Features of Computed Tomography Perfusion of Mediastinal Lymphadenopathies: a Pathology-based Retrospective Study
1Department of Radiology, The PLA 175thHospital & Affiliated Southeast Hospital of Xiamen University, Fujian 363000, China
2Department of Radiology, Nanjing General Hospital of Nanjing Military Command, Nanjing 200012, China
Features of Computed Tomography Perfusion of Mediastinal Lymphadenopathies: a Pathology-based Retrospective Study
Lin Ou-yang1and Guang-ming Lu2*
1Department of Radiology, The PLA 175thHospital & Affiliated Southeast Hospital of Xiamen University, Fujian 363000, China
2Department of Radiology, Nanjing General Hospital of Nanjing Military Command, Nanjing 200012, China
mediastinal lymphadenopathy; computed tomography perfusion; functional computed tomography
ObjectiveTo explore the features of various mediastinal lymphadenopathies using computed tomography perfusion (CTP).
MethodsCTP parameters (CTPs) of the selected mediastinal nodes from 59 patients with pathology-proven malignant lymph nodes and of those from 29 patients with clinically diagnosed or pathology-proven inflammatory lymphadenopathies were collected. Patients were divided into subgroups by etiology and phase of primary disease, including different pathological malignant nodes and diverse inflammatory nodes. CTPs were defined as blood flow (BF), blood volume (BV), mean transit time (MTT), permeability (PMB), and time to peak (TTP). Differences of CTPs were compared between malignant and benign nodes, and among subgroups, respectively.
ResultsIn the mediastinum, no significant differences of CTPs were found between malignant and benign groups (all P>0.05), the same for subgroups of malignant nodes (all P>0.05). Acute lymphadenitis had higher BF and BV than chronic inflammatory, lymphoid tuberculosis, sarcoidosis and malignant nodes. The BF of malignant nodes was markedly slower than that of acute lymphadenitis (P=0.01), but faster than chronic inflammatory nodes (P=0.04) and sarcoidosis (P=0.03), with no significant difference compared with lymphoid tuberculosis. Pneumonia-complicated lymphoid tuberculosis showed the longest MTT while sarcoidosis displayed the shortest MTT, and inflammatory nodes, lymphoid tuberculosis without complicated pneumonia and malignant nodes had moderate MTT.
ConclusionCTPs show promising potential in distinguishing various lymphadenopathies in the mediastinum, but more studies are needed to improve their specificity.
Chin Med Sci J 2015; 30(3):162-169
THE advent of computed tomography perfusion (CTP) technique broadens the functional value of CT in the clinic, permitting the measurement of vascular physiology in the focus of infection.1This technique has been used in clinic for staging cancer, discriminating diagnosis,2evaluating therapeutic response and predicting prognosis.3But few applications of this technique were performed for the mediastinal lymphadenopathy, especially differentiating malignant nodes from various inflammatory nodes. And for now, it is still unsatisfactory to identify the metastatic mediastinal nodes,4although the nature and status of the infected nodes are very important for patient to choose therapy strategy and fate from many uncertain selections. Therefore, this study was designed to observe the differences of various mediastinal lymphadenopathies in CTP.
PATIENTS AND METHODS
Subjectscollection
In this retrospective comparison analysis, inpatients of the PLA 175thHospital (Affiliated Southeast Hospital of Xiamen University) were selected from January 2010 to February 2014. A CTP technique was performed for patients with suspected primary or metastatic malignant lymph nodes and inflammatory nodes in the mediastinum before invasive diagnosis and therapy. The final diagnoses were based on the pathology by surgery, mediastinoscope, endobronchial ultrasound-guided transbronchial needle aspiration for all suspected malignant nodes and lymphadenopathies of unknown origin, and for clinically suspected inflammatory lympadenopathies, clinical situation, therapy and follow-up were integrated to gain the final diagnosis before using pathological methods. Complete data, including CTPs and pathology, were retrospectively collected for them in this study.
CT program
CT was performed using a Definition AS 4D multi-detector CT scanner (Siemens Somatom Definition AS 4D CT; Siemens Company, Germany). A preliminary non-contrast 5.0-mm-thick CT of the mediastinum region (from the superior aperture of the thorax to the diaphragmatic muscle) was performed to ensure a panoramic view of the entire anatomical region, thereby enabling recognition of anatomical landmarks. The target node was identified on the images. The table position at the central level of the marked target node was recorded while defining the scan range using an external laser alignment light. The alignment of the table position was confirmed with that recorded when the patient was centered. The following parameters were selected for the dynamic CTP scanning: tube current 50-200 mAs (automatic-adapted adjustment by machine), tube voltage 100 kV, slice 5.0 mm (acquisition 24×1.2 mm), number of scans 20, gantry rotation time 0.33 seconds, scan range was adjusted by size of the target node to contain the maximum volume of the target node, examination time 20.60-30.90 seconds, CT dose index volume (CTDIvol) 27.06-126.57 mGy, slice 1.5 mm. A standard reconstruction algorithm with no edge enhancement was used for dynamic scanning. A cradle scanning technique belonging to Siemens Somatom Definition AS 4D CT was used for controlling motion artifact.
Contrast agent techniques: Bolus injection of 45 ml of non-ionic iodinated contrast medium (Iopamidol injection 370 mgI/ml; Bracco Sine Pharmaceutical Corp. Ltd., Shanghai, China) at 5.5 ml/second, followed by 20 ml of saline at 2 ml/second via a 20-gauge cannula in the right antecubital vein. After contrast material administration, consecutive dynamic CT acquisitions were performed after a 4-second delay from the start of the injection, to-and-fro scanning at the same field of view with a breath-hold for 21-31 seconds of total duration and no delay.
Image data analysis
The obtained images and data were transferred to an image processing workstation (Syngo MultiModality Workplace, Series Number: 46 531) and analyzed by two experienced readers (17 and 11 years’ experience in CT diagnosis), who were blinded to the pathological results. Commercially available software (Syngo MMWP VE36A, Siemens AG) was used for the CTP analysis. In one of the series slices, a region of interest (ROI) that was sufficiently small to avoid partial volume effects (2 to 6 pixels) was placed in the aorta to calculate the arterial input, and a ROI was manually drawn along the visible margins of the node (Fig. 1). The software provided automated calculation of the following perfusion parameters in the ROI areas: blood flow (BF) in milliliters per 100 mg per minute, blood volume (BV) in milliliters per 100 mg, mean transit time (MTT) of the contrast medium through the ROI area in seconds, time to peak (TTP) of the contrast medium to reach the highest enhancement peak of the ROI in seconds, and permeability (PMB) product, which measures contrast medium extravasation into the extravascular space in milliliters per 100 mg per minute. Values of the perfusion parameters were averaged across three or four slices to minimize the variability resulted from the ROIs selection. These three ROI slices (node less than or equal to 10 mm)or four ROI slices (node larger than 10 mm) were selected from the target node by equal intervals. The set of slices avoided calcification, hemorrhage, necrosis and cyst regions.
Pathological evaluation
The node specimens were fixed with 10% neutral buffered formalin for 6 to 8 hours under vacuum at 37°C, then were bisected along the major axis and were sliced at 2-mm intervals and subsequently dehydrated, cleared, and embedded in paraffin. From the paraffin blocks, pairs of sections were cut at 50-μm intervals until complete sectioning of the nodes was achieved. One section of each pair was routinely stained with hematoxylin and eosin, whereas the mirror sections were kept unstained. Metastatic foci of up to 2 mm in the greatest dimension were reported as micro-metastases; nodes larger than 2 mm were defined as metastatic.
Statistical analysis
Statistical analyses were performed using SPSS 17.0 (SPSS Inc., IL, USA). Continuous variables were expressed as means±SD. The differences of CTPs between malignant and inflammatory nodes were evaluated using independent sample t-test. The differences of CTPs among subgroups of different pathological malignant nodes, among subgroups of different etiology and phase of inflammatory nodes, and between malignant and inflammatory subgroups were respectively statistically evaluated by multivariate F test of general linear mode. Adjustment for multiple comparisons with Bonferroni test was considered. P<0.05 was considered statistically significant.
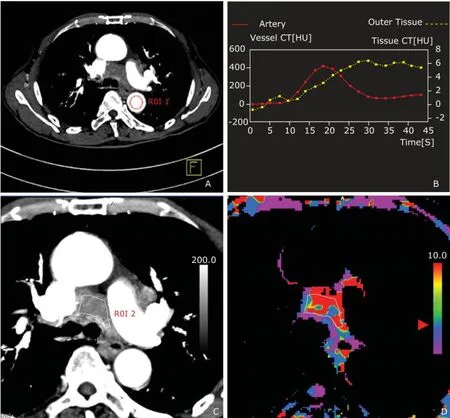
Figure 1. Images from one of the slices obtained by dynamic computed tomography centered on the enlarged mediastinum node from a patient with left upper lung lingular segment lung cancer. A region of interest (ROI 1) was positioned in the descending thoracic aorta (A) for calculation of the arterial contrast medium TDC (red curve) and outer tissue TDC (yellow curve) (B), and ROI 2 was hand-drawn along the margins of the target node for calculation of the CTP values of the target node (C). The colored scale (D, arrowhead) perfusion map designed using software showed how the perfusion of the target node was similar to that of the outer tissue.
RESULTS
Enrolled nodes and etiological diagnosis
According to the criteria, 59 patients with malignant mediastinal lymph nodes and 29 patients with mediastinal inflammatory lymphadenopathies were selected to analyse their CTPs features. The malignant nodes were 23 lung adenocarcinomas (LAC), 10 lung small cell carcinomas (LSCC), 7 lung squamous carcinomas (LSC), 6 mediastinal lymphomas (ML), 6 lung neuroendocrine tumors (LNET), and 7 esophagus cancers (EC). The inflammatory nodes included 14 lymphoid tuberculosis (LT), 10 lymphadenitis and 5 sarcoidosis. The lymphadenitis was sub-grouped into acute stage (n=5) and chronic stage lymphadenitis (n=5). The LT was divided into subgroups of complicated caseous pneumonia (n=8), and uncomplicated caseous pneumonia (n=6).
General characters of the mediastinal malignant and inflammatory lymph nodes
The sizes of malignant nodes ranged from 7.1 cm to 10.2 cm, and the sizes of inflammatory nodes from 3.5 cm to 10.9 cm. The mean diameters of the malignant and inflammatory nodes were 2.7±1.3 cm and 1.6 ± 0.7 cm, respectively. There was significant difference in size between the malignant and inflammatory nodes (P=0.002). The mean areas of the ROIs drawn along the target nodes were 157.5 mm2and 112.9 mm2for the malignant and inflammatory nodes, respectively. No significant difference in areas was found between the malignant and inflammatory nodes (P=0.29).
Differences of CTPs between the malignant and inflammatory nodes in the mediastinum
The CTPs values showed no significant differences between the whole malignant and inflammatory lymph nodes, with BF (F=0.71, P=0.40), BV (F=3.73, P=0.06), TTP (F=3.14, P=0.08), PMB (F=0.05, P=0.81) and MTT (F=0.05, P=0.81). And no significant differences were found in all CTPs among different pathological types of malignant nodes (Table 1).
In inflammatory nodes, there were no significant differences in terms of BF (F=2.47, P=0.10), BV (F=0.24, P=0.79), TTP (F=0.01, P=0.99), PMB (F=0.92, P=0.41) among lymphadenitis, LT and sarcoidosis, except MTT (F=7.35, P=0.00) being remarkably longer in LT than in both lymphadenitis (P=0.02) and sarcoidosis (P=0.00). Also, malignant nodes (Fig. 2) had no significant difference in CTPs from lymphadenitis, LT (Fig. 3) and sarcoidosis in the mediastinum. However, when inflammatory nodes were divided into subgroups by etiology and phase, acute lymphadenitis had higher BF than chronic lymphadenitis (P=0.00), LT without caseous pneumonia (P=0.01), and sarcoidosis (P=0.00). The BF of malignant lymph nodes was significantly slower than acute lymphadenitis (P=0.01), but faster than chronic lymphadenitis (P=0.04) and sarcoidosis (P=0.03). Moreover, acute phase lymphadenitis had the largest BV in all inflammatory and malignant subgroups. Malignant nodes had a moderate BV being larger than chronic proliferative inflammatory nodes and smaller than acute lymphadenitis. Furthermore, sarcoidosis had the shortest MTT and caseous pneumonia-complicated lymphoid tuberculosis had the longest MTT in these various lymphadenopathies (Tables 2, 3).
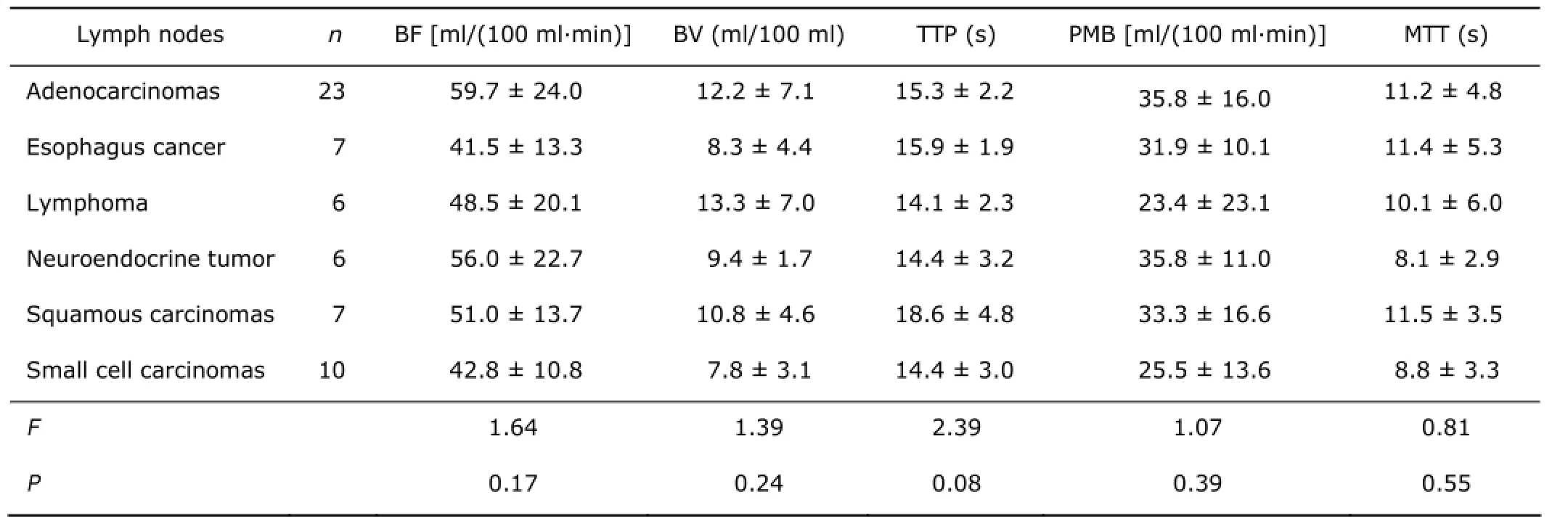
Table 1. Differences of CTPs among different pathological malignant nodes in the mediastinum§

Figure 2. CTP maps of mediastinal enlarged node from a 39-year-old female patient with left upper lung lower lingular segment lung cancer.A. Transverse material-enhanced CT image of target node and pathological immunohistochemical map (SP staining, ×200) (arrow);B-F. CTP color maps were BF [42.9 ml/(100 ml·min), B], BV (17.0 ml/100 ml, C), TTP (15.9 s, D), PMB [27.9 ml/(100 ml·min), E], and MTT (4.44 seconds, F), respectively. Functional color maps showed increased microvascular perfusion function induced by neo-angiogenesis compared with outer chest wall muscle tissue according to the color scale, and average CTPs values for ROIs were automatically obtained. At post-surgical pathology, the lymph node was determined to be metastatic poorly differentiated lung adenocarcinoma.
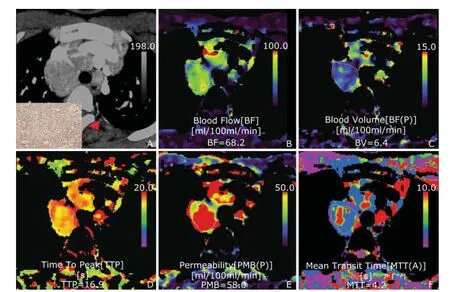
Figure 3. CTP maps of mediastinal enlarged node from a 27-year-old male patient with mediastinal lymphoid tuberculosis. A. Transverse material-enhanced CT image of target node and pathological immunohistochemical map (SP staining, ×200) (arrow);B-F. CTP color maps were BF [68.2 ml/(100 ml·min), B], BV (6.4 ml/100 ml, C), TTP (16.90 s, D), PMB [58.0 ml/(100 ml·min), E], and MTT (4.2 seconds, F), respectively. At post-thoracoscopy, the lymph node was pathologically determined to be lymphoid tuberculosis. The node presented higher perfusion function in both BF and PMB compared with outer chest wall muscle tissue according to the color scale, mimicking malignant CTP presentation. Because changes occurring in the vascular endothelium and changes in vessel function induced by cytokine-mediated inflammation and neo-angiogenesis were similar, so inflammatory proliferative lymph nodes could mimic malignant CTP presentation in their active phase.
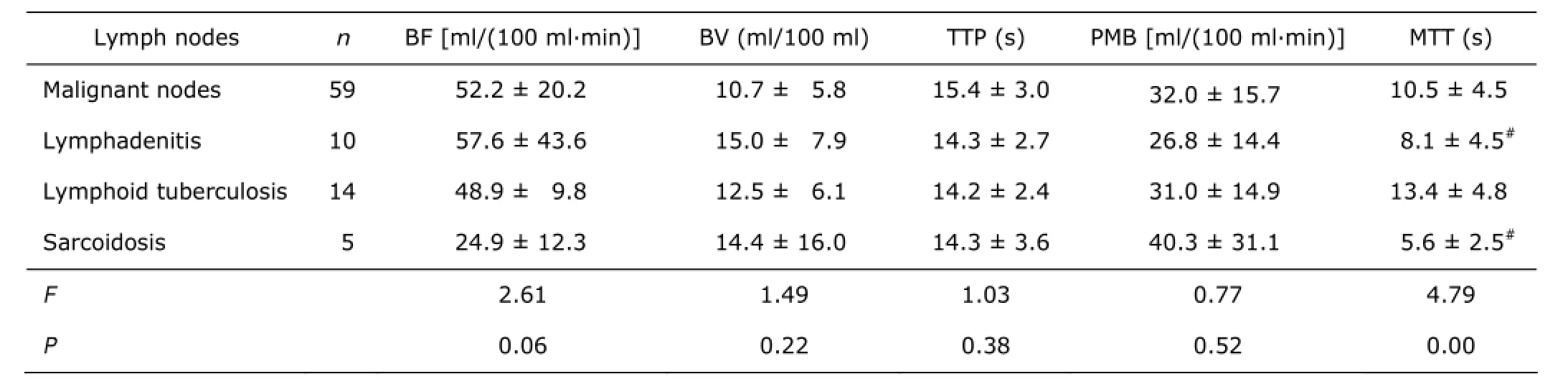
Table 2. Differences of CTPs between the malignant and different inflammatory nodes§
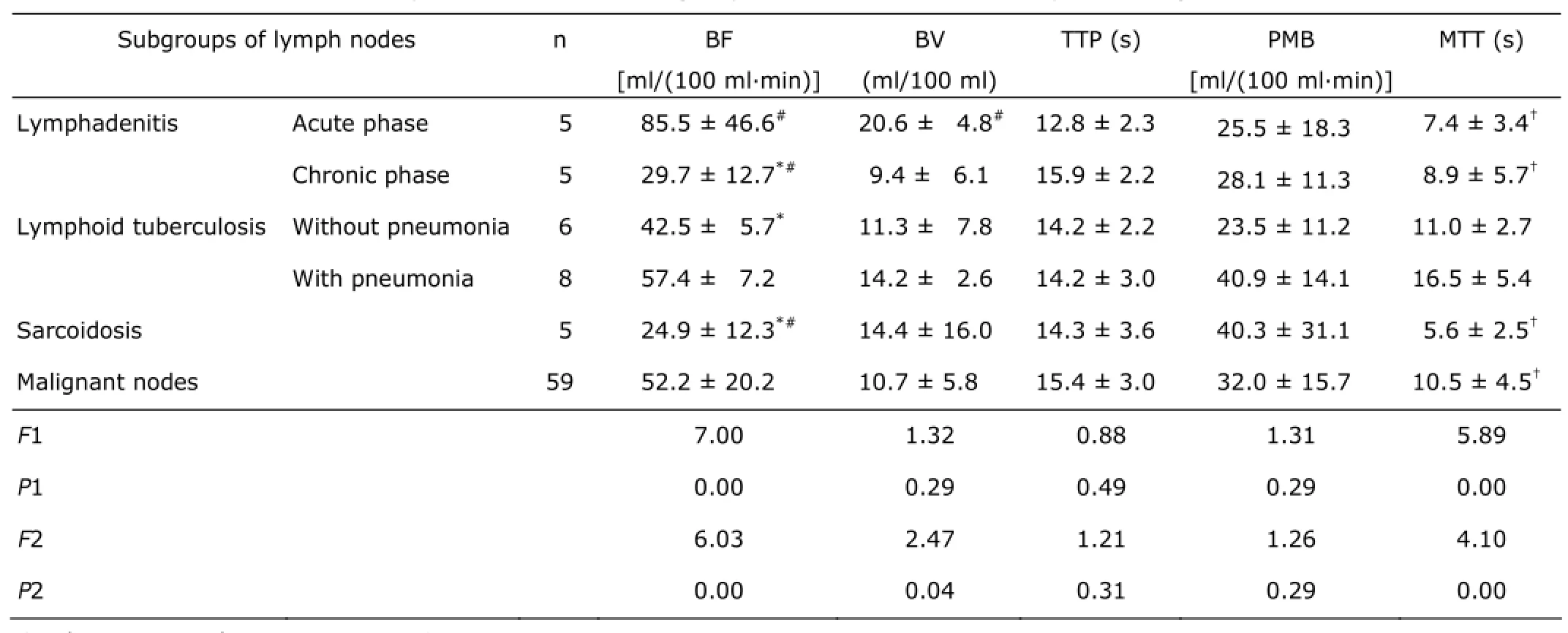
Table 3. Comparison of CTPs of subgroups of different inflammatory and malignant nodes§
DISCUSSION
In this study, when all malignant and inflammatory lymph nodes respectively taken as a group, all CTPs showed no significant differences between the malignant and inflammatory nodes. When malignant lymph nodes were divided into subgroups by the pathology of the primary tumor, all CTPs presented no significant differences among different subgroups. This result was consistent with Bisdas et al,5who declared that the differentiation of malignant lymph nodes was not possible using perfusion values, which had been obtained from a study using CTP to detect and differentiate primary and recurrent tumors as well as nodal disease in patients with oropharynx and oral cavity cancer.
Also, there were no significant differences of BF, BV among lymphadenitis, LT, sarcoidosis and malignant nodes. However, when inflammatory nodes were divided into subgroups by etiology and phase, interestingly, the results presented that acute lymphadenitis had the fastest BF and the biggest BV in all subgroups, including malignant nodes. LT with caseous pneumonia had the longest MTT and sarcoidosis had the shortest MTT (Table 3). This suggested that lymphadenopathies of different etiology and phase have very different presentation in CTPs.
The BF of malignant nodes was smaller than that of acute lymphadenitis but larger than that of proliferative nodes in chronic phase. Yun et al6also found that the mean BF of the metastatic nodes was higher than that of the inflammatory nodes (P<0.05) in differentiating metastatic enlarged axillary lymph nodes from inflammatory in patientswith breast cancer. And Trojanowska et al7differentiated malignant from non-malignant cervical lymph nodes in patients with squamous cell cancer of the hypopharynx and larynx using CTPs and demonstrated that compared to non-malignant nodes, malignant nodes showed significantly higher BF (P<0.05).
The basic theory of CT perfusion software is based on the measure of blood supply to the ROI and its leakage into intercellular spaces. With the contrast media leakage into the extravascular space, enhancement of the tumors was caused by the contrast media in both the intravascular and extravascular space.8The CTP mirrors tissue vascularization and might reflect angiogenic activity.9BF and BV are increased in newly developed vessels due to angiogenetic activity. MTT reflects the transit time via the vascular bed and is predominantly affected by the presence of shunts. PMB represents the transmission rate of the contrast media from the capillary endothelium to the interstitial space, which reflects the integrity of endothelial cells and permeability of vessels.
Thus, theoretically, it may be possible to differentiate neoplastic from non-neoplastic tissue. But in clinical practice, when metastatic cells were implanted in a lymph node and invading extravascular space, the changes occurring in the lymph nodes were most likely similar for different pathological tumors,10which might explain the absence of significant differences of CTPs among diverse malignant nodes. And an inflammatory hyperplasia lymph node usually mimics malignant nodes using imaging.11This situation may be due to the effect of cytokines, which induce vasodilatation and increase endothelial permeability. This hypotheses support these findings that acute lymphadenitis had higher BF and BV than chronic hyperplasia nodes as chronic lymphadenitis, LT and sarcoidosis, even than malignant nodes. LT with caseous pneumonia had relatively higher BF and BV than LT without caseous pneumonia, which may be due to caseous pneumonia contributing to an acute inflammation-like response (Tables 2, 3).
The changes induced by metastatic cells implanted in a lymph node are mainly expressed by blood vessel overgrowth and architectural disorganization.10When invading the extravascular space, inflammatory and neoplastic infiltrations are similar in size12,13and function to the lymph node, as demonstrated using USPIO-MRI imaging.14,15Because changes in the vascular endothelium and function induced by neo-angiogenesis and cytokinemediated inflammation were similar, these inflammatory lymph nodes could mimic malignant CTP presentation in their certain phase (Figs. 2, 3).
Unlike Bisdas et al,5who did not take into account of the variety of lymph nodes, this study sub-grouped the nodes by etiology. And unlike the studies of Liu et al6and Trojanowska et al7, the pathophysiological processes were not considered for the lymph nodes enrolled in whose studies, this study divided inflammatory nodes into subgroups by stage. Both etiology and stage are crucial for CTPs presentation, which most probably contributed to the discrepancy of results from these studies.
In this study, for CTPs, the mean areas of the ROIs drawn along the target nodes were 157.5 mm2and 112.9 mm2for malignant nodes and inflammatory nodes, respectively, with no significant difference (P=0.29). The injected contrast agent, injection site, dosage and flow rate were designed according to most studies.16,17A quiet respiration was requested during the scan, with patients in the prone position. The majority of the injected contrast media remained intravascular within 40 seconds (it was also affected by cardiac output and central blood volume); however, a greater proportion subsequently passed into the extravascular space. Eventually, equilibrium was reached and the blood returned into the intravascular space. The duration of this process lasted approximately 2 to 4 minutes.18By measuring the attenuation change within the input artery and tumor, an (input response function IRF) curve could be derived. The peak of this curve was regarded as the BF, while the BV was represented by the area under the curve. As a result, different acquisition times did not dramatically affect these three values because the shape of the curve was fixed.19
Furthermore, a long acquisition time can result in respiratory motion and a higher examination radiation dose. Taken together, the dynamic scan time was established at 40 seconds for this study, and at the same time, by automaticadapted adjustment by machine, the scan radiation dose was controlled by CTDI vol at 27.06-126.57 mGy.
There are several limitations in our study. First, the size of the study population was small and asymmetric in the inflammatory and malignant nodal subgroups, which potentially brought about bias in statistical analysis or non-significant differences among groups. Second, malignant nodes were not divided into subgroups according to TNM staging of primary tumor because of unequal pace of changes in nodes and primary tumor. Metastatic or malignant invasion in lymph node may affect the part or the whole, which would affect the homogeneity of CTP. Third, the contrast enhancement was set by a fixed mode, neglecting body surface area, heart rate, stroke volume and central blood volume, which results in calculation error of perfusion kinetics and is difficult to overcome.
Meaningfully, this study suggested the influence of etiology and stage on the CTP presentation of mediastinal lymph nodes, elucidated the principle of inconsistent results in previous studies, laying a foundation for further exploring the real clinical diagnostic application of CTP.
1. Song T, Shen YG, Jiao NN, et al. Esophageal squamous cell carcinoma: assessing tumor angiogenesis using multi-slice CT perfusion imaging. Dig Dis Sci 2012; 57: 2195-202.
2. Razek AA, Tawfik AM, Elsorogy LG, et al. Perfusion CT of head and neck cancer. Eur J Radiol 2014; 83: 537-44.
3. Preda L, Calloni SF, Moscatelli ME, et al. Role of CT perfusion in monitoring and prediction of response to therapy of head and neck squamous cell carcinoma. Biomed Res Int 2014; 2014: 917150.
4. Kazmierczak PM, Nikolaou K, Rominger A, et al. Radiological diagnostics in CUP syndrome. Radiology 2014; 54: 117-23.
5. Bisdas S, Baghi M, Smolarz A, et al. Quantitative measurements of perfusion and permeability of oropharyngeal and oral cavity cancer, recurrent disease, and associated lymph nodes using first-pass contrastenhanced computed tomography studies. Invest Radiol 2007; 42: 172-9.
6. Liu Y, Bellomi M, Gatti G, et al. Accuracy of computed tomography perfusion in assessing metastatic involvement of enlarged axillary lymph nodes in patients with breast cancer. Breast Cancer Research 2007; 9: R40.
7. Trojanowska A, Trojanowski P, Bisdas S, et al. Squamous cell cancer of hypopharynx and larynx-evaluation of metastatic nodal disease based on computed tomography perfusion studies. Eur J Radiol 2012; 8: 1034-9.
8. Sahani DV, Kalva SP, Hamberg LM, et al. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology 2005; 234: 785-92.
9. Feng ST, Sun CH, Li ZP, et al. Evaluation of angiogenesis in colorectal carcinoma with multidetector-row CT multislice perfusion imaging. Eur J Radiol 2010; 75: 191-6.
10. Hoshida T, Isaka N, Hagendoorn J, et al. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res 2006; 66: 8065-75.
11. Ouyang L, Zhou ST, Chen MH, et al. CT perfusion time-density curve morphosis of lymphadenopathy and its clinical significance. Chin J Med Imaging Technol 2012; 28: 144-8.
12. Ahmed HG, Nassar AS, Ginawi I. Screening for tuberculosis and its histological pattern in patients with enlarged lymph node. Patholog Res Int 2011; 2011: 417635.
13. Kuo CH, Chen HC, Chung FT, et al. Diagnostic value of EBUS-TBNA for lung cancer with non-enlarged lymph nodes: a study in a tuberculosis-endemic country. PLoS One 2011; 6: e16877.
14. Pollard RE, Garcia TC, Stieger SM, et al. Quantitative evaluation of perfusion and permeability of peripheral tumors using contrast-enhanced computed tomography. Invest Radiol 2004; 39: 340-9.
15. Koh DM, Brown G, Temple L, et al. Rectal cancer: mesorectal lymph nodes at MR imaging with USPIO versus histopathologic findings initial observations. Radiology 2004; 231: 91-9.
16. d'Assignies G, Couvelard A, Bahrami S, et al. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology 2009; 250: 407-16.
17. Wu GY, Ghimire P. Perfusion computed tomography in colorectal cancer:Protocols, clinical applications and emerging trends. World J Gastroenterol 2009; 15: 3228-31.
18. Spira D, Adam P, Linder C, et al. Perfusion and flow extraction product as potential discriminators in untreated follicular and diffuse large B cell lymphomas using volume perfusion CT with attempt at histopathologic explanation. AJR Am J Roentgenol 2012; 198: 1239-46.
19. Zhang H, Pan ZL, Du LJ, et al. Advanced gastric cancer and perfusion imaging using a multidetector row computed tomography: correlation with prognostic determinants. Radiology 2008; 9: 119-27.
Received for publication November 3, 2014.
*Corresponding author Tel: 86-25-80860185, E-mail: cjr.luguangming@vip.163.com
 Chinese Medical Sciences Journal2015年3期
Chinese Medical Sciences Journal2015年3期
- Chinese Medical Sciences Journal的其它文章
- Propofol Affects Different Human Brain Regions Depending on Depth of Sedation△
- Outcomes of T3a Prostate Cancer with Unfavorable Prognostic Factors Treated with Brachytherapy Combined with External Radiotherapy and Hormone Therapy
- Accuracy of a Simple Digital Templating in Primary Uncemented Total Hip Arthroplasty△
- Placement of a Long Intestinal Tube in Patients with Early Postoperative Small Bowel Obstruction under Fluoroscopic Guidance
- Cerebrospinal Fluid Biomarkers in Dementia Patients with Cerebral Amyloid Angiopathy
- Double Roots of Mandibular Premolar in Full-mouth Periapical Films
