The Analytic Hierarchy Process [M].NewYork: McGraw-Hill, Inc., 1980.
[2] BIAN Bo-yang, CHANG Feng, SHAO Rong. Enlightenment of America’sDrug Safety Risk Management on China [J]. Chinese Pharmacy, 2007,21 (12): 956-957, 985.
[3] CHEN Xiao-li. Analysis of Drug Production and Supervision from “Xinfu” and “Qieryao” [J].Chinese Pharmacy, 2008,22 (10): 871-873.
[4] YANG Yue, WU Zhi-ang, WEI Jing. The Legal Thought onCivil Liability from Qieryao Event[J]. China’s Pharmacy, 2009,20 (7): 481-483.
[5] ZHANG Nian-xian. Analysis of Supply Chain Management in Chinese Medicinal Materials from Polluted Diethylene Glyco [J]. Herald of Medicine, 2008,9 (27): 1138-1140.
[6] YI Na, QIN Zheng-bi, XIONG Dao-jun,RiskManagement of Traditional Chinese Medicine from “Houttuynia Incident” [J]. Herald of Medicine, 2008,27, (9): 1136-1138.
[7] FU Yu, LIANG Ze-nan. Analysis of the Special Customs Supervision from Jiangxi Immunoglobulin Events [J]. Chinese Inspection and Quarantine, 2008,12: 27.
Author’s information: SHAO Rong, Professor. Major research area: Social pharmacy. Tel: 025-86185038, E-mail: shaorong118@163.com

