Magnesium production by carbothermic reduction in vacuum☆
,*
aNational State Key Laboratory of Complex Nonferrous Metal Resources Clear Utilization,Kunming University of Science and Technology,Kunming 650093,China
bNational Engineering Laboratory for Vacuum Metallurgy,Kunming University of Science and Technology,Kunming 650093,China
cKey Laboratory of Non-Ferrous Metals Vacuum Metallurgy of Yunnan Province,Kunming 650093,China
dCollege of Metallurgy and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,China
Magnesium production by carbothermic reduction in vacuum☆
Yang Tiana,b,c,d,Bao-qiang Xua,b,c,d,Bin Yanga,b,c,d,*,Cheng-b Yangb,c,Tao Qua,b,c,d, Da-chun Liua,b,c,d,Yong-nian Daia,b,c,d
aNational State Key Laboratory of Complex Nonferrous Metal Resources Clear Utilization,Kunming University of Science and Technology,Kunming 650093,China
bNational Engineering Laboratory for Vacuum Metallurgy,Kunming University of Science and Technology,Kunming 650093,China
cKey Laboratory of Non-Ferrous Metals Vacuum Metallurgy of Yunnan Province,Kunming 650093,China
dCollege of Metallurgy and Energy Engineering,Kunming University of Science and Technology,Kunming 650093,China
In present work,we investigate production of magnesium by carbothermic reduction under vacuum conditions.The process was divided into two parts,one is reduction process,and the other one is condensation process.The experimental results revealed that during reduction process,the gas-solid reaction between MgO and CO was not occurred at a temperature and pressure of 1723 K and 30-100 Pa respectively.So the main reduction reaction was MgO(s)+C(s)=Mg(g)+CO(g)(under vacuum)and reaction type belonged to solid-solid reaction.In Condensation Process,according to a contrast and analysis,the condensation quality of magnesium is associated with CO concentration.The resultant product C was formed and it followed magnesium vapor condensation which prevents mutual combination of two metal droplets to forms the compact condensation produces.Therefore,in order to compact morphological forms of magnesium crystal whiskers,we must control the technical conditions and fnd the method to separate the magnesium vapor and carbon monoxide.That's the key factor to get better crystalline structure. Copyright 2015,National Engineering Research Center for Magnesium Alloys of China,Chongqing University.Production and hosting by Elsevier B.V.All rights reserved.
Metal magnesium;Carbothermic reduction;Condensation;Vacuum
1.Introduction
Generally,magnesium is produced by two main processes: electrolysis of molten magnesium chloride and thermal reduction of magnesia[1].Electrolysis is the pre-dominant route in western countries,accounting for about 77 pct of western countries total production,however,this process is characterized by high-energy requirements[2].In recent years,production of magnesium in China by the Pidgeon process has dominated world production.In this process, ferrosilicon reduces magnesia from calcined dolomite under vacuum.The process suffers from high energy usage and low productivity[3].Our research group is exploring the carbothermic reduction of magnesia area of study for many years, and the process of carbothermic reduction and magnesium vapor condensation were the important link to solve the problem of industrialization.The reaction between MgO and C may provide a new route toward the production of magnesium.But for the carbothermic reduction reaction type,one point of view was that the main reaction is solid-solidreaction,the other was that the main reaction is gas-solid reaction[4-6].
Qu analyzed the uniform nucleation of water from nucleation rate and randomness,while investigating magnesium vapor condensation process.Zhang theoretically studied the gas bubble nucleation of molten metal.Zeng evaluated the properties of metastable equilibrium,and the mechanism of gas bubble nucleation through thermodynamics and kinetic theory.Izmailov studied the statistical mechanics of nucleation.Ring researched the reason of nano-clusters nucleation. Leubner established nucleation and growth model.In Lu¨mmen's paper,the uniform nucleation of supersaturation iron vapors has been investigated by molecular dynamics simulation method[7-15].Therefore,analysis of the magnesium vapor nucleation in theory is helpful to fnd the key factors which affect the way of magnesium vapor condensation.In addition the reactions of carbothermic reduction process were discussed.In order to investigate the mechanism of the carbothermic reduction process in vacuum,XRD analysis was also proposed.
2.Reduction process
2.1.Raw material
Analytical-grade magnesia and carbon were used as the raw materials in our experiments.
2.2.Schematic diagram of vacuum furnace
The experiments were carried out in a self-made vacuum furnace shown in Fig.1.
2.3.Experimental procedure
The raw materials,magnesia(20 g)and carbon(12 g) grounded to-140 mesh after drying were mixed in a molar ratio(MgO/C=1/2).The mixed materials were compacted into pieces of Φ30 mm×10 mm dimensions under 6 MPa. The compacts were placed in a graphite crucible and heated at the heating rate of 15 K×min-1.The system temperature needed to be kept at 1723 K for 1-2 h under vacuum.At high reduction temperature,Mg vapors and CO were produced and moved into the upper condensing towers.The temperature of the condensing surfaces was kept between 773 and 973 K.The reversion reaction occurred during the condensing process.
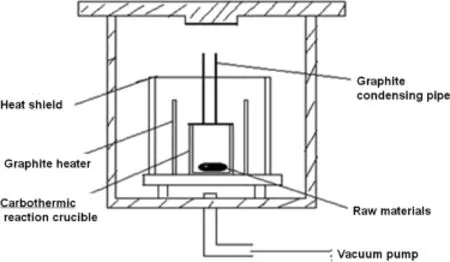
Fig.1.Schematic diagram of vacuum furnace.
2.4.Analysis method
The crystalline phase of the resulted products was identifed by X-ray diffraction(XRD)instrument(D/max-3B)using Cu Kαradiation with a scanning rate of 2(°)/min which was made by Rigaku Corporation of Japan.
The mass loss ratio(α)is defned as

whereM0is the mass at the start of the reduction reaction andM1is the reaction equilibrium mass at the end of reduction. The termM0-M1/M1,represents the reduction degree of magnesia available for reaction.
2.5.Analysis of experimental results
Carbothermic reduction was carried out under the following conditions:MgO:C = 1:2 (molar ratio),temperature 1073-1773 K,hold temperature time 1-2 h,system pressure 30-100 Pa,compacting pressures 6 MPa of the blend MgO. During the thermo gravimetric reduction process,the mass loss of the sample was monitored as a function of time.It was calculated by formula(1).The photographs of materials (MgO+C)before and after reaction at 1723 K are shown in Fig.2.A typical mass-loss percentage vs.temperature curve of reduction of magnesia by carbon is shown in Fig.3.
Fig.2 shows that the structure of briquetted materials was changed into porous and loose after reduction reaction,which revealed that the solid-solid reaction occurred between MgO and C.It can be noticed that there was no sharply mass loss detected when the temperature was below 1553 K as shown in Fig.3.The values below 15%indicate that there was not any reduction reaction.When the temperature was higher than 1553 K,the sample weight changes sharply with increase of temperature,and there was an obvious infection point of mass loss at 1553 K.Therefore,this temperature was considered as initial reaction temperature.It accorded with the theoretical calculation.It can also be seen that the maximum of mass loss reached 75%in the experimental temperature range,the solid-solid reduction reaction was completely fnished under the experimental condition.
The reaction between MgO and CO may provide a new method toward investigation of gas-solid reaction.A stomatal dish was added in the carbothermic reaction crucible to divide reduction zone into two parts.The pieces of MgO were placed on the Al2O3fats(above the stomatal dish)in order to avoid contacting the graphite,so that the reaction between MgO and crucible(C)was not occurred.Under the stomatal dish, magnesia and carbon as the starting materials were mixed in a molar ratio(MgO/C=1/2)with addition of 5%CaF2andestimated.The photograph of material(MgO+CO)before and after reaction at 1723 K is shown in Fig.4.A typical mass-loss percentage vs.temperature curve of reduction of magnesia by CO is shown in Fig.5.

Fig.2.Photographs of material(MgO+C)before(a),and after reaction(b)at 1723 K.
The morphology of MgO was the same after a gas-solid reaction,as shown in Fig.4.No pore was appeared inside MgO.Through the calculation of mass loss ratio,the α of MgO is shown in Fig.5.It showed that the value of α was less than 20%,the result was in the range of α of pure MgO,and no condensing product was obtained in condensation zone too. It was accorded with the theoretical calculation of gas-solid reaction.Therefore,the gas-solid reaction was not occurred in the carbothermic reduction process under the condition of 10-60 Pa and 300-2000 K. were made into pieces under compaction pressures of 6 MPa. When the system temperature needed to be kept at 1723 K for more than 2 h,a mass of CO was produced by mixed MgO and C under the stomatal dish and moved through the stomatal dish to the gas-solid reaction zone(Above the stomatal dish). The Analytical-grade magnesia could make a good contact with CO,and then the reaction between MgO and CO was
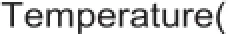
Fig.3.Effect of reduction temperature on material(MgO+C)weight loss.
3.Condensation process
3.1.The condensation of magnesium in carbothermic reduction of magnesia
As reverse reaction between magnesium vapor and CO occurred:
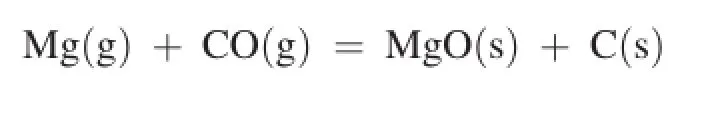

Fig.4.Photograph of material(MgO+CO)before(a)and after reaction(b)at 1723 K.
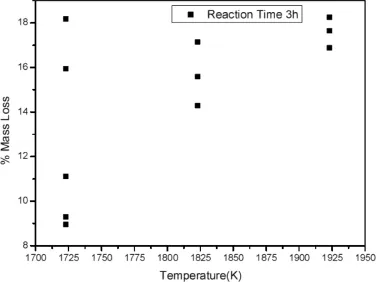
Fig.5.Effect of reduction temperature on material(MgO+CO)weight loss.
The raw materials and reduction product samples are shown in Fig.6.Fig.7 shows the XRD patterns of the condensing product in carbothermic reduction,it can be seen that metal magnesium was obtained.Meanwhile,magnesia was also found as indicated by peaks.
The method of vacuum distillation was used to separate the metal magnesium,MgO,and Mg2C3.In the distillation process,the condensing product was used as raw material and heated from room temperature to 1373 K.After distillation for 1 h or 2 h,the temperature of distillation product cooled to room temperature and took out.After the vacuum distillation process,MgO,Mg2C3and carbon were stayed loaded into the reaction crucible,high purity metal magnesium was produced in the condensing tower,which called distillation product.
The SEM images of condensing product which were obtained from condensing tower are shown in Fig.8.The image of condensing product(a)shows irregular shape with an incompact structure which had a lot of small openings,where the carbon can be found.But the distillation product(b)shows irregular shape with a compact structure and good crystalline feature.
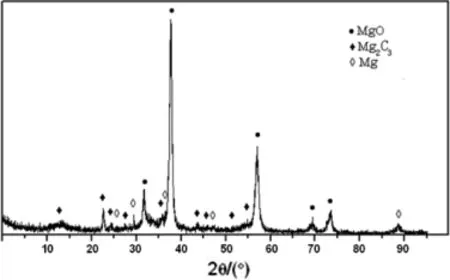
Fig.7.XRD patterns of the condensing product in carbothermic reduction.
As the BESECOMP image of condensing product is shown in Fig.9,the loose crystallization with chinky structure was observed which is due to the carbon and magnesia,obtained by reverse reaction.It means that the carbon and magnesia (obtained by reverse reaction)were simultaneously in the condensing product blocks the atomic aggregation and coacervation,decreases the rate of metal magnesium nucleation and the rate of crystal growth.Then,the different appearance of magnesium crystal from block-like to loose-like was obtained.
3.2.Analysis of reverse reaction ratio

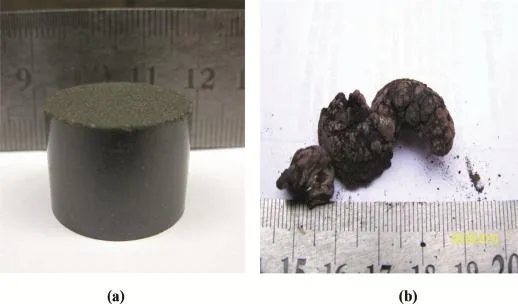
Fig.6.The picture of raw materials(a)and reduction product(b).
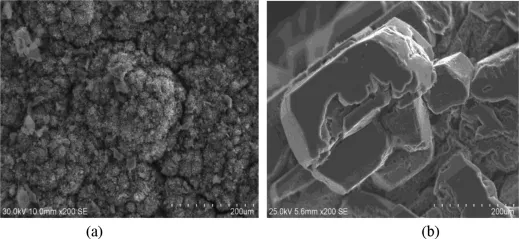
Fig.8.SEM images of condensing product(a)and vacuum distillation product of purity magnesium(b).

Fig.9.The BSECOMP image of condensing product.
The reversion reaction ratio(γ)was defned as whereW0represents the total mass of magnesium and magnesia in the condensing product,andW1the mass of magnesia in distillation residual.
The reverse reaction ratio was calculated using formula(2), as shown in Fig.10,it is clear that the reverse reaction ratio changed slowly with the incremental molar ratio,the reverse reaction ratio increased with increasing reaction time.However,the peak value was less than 9%,it can be deduced that most magnesium vapors transformed into metal magnesium, the condensing condition of the vacuum equipment was appropriate for the carbothermic reduction.
4.Conclusions
1)In reduction process,the analysis of experimental results revealed that the gas-solid reaction between MgO and CO was not occurred at 1723 K temperature and 30-100 Pa pressure. So the main reduction reaction was MgO(s)+C(s)=Mg(g)+CO(g)in vacuum.The reaction type belonged to solid-solid reaction.
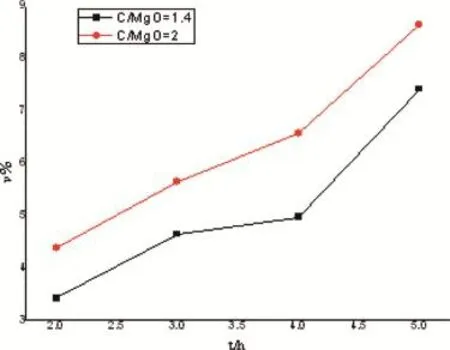
Fig.10.Relationships between reversion ratio and reaction time in different molar ratios.
2)In Condensation Process,according to a contrast and analysis,the condensation quality of magnesium is associated with CO concentration.The resultant product C was formed and it followed magnesium vapor condensation which prevents mutual combination of two metal droplets to forms the compact condensation produces.That's the reason of the structure decentralized products obtained which loose metal luster.Therefore,in order to compact morphological forms of magnesium crystal whiskers,we must control the technical conditions and fnd the method to separate the magnesium vapor and carbon monoxide.That's the key factor to get better crystalline structure.
[1]Rong-ti Li,Wei Pan,Masamichi Sano,Metall.Mater.Trans.8(2003) 433-437.
[2]A.Froats,Light Metal,Metallurgical Society of AIME,New York,NY, 1980,pp.969-979.
[3]S.Ramakrishnan,P.Koltun,Resour.Conserv.Recycl.42(2004)49-64.
[4]M.Sakamoto,S.Akiyama,J.Mater.Sci.Lett.16(12)(1997) 1048-1050.
[5]Ding-cheng Mo,Metallurgical Kinetics,Central South University Press, Changsha,1987,pp.173-326.
[6]Dong Yang,Chao-wan Wu,Zhong-yi Wang,Special Metallurgical Problems 10,China Industry Press,Beijing,1963,pp.58-84.
[7]Jiu-sheng Bao,Tiong-gang Liu,Eng.Mech.Mater.32(2)(2008)4-7.
[8]Lan Peng,Chao Liu,J.Eng.Thermophys.21(6)(2000)683-685.
[9]Kai-yang Qu,Yi Jiang,J.Phys.49(11)(2000)2214-2219.
[10]Hua-wei Zhang,Yan-xiang Li,J.Phys.56(8)(2007)4864-4871.
[11]Dan-Qin Zeng,J.Chongqing Univ.18(2)(1995)1-8.
[12]Alexander F.Izmailov,Allan S.Myerson,Stephen Arnold,J.Cryst. Growth 196(1999)234-242.
[13]Terry A.Ring,Adv.Colloid Interf.Sci.91(2001)473-499.
[14]Ingo H.Leubner,Curr.Opin.Colloid Interf.Sci.5(2000)151-159.
[15]N.Lu¨mmen,T.Kraska,Aerosol Sci.36(2005)1409-1426.
Received 1 July 2014;revised 9 April 2015;accepted 10 April 2015 Available online 21 May 2015
☆Foundation item:Project supported by National Natural Science Foundation of China(No.51304095);the Personnel Training Funds of Kunming University of Science and Technology,China(No.14118665)and Educational Commission of Yunnan Province,China(No.14051618).
*Corresponding author.College of Metallurgy and Energy engineering, Kunming University of Science and Technology,Kunming 650093,China. Tel.:+86 871 65161583.
E-mail address:kgyb2005@126.com(B.Yang).
Peer review under responsibility of National Engineering Research Center for Magnesium Alloys of China,Chongqing University.
http://dx.doi.org/10.1016/j.jma.2015.04.001.
2213-9567/Copyright 2015,National Engineering Research Center for Magnesium Alloys of China,Chongqing University.Production and hosting by Elsevier B.V.All rights reserved.
 Journal of Magnesium and Alloys2015年2期
Journal of Magnesium and Alloys2015年2期
- Journal of Magnesium and Alloys的其它文章
- GUIDE FOR AUTHORS
- Taguchi optimization of process parameters in friction stir processing of pure Mg
- Processing,microstructure and mechanical properties of bimodal size SiCp reinforced AZ31B magnesium matrix composites
- Microstructure and room temperature tensile properties of 1 μm-SiCp/AZ31B magnesium matrix composite
- A comparative corrosion behavior of Mg,AZ31 and AZ91 alloys in 3.5% NaCl solution
- Dynamic compressive property and failure behavior of extruded Mg-Gd-Y alloy under high temperatures and high strain rates
