Propofol Affects Different Human Brain Regions Depending on Depth of Sedation△
Xiang Quan, Tie-hu Ye, Si-fang Lin, Liang Zou, and Shou-yuan Tian
Department of Anesthesiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
Propofol Affects Different Human Brain Regions Depending on Depth of Sedation△
Xiang Quan, Tie-hu Ye*, Si-fang Lin, Liang Zou, and Shou-yuan Tian
Department of Anesthesiology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College, Beijing 100730, China
propofol; bispectral index; functional magnetic resonance imaging; anesthesia
ObjectiveTo investigate the effect of propofol on brain regions at different sedation levels and the association between changes in brain region activity and loss of consciousness using blood oxygen level-dependent functional magnetic resonance imaging (BOLD-fMRI) and bispectral index (BIS) monitoring.
MethodsForty-eight participants were enrolled at Peking Union Medical College Hospital from October 2011 to March 2012 and randomly assigned to a mild or a deep sedation group using computergenerated random numbers. Preliminary tests were performed a week prior to scanning to determine target effect site concentrations based on BIS and concomitant Observer’s Assessment of Alertness/Sedation scores while under propofol. Within one week of the preliminary tests where propofol dose-response was established, BOLD-fMRI was conducted to examine brain activation with the subject awake, and with propofol infusion at the sedation level.
ResultsMild propofol sedation inhibited left inferior parietal lobe activation. Deep sedation inhibited activation of the left insula, left superior temporal gyrus, and right middle temporal gyrus. Compared with mild sedation, deep propofol sedation inhibited activation of the left thalamus, precentral gyrus, anterior cingulate, and right basal nuclei.
ConclusionMild and deep propofol sedation are associated with inhibition of different brain regions, possibly explaining differences in the respective loss of consciousness processes.
Chin Med Sci J 2015; 30(3):135-142
PROPOFOL is a short-acting intravenous anesthetic that has been widely used for many years in clinical settings. Yet there is no definitive explanation for how propofol exerts its effects on the human brain leading to loss of consciousness. Previous studies have mainly focused on molecular and cellular targets of propofol.1,2However, the functional target of propofol in human brain on a systemic level is not fully understood.
Blood oxygen level-dependent functional magnetic resonance imaging (BOLD-fMRI) provides a method for non-invasive real-time exploration of the brain areas thatare activated under sedation or anesthesia.3,4Of particular interest are the areas of the human brain that are involved in propofol-induced behavioral changes such as loss of consciousness and amnesia.5,6Recent studies suggested that inhibition of the temporal lobe and thalamus could result in a reduction of consciousness.6,7However, these studies did not objectively measure the depth of sedation or differentiate levels of consciousness.
In the present study, bispectral index (BIS) and modified Observer’s Assessment of Alertness/Sedation (OAA/S) scoring8were used to monitor the depth of sedation and to verify loss of consciousness. BOLD-fMRI was employed to detect the brain areas underlying the sedative effect of propofol, and to delineate the brain areas that are associated with loss of consciousness.
SUBJECTS AND METHODS
Participant selection
Forty-eight healthy right-handed male volunteers (aged 20-39 years, weight 50-80 kg, body mass index 18-25 kg/m2) were recruited from Clinical Pharmacology Center of Peking Union Medical College Hospital from October 2011 to March 2012. None of the participants had any metal implants, history of allergies or snoring, history or physical evidence of neurological or psychiatric disease, or was taking any medications at the time of the present study. Based on a previous fMRI cohort analysis, 20 subjects or more are required in fMRI studies in order to have sufficient reliability.9Considering the possible loss of data, 48 volunteers were included in this study, 24 in each group.
The protocol of this study was approved by the Research Ethics Committee of Peking Union Medical College Hospital. All the participants provided written informed consent.
Groups and sedation levels
The participants were randomly assigned to a mild or a deep sedation group (n = 24 in each group) using computergenerated random numbers (Excel 2003; Microsoft, Redmond, WA, USA) in sealed envelopes. Mild sedation was defined as a modified OAA/S of 3 (i.e., responding only after their names were called loudly or repeatedly) and a BIS of 60-80. Deep sedation was defined as a modified OAA/S of 1 (not responding to mild prodding or shaking) and a BIS of 40-60.10
On the first visit (i.e., preliminary tests), the participants received propofol outside the magnetic resonance (MR) scanner. The target effect-site concentration (ESC) of propofol was slowly increased until the designated sedation level (mild or deep) was achieved. On the second visit (i.e., BOLD-fMRI scanning tests), propofol was delivered to the target ESC based on the trial in the first visit, and the target ESC was maintained for 3 minutes during BOLD-fMRI scanning.
Preliminary tests
Preliminary tests were performed a week prior to BOLD-fMRI scanning to determine target ESCs based on BIS and concomitant OAA/S scores while under propofol. Before the test, food intake was inhibited for 8 hours, and water intake for 4 hours. During the tests, all the participants received supplemental oxygen (6 L/min) via a nasal cannula to ensure peripheral oxygen saturation (SpO2) ≥ 92%.11The blood pressure, heart rate, SpO2, and end-tidal carbon dioxide concentration (ETCO2) of all the participants were measured, and electrocardiography (ECG) was performed. BIS was measured throughout the first visit using a BISXPmonitor (model A-2000, Aspect Medical Systems, Natick, MA, USA).
Lactated Ringer’s solution was injected at 10 ml·kg-1·h-1into the antecubital vein.12Propofol (10 mg/ml, Astra-Zeneca SpA, Caponago, Milano, Italy) was administered through a Graseby 3500 Diprifusor Syringe Pump (SIMS Graseby, Watford, UK). The initial target plasma concentration was set at 1.0 μg/ml, and the plasma concentration was increased by 0.3 μg/ml every 10 minutes until the target BIS value was reached. The target ESC of propofol was the average ESC required to reach a BIS between 80 and 60 for the mild sedation group, or a BIS between 60 and 40 for the deep sedation group. The target ESC was maintained for 10 minutes, and an OAA/S was recorded. After the test, each volunteer was allowed to leave only after meeting the criteria for safe discharge according to Aldrete’s score.13
BOLD-fMRI scanning
Scanning was performed within one week after the preliminary tests. Before scanning, each participant was positioned supine on the bed at rest for 30 minutes with eyes closed and instructed to relax and think about nothing. Their ears were then covered with earmuffs, and heads were fixed with sponge pads and tape to avoid movements. Blood pressure, heart rate, ECG, and SpO2were monitored using a magnetic-resonance-compatible monitor (Medrad 9500 Multigas Monitor, PA, USA), and ETCO2with a gas monitor (GE Detax-Ohmeda, USA). All the participants received supplemental oxygen (6 L/min) via a nasal cannula. BIS monitoring was not performed, as the monitor was not compatible with the MR room.
Lactated Ringer’s solution was injected at 10 ml·kg-1·h-1into the antecubital vein. The entire scanning process included 60 cycles (Cy) for 20 minutes. The participants were first scanned under wakeful baseline conditions for 1.5 minutes (0-5 Cy). At the beginning of the fifth cycle, propofol was infused using a target-controlled infusion system (Graseby 3500 Diprifusor Syringe Pump), until the target ESC was reached (5-50 Cy). The target ESC was maintained for 3 minutes during sedation scanning (50-60 Cy). The OAA/S was obtained immediately after scanning.
Structural imaging was conducted on a 3.0 T wholebody MR scanner (Signa VHi Excite II, GE, USA) and with an 8-channel phased array head coil. The slice thicknesses of the anatomical and functional images were both 10 mm and the interval was 0 mm. Turbo spin-echo sequences were used to get two-dimensional images with 256×256 matrix. Field of view was 300 mm×300 mm, scanning matrix was 96×96, and reconstructed matrix was 128×128. The parameters of echo-planar imaging scanning were repetition time/echo time at 3000/50 ms and flip angle at 90°. The spatial resolution was 0.5×0.5×10 mm3. The scanning was performed from calotte to medulla oblongata, and the functional imaging was performed with automatic shimming and lipid suppression technology.
Statistical analysis
In each cycle, 10 image planes were collected, producing a total of 500 image planes. All images and data from the fMRI were analyzed using SPM8 software (http://www. fil.ion.ucl.ac.uk/spm/). For each volunteer, the brain regions activated at wakeful baseline and under the desired sedation level were identified through space preprocessing and statistical analysis. The purpose of the intra-group comparison of activated regions in the wakeful baseline and under sedation was to reveal the brain regions inhibited while under sedation, and the inter-group comparison of activated regions showed the brain regions that were inhibited with increasing depth of sedation. Statistical parametric maps of the t-statistics (SPMt) were obtained and corrected for multiple comparisons based on Gaussian Random Field theory [Family-wise error (FWE) correction, voxel >10, P < 0.01]. The anatomical location of brain regions were identified using MNI coordinates. For fMRI data analysis, voxel >10 was considered statistically significant, because cluster-level thresholding is more reliable than voxel-based thresholding.9FWE correction and P < 0.01 was applied to control the error rate to ensure that every voxel activated was a true active voxel.5,9
General data were analyzed using SPSS 13.0 software. All values are presented as means ± standard deviation. Unpaired t-test was applied for inter-group comparisons of demographics and clinical data; paired t-test for intra-group comparisons of clinical data. P < 0.05 was considered statistically significant.
RESULTS
Forty participants completed the study, 20 in each group. The data from eight participants were discarded due to excessive head movement (n = 3), oxygen saturation <92% (n = 2), or OAA/S and BIS mismatch (OAA/S of 1 with BIS at 60-80, n = 3).
General information
No significant differences in mean age, weight or body mass index between the two groups were found (all P >0.05, Table 1). The target ESCs of propofol for the mild and deep sedation groups were 1.52 ± 0.28 and 2.50 ± 0.55 μg/ml, respectively. After propofol infusion, no significant changes in hemodynamic indices or intra-group and intergroup ETCO2were found (P > 0.05, Table 1).

Table 1. Demographics and clinical data of the mild and deep sedation groups§(both n=20)
Imaging data
Mild sedation inhibited the activation of the left inferior parietal lobe (Fig. 1, Table 2). Deep sedation inhibited the activation of the left insula, the left superior temporal gyrus, and the right middle temporal gyrus (Fig. 2, Table 2). Compared with mild sedation, deep sedation inhibited the activation of the left thalamus, precentral gyrus, anterior cingulate, and the right basal nuclei (Fig. 3, Table 2).

Figure 1. Mild sedation inhibited the activation of the left inferior parietal lobe. The images consist of a color-rendered statistical map of activation registered onto high-resolution structural scans that have been transformed to standardized brain geometry (MNI space). Colors are used to display the T scores for voxel activation, from red (the lowest T) to yellow (the highest T). A low T reflects low activation and vice versa.
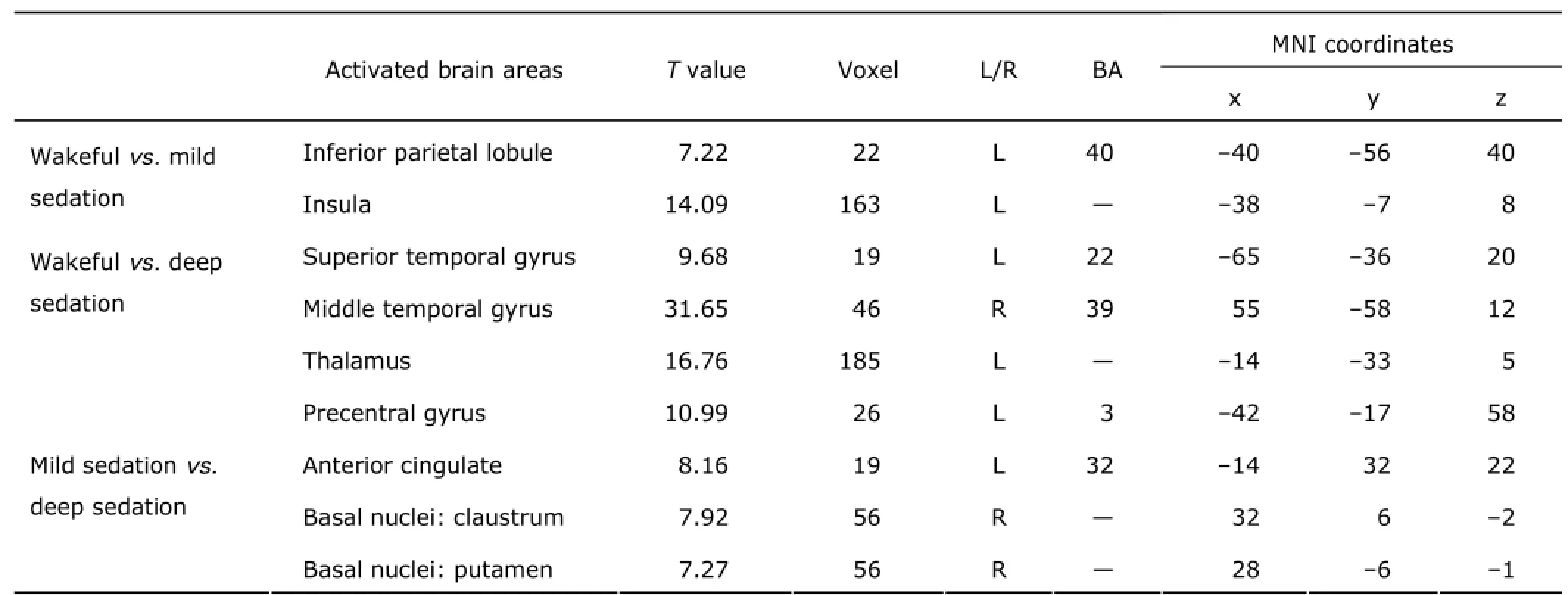
Table 2. Comparison of activated brain areas between different sedative states in the mild and deep sedation groups (both n=20)
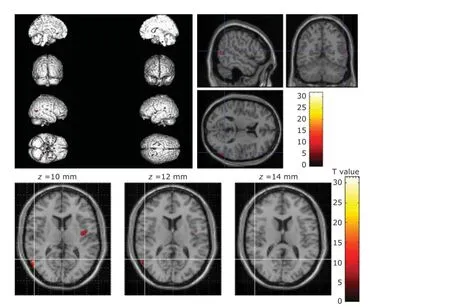
Figure 2. Deep sedation inhibited the activation of the left insula, the left superior temporal gyrus, and the right middle temporal gyrus.

Figure 3. Compared with mild sedation, deep sedation inhibited the activation of the left thalamus, precentral gyrus, anterior cingulate, and the right basal nuclei.
DISCUSSION
This study showed that mild sedation (OAA/S = 3; BIS, 60-80) inhibited the activation of the left inferior parietal lobe, suggesting that this brain region may be important for mild propofol sedation; deep sedation (OAA/S =1; BIS, 40-60) inhibited the activation of the left insula, the left superior temporal gyrus, and the right middle temporal gyrus, indicating the importance of these brain regions in deep propofol sedation. Increasing the depth of sedation inhibited the activation of the left thalamus, precentral gyrus, anterior cingulate, and the right basal nuclei, implicating these brain regions in increasing the depth of sedation. Because deepening sedation induced loss of consciousness (determined by decreasing BIS and OAA/S’s), these brain regions may be involved in the loss of consciousness.
The BIS is the first parameter approved by the United States Federal Drug Administration (1996) for measuring depth of anesthesia, initially to assess the hypnotic component of anesthesia. It is currently used as a measure of the level of consciousness during sedation, with a dimensionless number that ranges from zero (electroencephalographic silence) to 100 (fully awake and alert). The recommended BIS for general anesthesia is 40 to 60, when consciousness is lost.14In this study, mild and deep sedation were defined as BIS scores of 60-80 and 40-60, respectively. Expanding the range of BIS scores that defined each group increased the number of brain regions corresponding to each state of consciousness, thus making the inter-group comparisons more salient. Because the BIS monitor is not compatible with the MR room in our hospital, preliminary tests were performed outside the MR room to determine the ESC of propofol required to achieve the desired BIS score, rather than monitoring real-time BIS during scanning. The sedative state of a volunteer could be inferred by applying the target ESC during fMRI scanning in the following week.
The design of this study also included an increased separation of OAA/S’s between the mild and deep sedation groups of 3 and 1, respectively. Three participants in the mild sedation group (BIS 60 to 80) were found with an OAA/S = 1 as their ESC reached the target ESC. To keep a clear demarcation between the two groups, data from these three volunteers were excluded. In this way, both the BIS and OAA/S’s of the two groups allowed for adequate differentiation of the responses to treatment. In general, an OAA/S score of 3 indicates partial consciousness, while an OAAS of 1 indicates loss of consciousness.8Thus according to the BIS and OAA/S, the volunteers in the mild sedation group were conscious, while those in the deep sedation group were unconscious. Loss of consciousness was the key difference between the two groups; the brain regions important to loss of consciousness were thus identified by comparing the activated brain regions.
Under the influence of propofol, activity in the left inferior parietal lobe was inhibited only during mild sedation. The inferior parietal lobe is known to be involved in advanced language integration.15The inhibition of this area found in the present study suggests the suppression of integrative functions related to language, and may explain patients’ unclear speech during mild sedation observed in clinical settings. Activity in the left insula, the left superior temporal gyrus, and the right middle temporal gyrus was suppressed during deep sedation. The exact function of the insula is unclear. Since the temporal lobe has been associated with auditory information processing and memory,5the results of this study imply that deep sedation might inhibit hearing and memory. Previous studies have shown that memory loss occurs at BIS 40-60 and an OAA/S of 1.5The present study verifies memory inhibition during deep sedation based on anatomical location, thus adding evidence to the conclusion of previous studies.
Compared with mild sedation, deep sedation inhibited the activity of the left thalamus, precentral gyrus, anterior cingulate, and right basal nuclei, suggesting that these four regions may be the key to loss of consciousness, in logical concordance with the findings of other studies. For example, it has been shown in previous studies that the thalamus is functionally related to propofol-induced unconsciousness.6,16The human primary motor cortex is located on the precentral gyrus, so reduced activation in this region with increasing depth of sedation in the present study implies suppression of motor control, which is similar to the finding of a previous study.17
The anterior cingulate appears to have conscious experience functions.18It has roles in a wide variety of autonomic functions such as regulating blood pressure and heart rate, as well as rational cognitive functions such as reward anticipation, decision-making, empathy, impulse control, and emotion.19In the present study, the inhibition of the anterior cingulate with increasing sedative depth suggests that rational cognitive function was significantly reduced. In the present study, deep sedation also inhibited activity of the right basal nuclei. The claustrum is an important part of a network that subserves consciousness,20and the main function of the putamen is to regulate movements21and influence various types of learning.22Suppression of this region under sedation may imply reduced movement regulation and learning ability.
All BOLD-fMRI studies are limited in that results are an indirect reflection of neuronal mass activity.23The rationale underlying fMRI is that brain activity may be measured by detecting associated changes in blood flow. Therefore, other factors that affect the cerebral blood flow, such as hemodynamic indices and partial pressure of CO2in arterial blood (PaCO2), may influence the results.24There were no significant changes in the hemodynamic indices or ETCO2before and after the infusion of propofol in this study, and therefore these factors probably had marginal influence on cerebral blood flow and changes in regional cerebral blood flow observed in this study were mainly due to the functional activity of the brain. With the aim of distinguishing between the mild and deep sedation groups in this study, we did not allocate a moderate sedation group, so as to examine the brain area associated with loss of consciousness, which was the key difference between the two groups. In addition, the BIS value could not be monitored during the fMRI scanning due to equipment incompatibility. In addition, the BIS value from the first visit (preliminary tests) was used instead, which is a reasonable substitute for real-time BIS value. To ensure the credibility of the data, information was discarded when the OAA/S and BIS did not match.
In conclusion, mild propofol sedation inhibits the left inferior parietal lobe, while deep sedation inhibits the left insula, superior temporal gyrus, and right middle temporal gyrus. The left thalamus, precentral gyrus, anterior cingulate, and right basal nuclei may be involved in the loss of consciousness.
Acknowledgements
The authors are grateful to Hui You, MD (Department of Radiology, Peking Union Medical College Hospital) for assistance in MRI data acquisition and Wen-ping You, PhD (Department of Psychology, Beijing Normal University, Beijing, China) for assistance in statistical analysis of the MRI data.
1. Zhu X, Hao X, Luo J, et al. Propofol inhibits inflammatory cytokine-mediated glutamate uptake dysfunction to alleviate learning/memory impairment in depressed rats undergoing electroconvulsive shock. Brain Res 2015; 1595: 101-9.
2. Baker R, Gent TC, Yang Q, et al. Altered activity in the central medial thalamus precedes changes in the neocortex during transitions into both sleep and propofol anesthesia. J Neurosci 2014; 34: 13326-35.
3. Hudetz AG, Liu X, Pillay S. Dynamic repertoire of intrinsic brain states is reduced in propofol-induced unconsciousness. Brain Connect 2015; 5: 10-22.
4. Schroeter A, Schlegel F, Seuwen A, et al. Specificity of stimulus-evoked fMRI responses in the mouse: the influence of systemic physiological changes associated with innocuous stimulation under four different anesthetics. Neuroimage 2014; 94: 372-84.
5. Quan X, Yi J, Ye TH, et al. Propofol and memory: a study using a process dissociation procedure and functional magnetic resonance imaging. Anaesthesia 2013; 68: 391-9.
6. Liu X, Lauer KK, Ward BD, et al. Differential effects of deep sedation with propofol on the specific and nonspecific thalamocortical systems: a functional magnetic resonance imaging study. Anesthesiology 2013; 118: 59-69.
7. Song XX, Yu BW. Anesthetic effects of propofol in the healthy human brain: functional imaging evidence. J Anesth 2015; 29: 279-88.
8. Chernik DA, Gillings D, Laine H, et al. Validity and reliability of the Observer's Assessment of Alertness/ Sedation Scale: study with intravenous midazolam. J Clin Psychopharmacol 1990; 10: 244-51.
9. Thirion B, Pinel P, Meriaux S, et al. Analysis of a large fMRI cohort: statistical and methodological issues for group analyses. Neuroimage 2007; 35: 105-20.
10. Apfelbaum JL, Silverstein JH, Chung FF, et al. Practice guidelines for postanesthetic care: an updated report by the American Society of Anesthesiologists Task Force on Postanesthetic Care. Anesthesiology 2013; 118:291-307.
11. Scott RP, Saunders DA, Norman J. Propofol: clinical strategies for preventing the pain of injection. Anaesthesia 1988; 43: 492-4.
12. Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth 1995; 7: 89-91.
13. Glass PS, Bloom M, Kearse L, et al. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology 1997; 86: 836-47.
14. Pilge S, Kreuzer M, Kochs EF, et al. Monitors of the hypnotic component of anesthesia-correlation between bispectral index and cerebral state index. Minerva Anestesiol 2012; 78: 636-45.
15. Benn Y, Zheng Y, Wilkinson ID, et al. Language in calculation: a core mechanism? Neuropsychologia 2012; 50: 1-10.
16. Xie G, Deschamps A, Backman SB, et al. Criticalinvolvement of the thalamus and precuneus during restoration of consciousness with physostigmine in humans during propofol anaesthesia: a positron emission tomography study. Br J Anaesth 2011; 106: 548-57.
17. Jankowski J, Paus S, Scheef L, et al. Abnormal movement preparation in task-specific focal hand dystonia. PLoS One 2013; 8: e78234.
18. Taylor SF, Martis B, Fitzgerald KD, et al. Medial frontal cortex activity and loss-related responses to errors. J Neurosci 2006; 26: 4063-70.
19. Jackson PL, Brunet E, Meltzoff AN, et al. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia 2006; 44: 752-61.
20. Koubeissi MZ, Bartolomei F, Beltagy A, et al. Electrical stimulation of a small brain area reversibly disrupts consciousness. Epilepsy Behav 2014; 37: 32-5.
21. Marchand WR, Lee JN, Thatcher JW, et al. Putamen coactivation during motor task execution. Neuroreport 2008; 19: 957-60.
22. Ell SW, Marchant NL, Ivry RB. Focal putamen lesions impair learning in rule-based, but not informationintegration categorization tasks. Neuropsychologia 2006; 44: 1737-51.
23. Logothetis NK. What we can do and what we cannot do with fMRI. Nature 2008; 453: 869-78.
24. Chamberlin NL, Eikermann M. This is no humbug: anesthetic agent-induced unconsciousness and sleep are visibly different. Anesthesiology 2010; 113: 1007-9.
Received for publication March 13, 2015.
△Supported by National Natural Science Foundation of China (30672030).
*Corresponding author Tel: 86-10-69155582, E-mail: quan79102@188. com
 Chinese Medical Sciences Journal2015年3期
Chinese Medical Sciences Journal2015年3期
- Chinese Medical Sciences Journal的其它文章
- Paraneoplastic Dermatomyositis Accompanying Nasopharyngeal Carcinoma
- Genetic Effects on Sensorineural Hearing Loss and Evidence-based Treatment for Sensorineural Hearing Loss
- Serum Myeloperoxidase Level in Systemic Lupus Erythematosus
- Pulmonary Carcinosarcoma with Intracardiac Extension: a Case Report
- Postpartum Atypical Hemolytic Uremic Syndrome: an Unusual and Severe Complication Associated with IgA Nephropathy
- Double Roots of Mandibular Premolar in Full-mouth Periapical Films
