Ulinastatin suppresses endoplasmic reticulum stress and apoptosis in the hippocampus of rats with acute paraquat poisoning
Hai-feng Li, Shi-xing Zhao, Bao-peng Xing, Ming-li Sun
Department of Emergency Medicine, the First Hospital of Jilin University-the Eastern Division, Changchun, Jilin Province, China
Ulinastatin suppresses endoplasmic reticulum stress and apoptosis in the hippocampus of rats with acute paraquat poisoning
Hai-feng Li, Shi-xing Zhao, Bao-peng Xing, Ming-li Sun*
Department of Emergency Medicine, the First Hospital of Jilin University-the Eastern Division, Changchun, Jilin Province, China
Lung injury is the main manifestation of paraquat poisoning. Few studies have addressed brain damage after paraquat poisoning. Ulinastatin is a protease inhibitor that can effectively stabilize lysosomal membranes, prevent cell damage, and reduce the production of free radicals. This study assumed that ulinastatin would exert these effects on brain tissues that had been poisoned with paraquat. Rat models of paraquat poisoning were intraperitoneally injected with ulinastatin. Simultaneously, rats in the control group were administered normal saline. Hematoxylin-eosin staining showed that most hippocampal cells were contracted and nucleoli had disappeared in the paraquat group. Fewer cells in the hippocampus were concentrated and nucleoli had disappeared in the ulinastatin group. Western blot assay showed that expressions of GRP78 and cleaved-caspase-3 were signifcantly lower in the ulinastatin group than in the paraquat group. Immunohistochemical fndings showed that CHOP immunoreactivity was signifcantly lower in the ulinastatin group than in the paraquat group. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling staining showed that the number of apoptotic cells was reduced in the paraquat and ulinastatin groups. These data confrmed that endoplasmic reticular stress can be induced by acute paraquat poisoning. Ulinastatin can effectively inhibit this stress as well as cell apoptosis, thereby exerting a neuroprotective effect.
nerve regeneration; paraquat; poisoning; rats; endoplasmic reticulum stress; apoptosis; ulinastatin; CHOP; GRP78; caspase-3; hippocampus; reactive oxygen species; neural regeneration
Funding:This study was supported by a grant from the National Key Specialty Construction Project in China in 2012, No. [2012]650.
Li HF, Zhao SX, Xing BP, Sun ML (2015) Ulinastatin suppresses endoplasmic reticulum stress and apoptosis in the hippocampus of rats with acute paraquat poisoning. Neural Regen Res 10(3)∶467-472.
Introduction
The hippocampus is sensitive to ischemia and hypoxia (Dinis-Oliveira et al., 2006; Feng, 2008). Infammatory responses are considered an important pathological process that occurs after cerebral ischemia/reperfusion injury. Its “waterfall-like” reaction triggers pathological damage, which then leads to brain damage (Houze et al., 1990). Recently, an apoptosis pathway mediated by endoplasmic reticular stress has been reported (Akiyama et al., 2011). Enhancer binding protein homologous protein (CHOP), also known as growth arrest- and DNA damage-inducible gene 153 (GADD153) serves as a marker of endoplasmic reticular stress and plays an important role in neuronal apoptosis. In contrast, caspase-3 is a key enzyme and executor of apoptosis. The activation of caspase-3 inevitably leads to cell apoptosis (Dringen, 2005).
Paraquat is a highly toxic, non-selective, contact herbicide. After oral poisoning, paraquat rapidly distributes in tissues and organs, primarily the lungs. The concentration of paraquat in the lungs is 10 times that in the plasma (Liu et al., 2009). The mechanism of paraquat poisoning is considered to be related to oxidative damage induced by reactive oxygen species, immune activation, and inflammatory mediators. Reactive oxygen species play an important role in inflammatory stress (Wang et al., 2003). Additionally, free radicals are also involved in the pathophysiology of many diseasesin vivo. Excessive free radicals can produce lipid peroxidation and damage the structure of neuronal membranes (Boyce et al., 2005). Paraquat is a non-selective contact herbicide in the pyridine group with high efficiency, and is widely used around the world. As a contact herbicide, paraquat can rapidly degrade, and does not produce any environmental pollution when it comes in contact with solids. For the commonly sold 20%-paraquat aqueous solution, the lethal oral dose is 2—3 g and the median lethal dose is about 50 mg/kg (Houze et al., 1990; Feng, 2008). Moreover, mortality can reach 80%, because there is no specifc antidote. According to a report from Vale in 1987, paraquat poisoning can be divided into three categories: (1) mild poisoning, when the oral dose is < 20 mg/kg body mass, patients usually fullyrecover; (2) mild-moderate poisoning, when the oral dose is 20—50 mg/kg body mass, most patients experience acute lung injury, pulmonary fbrosis, and renal failure, and die of poisoning after 2—3 weeks; and (3) acute poisoning, when the oral dose is over 50 mg/kg body mass, the majority of patients die of multiple organ failure within 3 days of poisoning (Huang et al., 2012). Although most patients are sent to the hospital more than 6 hours after paraquat poisoning, after paraquat has already distributed throughout the body, its concentration in the blood is very low. Therefore, oral dosages and clinical manifestations are the main factors used for confrming the degree of poisoning.
After oral absorption, paraquat is delivered to the liver, lungs, kidneys, thyroid, various body fuids, and cerebrospinal fluid. After poisoning, the highest amount of paraquat and the greatest amount of damage are in the lungs. For severe paraquat poisoning, nervous system injuries can manifest as mental disorders, lethargy, facial paralysis, hand tremors, hydrocephalus, and cerebral hemorrhage.
The endoplasmic reticulum is a newly discovered site for an apoptotic signaling pathway, which are involved in many diseases, such as neurodegeneration, diabetes, infections, and cerebral ischemia-reperfusion injury. This pathway also plays an important role in maintaining Ca2+balance and controlling protein synthesis and folding. When cells are subjected to ischemia, hypoxia, free radical damage, or poison, excessive accumulation of unfolded proteins will trigger a response that tries to restore normal function of the endoplasmic reticulum. Endoplasmic reticular stress can be initiated by activating PERK, IRE1 and ATF6.
Under physiological conditions, these three proteins can bind to glucose-regulated protein 78 (GRP78, also known as immunoglobulin heavy chain binding protein, Bip) at an inactivated state. GRP78/Bip is a member of the heat shock protein 70 protein family, and is expressed on the endoplasmic reticulum. It is composed of an adenosine triphosphate enzyme domain, a peptide-binding domain, and an endoplasmic reticulum retention signal (Yang and Sun, 1998). GRP78 is generally associated with Ca2+, and is a signal regulator of endoplasmic reticular stress that promotes proper protein folding and degradation of misfolded proteins, and blocks the accumulation of intermediates (Farrington et al., 1973). GRP78 has a low expression in normal adult organs, such as the brain, lungs, and heart (Haddad, 2004). In mammals, when unfolded proteins accumulate, GRP78 departs from the receptor, transfers a signal to the nucleus, and starts a response to unfolded proteins that acts to protect cells through a series of reactions, such as reducing the level of protein translation, up-regulating the expression of GRP78 chaperonin, and enhancing the ERAD pathway that helps stabilize the abnormal function of the endoplasmic reticulum caused by misfolded proteins (Yasaka et al., 1981). When the duration of endoplasmic reticular stress is too long, the functional response appears to trigger endoplasmic reticulum stress-mediated cell apoptosis.
Caspase-12 is located on the endoplasmic reticulum and is a key molecule for endoplasmic reticulum stress-mediated apoptosis, which can be specifically activated by endoplasmic reticular stress (Tawara, 1996). Under physiological conditions, caspase-12 exists in an inactive precursor form, but when activated by endoplasmic reticular stress, sequential activation of caspase-12, caspase-9 and caspase-3 eventually leads to cell apoptosis (Whiteside and Israel, 1997).
Our previous studies suggested that acute paraquat poisoning caused acute lung injury, endoplasmic reticular stress, cell autophagy, and apoptosis in rats. Few studies have dealt with brain damage incurred from acute paraquat poisoning. Ulinastatin is a protease inhibitor, and inhibits many types of hydrolase activity (for proteins, lipids, and carbohydrates) and protects organs. This study observed changes in endoplasmic reticular stress and neuronal apoptosis in brain tissues and further identifed effects of paraquat poisoning on brain tissues. It also determined the effects of ulinastatin on paraquat poisoning and investigated the mechanism underlying the neuroprotective effect of ulinastatin on acute paraquat poisoning rats.
Materials and Methods
Experimental animals
A total of 150 Wistar rats of either sex aged 10 months old and weighing 250 ± 10 g, were purchased from the Experimental Animal Center of Norman Bethune Health Science Center of Jilin University, China (SCXK-(Ji)2008-0005). Experiments were approved by the Ethics Committee of First Hospital of Jilin University in China.
Model preparation
One hundred and ffty rats were equally and randomly divided into three groups. Control group: intragastric administration of 1 mL normal saline followed by intraperitoneal injection of 1 mL normal saline, twice daily. Paraquat group: intragastric administration of 40 mg/kg paraquat solution (Feng, 2008), followed by intraperitoneal injection of 1 mL normal saline, twice a day. Ulinastatin group: intraperitoneal administration of 1.2 × 105U/kg ulinastatin, twice a day, after acute paraquat poisoning by intragastric administration of paraquat 40 mg/kg.
Preparation of paraffn sections
Seven days after poisoning, rats were intraperitoneally anesthetized using 10% chloral hydrate and infused with PBSTween. The brain tissue was removed and fxed in 10% neutral formalin for 48 hours, followed by hematoxylin-eosin staining, terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining, and immunohistological analysis. A portion of brain tissue was cryopreserved in liquid nitrogen for protein detection.
Hematoxylin-eosin staining
The sections were deparaffinized, stained, dehydrated with increasing concentrations of ethyl alcohol, permeabilized, and mounted. Five felds per section were randomly selected under 400× optical magnification (BX51; Olympus, Tokyo, Japan).
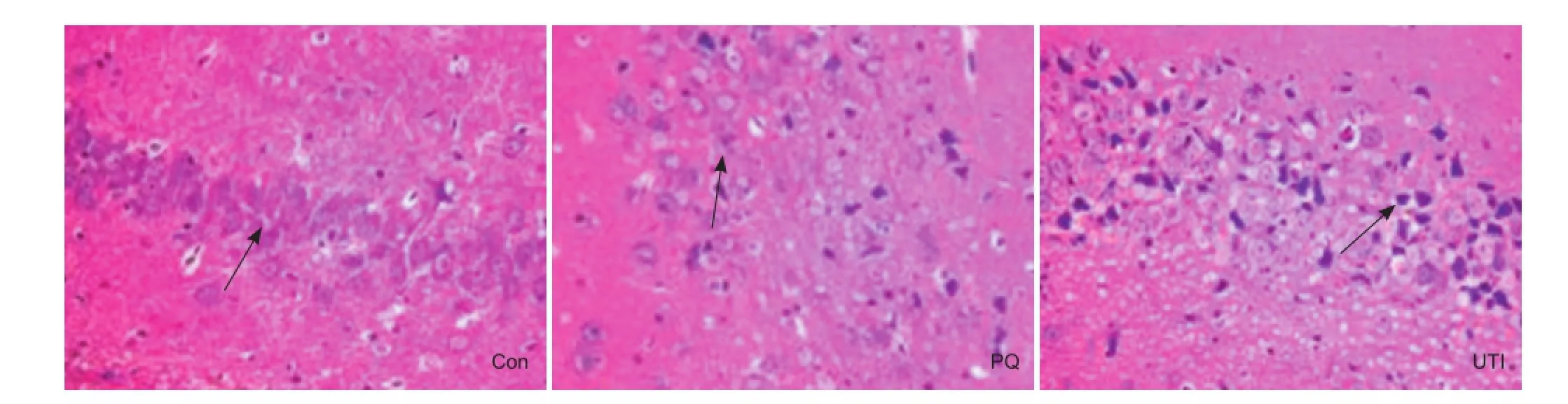
Figure 1 Morphology of hippocampal cells in rats after paraquat poisoning (hematoxylin-eosin staining, × 40).
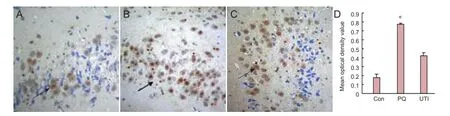
Figure 3 The immunoreactivity of CHOP in the rat hippocampus after paraquat poisoning (immunohistochemical staining, × 40).
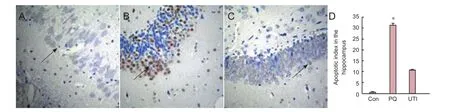
Figure 4 The apoptotic cells in the rat hippocampus after paraquat poisoning (TUNEL staining, optical microscope, × 40).
western blot assay of GRP78 and caspase-3 expression
Protein content of the samples was determined using the Bio-Rad method. Proteins were electrophoresed on a gel, and then transferred to a membrane. The membrane was incubated with primary antibody rabbit anti-rat monoclonal antibody (1:200; Boster, Wuhan, Hubei Province, China) at 4°C overnight, treated with secondary antibody goat anti-rat IgG (1:200; Boster) at room temperature or 37°C for 30 minutes to 1 hour. Horseradish peroxidase-labeled rat β-actin monoclonal antibody (1:200; Boster) served as internal reference. After exposure to X-rays, developing, and fxing, the ratio of target protein/β-actin in each group was determined by the ratio of the respective values of the gray regions, and was considered the relative value of target protein expression.

Figure 2 GRP78 and caspase-3 protein expression in the rat hippocampus 7 days after poisoning (western blot assay).
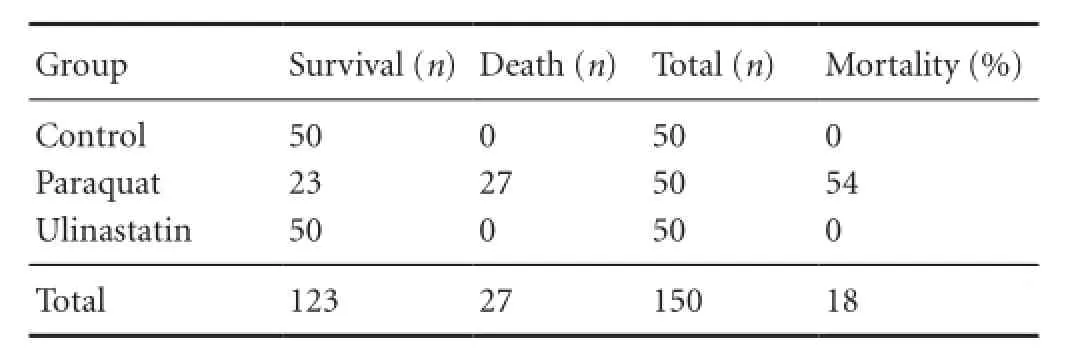
Table 1 Mortality of experimental rats suffering from poisoning
Immunohistochemical detection of CHOP expression
Sections were dewaxed and dehydrated through a graded alcohol series that was followed by antigen retrieval. After blocking with goat serum, sections were incubated with primary antibody rabbit anti-rat CHOP monoclonal antibody (1:200; Boster) at 4°C overnight, washed with PBS for 5 minutes × 3. The sections were then incubated with secondary antibody biotinylated rabbit anti-rat IgG (CHOP monoclonal antibody, 1:200; Boster) at room temperature or 37°C for 30 minutes to 1 hour. Horseradish peroxidase-labeled rat β-actin monoclonal antibody (1:200; Boster) served as internal reference. After washing with PBS for 5 minutes × 3, samples were incubated with horseradish peroxidase-labeled streptavidin working solution at 37°C for 10 minutes, visualized with 3,3′-diaminobenzidine (Tianjin Biohao Biotechnology Co., Ltd., Tianjin, China), and counterstained. Five fields of each section were randomly selected under 400× magnification. Samples were then photographed with an optical microscope (BX51; Olympus, Japan). Mean optical density of each section was analyzed using a Motic Image Advanced 3.2 image analytical system. Brown nuclei indicated a positive reaction. Higher optical density represents higher expression.
TUNEL detection of cell apoptosis
TUNEL staining was conducted in accordance with the manual for the TUNEL assay kit (Promega (Beijing) Biotechnology, Beijing, China). Samples were treated with streptavidin-horseradish peroxidase solution 100 µL at room temperature for 3—5 minutes, washed with PBS for 5 minutes × 3, visualized with 3,3′-diaminobenzidine, washed twice with ddH2O, counterstained with methyl green, washed with ddH2O, dehydrated through a graded alcohol series, permeabilized with xylene, mounted with neutral resin, and observed and photographed using the optical microscope. Five felds of each section were randomly selected under 400× magnification. The total number of cells and the number of apoptotic cells were counted in each field, and the average number of apoptotic cells was calculated. Cells with brown nuclei indicated TUNEL-positive cells. The apoptotic index was calculated as the percentage of apoptotic cells (number of apoptotic cells/the total number of cells × 100%).
Statistical analysis
All measurement data are expressed as the mean ± SD, and statistically processed using SAS 9.2 software (University of Notre Dame, IN, USA). One-way analysis of variance and Student-Newman-Keuls test were performed for intergroup comparisons. A value ofP< 0.05 was considered statistically signifcant.
为实现多轮清点的协同工作,定义状态标志,该标志可以表示三个状态,不妨将三个状态表示成S0、S1和S2。标签中保存这个状态标志,三个状态中一个为初始态,另两个为中间态,为了后续的描述方便,不妨设S0为初始态,S1和S2为中间态。标签的该标志在供电的情况下,如果没有改变状态的需求,则标签应维持该标志的状态不变,即S1或S2,在没有供电的情况下,该标志应能在一定持续时间内保持不变,即S1或S2,当没有供电超过了持续时间,标签的标志状态恢复为初始状态,即S0。
Results
Behaviors in rats suffering from poisoning
In the paraquat group, the rats experienced shortness of breath, perioral cyanosis, poor appetite, weight loss, fatigue, dysphoria, and difficulty in activities within 3 hours to 7 days. Mortality rates differed across groups. Before death, rats presented with shortness of breath and obvious systemic cyanosis that was most obvious within 24—72 hours. In the ulinastatin group, no signifcant dyspnea or cyanosis occurred, and eating, drinking, and motor activity were fair.No obvious decline in body mass was observed and hair appeared normal and shiny. Moreover, no rats in the ulinastatin group died (Table 1).
Morphology of hippocampal neurons in rats after acute paraquat poisoning
Seven days after poisoning, hematoxylin-eosin staining showed that in the control group, hippocampal neurons were arranged tightly with abundant cytoplasm and clearly visible nucleoli. In the paraquat group, most hippocampal cells were concentrated and nucleoli had disappeared. In the ulinastatin group, some cells in the hippocampus were concentrated and nucleoli had disappeared (Figure 1), but the extent appeared less than in the paraquat group
Expression of GRP78 and caspase-3 protein in the rat hippocampus after acute paraquat poisoning
Seven days after poisoning, results from the western blot assay showed that expression of GRP78 and cleaved-caspase-3 was significantly higher in the paraquat group than in the control group (P< 0.05). GRP78 protein expression was lower in the ulinastatin group than in the paraquat group (P<0.05; Figure 2).
CHOP immunoreactivity in the rat hippocampus after acute paraquat poisoning
Immunohistochemical results showed that brown reaction products of CHOP protein were primarily expressed in the nuclei of neuroglial cells, with some expression in the cytoplasm. Mean optical density values of CHOP in rat hippocampi showed signifcant differences among groups (P<0.05). Immunohistochemical fndings suggested that CHOP immunoreactivity was significantly higher in the paraquat group than in either the control group (P< 0.05) or the ulinastatin group (P< 0.05; Figure 3).
TUNEL-positive cells were rare in the control group. The number of apoptotic cells was significantly greater in the paraquat and ulinastatin groups than in the control group. Positive cells were indicated by brown nuclei, and their reaction products aggregated around the nuclear membrane. More cells were positive for TUNEL in the paraquat group than in either the control group (P< 0.05) or ulinastatin group (P< 0.05; Figure 4).
discussion
In this experiment, we found that compared with the control group, the expression of GRP78 and the number of apoptotic cells were higher in rats with paraquat poisoning, indicating that paraquat induces endoplasmic reticular stress in the brain tissue of poisoned rats. These fndings also demonstrated that increased GRP78 expression in the endoplasmic reticulum was not enough to restore its homeostasis.
Endoplasmic reticular stress without remission, excessive accumulation of unfolded proteins, and numerous unfolded proteins will trigger endoplasmic reticulum stress-mediated apoptosis. CHOP is the main marker for this process (Yasaka et al., 1986). It is weakly expressed under physiological conditionsin vivo, but is highly up-regulated when endoplasmic reticular stress occurs (Cocheme and Murphy, 2008). Inhibiting CHOP expression might relieve this type of apoptosis and thus protect cells (Costantini et al., 1995). When the regulation of unfolded protein response fails, it can promote cell death through apoptotic pathways (Yamada and Fukushima, 1993). The results from our study showed that CHOP expression significantly increased in the brain tissue of rats after 1 week of paraquat poisoning, as did cell apoptosis, indicating that paraquat poisoning-induced endoplasmic reticular stress results in cell apoptosis through activation of CHOP. These fndings show that after paraquat poisoning, the endoplasmic reticulum cannot function normally because of long-term stress, and the CHOP-dependent apoptotic pathway is eventually activated.
Caspase-3 is the most important effect-type caspase, and belongs to the downstream of caspase cascade that leads to cell apoptosis. Therefore, cleaved-caspase-3 was selected as a detection target in our study. Western blot assay showed that cleaved-caspase-3 expression increased after paraquat poisoning, and we deduced that the poisoning successively induced endoplasmic reticular stress, which lead to caspase-dependent apoptosis. Because of the long-term and strong endoplasmic reticular stress, the endoplasmic reticulum could not compensate for the damage, which resulted in the apoptotic response that ultimately led to brain damage.
Ulinastatin is a glycoprotein extracted from the urine of healthy adult males, and is a broad-spectrum protease inhibitor. It has a protective role in the lungs, liver, heart, kidneys, and brain. Animal experiments have shown that ulinastatin can reduce edema, degeneration, necrosis, and apoptosis in the brain tissue of neonatal rats with hypoxic-ischemic injury (Schoonbroodt and Piette, 2000). Ulinastatin is also reported to reduce neuronal cell death in rats with brain injury, thus playing a signifcant neuroprotective effect (Rio and Velez-Pardo, 2008). By inhibiting tumor necrosis factor-α, interleukin-6, and interleukin-10, ulinastatin can suppress lipid peroxidation, scavenge oxygen free radicals, and reduce the permeability of the blood-brain barrier, thereby alleviating brain damage (Lou et al., 2010; Roberts et al., 2011; Tomenson and Campbell, 2011). Additionally, ulinastatin has an anti-apoptotic effect through up-regulation of Bcl-2 protein and down-regulation of heat shock protein 70 (Ding et al., 2001; Manning-Bog et al., 2002). Ulinastatin can reduce the endoplasmic reticular stress and inhibit the expression of caspase-3, which in turn inhibits neuronal apoptosis. Here, we prepared a rat model of paraquat poisoning to observe the effect of paraquat poisoning on endoplasmic reticular stress and apoptosis. Compared with the paraquat group, the expressions of GRP78, CHOP, and cleaved-caspase3 were all reduced, and TUNEL assay results showed reduced apoptosis, indicating that ulinastatin exerts an inhibitory effect on endoplasmic reticular stress and apoptosis in the brain tissue of poisoned rats. Hematoxylin-eosin staining showed that ulinastatin relieved brain damage, suggesting that ulinastatin can protect the brain tissue of rats against paraquatpoisoning. This effect is probably associated with the role of ulinastatin in inhibiting endoplasmic reticular stress and cell apoptosis in the brain tissue of rats. Alternatively, it could result from scavenging oxygen free radicals and inhibiting the inflammatory response. Further studies are needed to determine the actual mechanism.
Paraquat poisoning is a continuous process. Amounts of GRP78, CHOP, and cleaved-caspase3 increased with increased poisoning duration, as did the number of apoptotic cells as shown by the TUNEL assay. These fndings indicate that endoplasmic reticular stress was followed by apoptosis, and indicate that the endoplasmic reticulum could not regain its normal function, and thus, the hippocampal tissue was damaged. Compared with the paraquat, GRP78, CHOP, and cleaved-caspase3 expression was less in the ulinastatin group. Hematoxylin-eosin staining and TUNEL staining revealed that in the ulinastatin group, tissue damage was less extensive, and cell apoptosis reduced. We therefore inferred that ulinastatin probably reduced the number of damaged cells by inhibiting endoplasmic reticular stress and thus inhibiting endoplasmic reticulum stress-mediated apoptosis. As a result, we conclude that ulinastatin has an overall protective effect in the hippocampus.
Endoplasmic reticular stress may be related to oxidative stress, infammation, and apoptosis during paraquat poisoning. Ulinastatin, which protects the brain against paraquat poisoning by inhibiting the activity of various enzymes, scavenging oxygen free radicals, and improving microcirculation, also appears to inhibit endoplasmic reticular stress and thus subsequent endoplasmic reticulum stress-mediated apoptosis. Thus, it is expected to play a protective role in multiple organ damage.
Acknowledgments:We are very grateful to Yan-wei Rao from Jilin Province People’s Hospital in China for providing suggestions and help on experimental manipulation and data analysis.
Author contributions:MLS designed the study. HFL analyzed data and wrote the paper. SXZ performed experiments. BPX conducted statistical analysis. All authors approved the final version of the paper.
Conficts of interest:None declared.
Akiyama K, Tone J, Okabe M, Nishimoto S, Sugahara T, Kakinuma Y (2011) Inhibition of myotube formation by paraquat in the myoblast cell line C2C12. J Toxicol Sci 36:243-246.
Boyce M, Bryant KF, Jousse C (2005) A selective inhibitor of eIF2alpha dephosphorylation protects cells from ER stress. Science 307:935-939.
Cocheme HM, Murphy MP (2008) Complex I is the major site of mitochondrial superoxide production by paraquat. J Biol Chem 283:1786-1798.
Costantini P, Petronilli V, Colonna R, Bernardi P (1995) On the effects of paraquat on isolated mitochondria. Evidence that paraquat causes opening of the cyclosporin A-sensitive permeability transition pore synergistically with nitricoxide. Toxicology 99:77-88.
Ding ZT, Ren HM, Jiang YP, Cai ZL, Zhu QY (2001) Infuence of paraquat on the system of substantial nigra and striatum in c57bl mice. Fudan Yixue: Yixue Kexue Ban 28:28.
Dinis-Oliveira RJ, Sarmento A, Reis P, Amaro A, Remiao F, Bastos ML, Carvalho F (2006) Acute paraquat poisoning: Report of a survival case following intake of a potential lethal dose. Pediatr Emerg Care 22:537-540.
Dringen R (2005) Oxidative and antioxidative potential of brain microglial cells. Antioxid Redox Signal 7:1223-1233.
Farrington JA, Ebert M, Land EJ, Fletcher K (1973) Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta 314:372-381.
Feng HJ (2008) Research progress of acute paraquat poisoning. Zhongguo Meitan Gongye Yixue Zazhi 11:1105.
Haddad JJ (2004) Redox and oxidant-mediated regulation of apoptosis signaling pathways: Immunoconception of oxidative siege versus cell death commitment. Int Immunopharmacol 4:475-493.
Houze P, Baud FJ, Mouy R, Bismuth C, Bourdon R, Scherrmann JM (1990) Toxicokinetics of paraquat in humans. Hum Exp Toxicol 9:5-12.
Huang CL, Lee YC, Yang YC, Kuo TY, Huang NK (2012) Minocycline prevents paraquat-induced cell death through attenuating endoplasmic reticulum stress and mitochondrial dysfunction. Toxicol Lett 209:203-210.
Liu XL, Sun ML, Ma DH (2009) Ulinastatin and Xuebijing in combination as a novel life-saving therapeutic strategy for acute paraquat poisoning: a case report. Crit Care Med 37:1025.
Lou D, Chang XL, Zhou ZJ (2010) Progress in research on Mechanism of Parkinson’s disease induced by paraquat exposure. Dulixue Zazhi 24:76-79.
Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink Al, Di Monte DA (2002) The herbicide paraquat causes up regulation and aggregation of alpha-synuclein in mice paraquat and α-synuclein. J Biol Chem 277:1641.
Rio MJ, Velez-Pardo C (2008) Paraquat induces apoptosis in human lymphocytes: protective and rescue effects of glucose, cannabinoids and insulin-like growth factor-1. Growth Factors 26:49-60.
Roberts DM, Wilks MF, Roberts MS, Swaminathan R, Mohamed F, Dawson AH, Buckley NA (2011) Changes in the concentrations of creatinine, cystatin C and NGAL in patients with acute paraquat self-poisoning. Toxicol Lett 202:69-74.
Schoonbroodt S, Piette J (2000) Oxidative stress interference with the nuclear factor-kappa B activation pathways. Biochem Pharmacol 60:1075-1083.
Tomenson JA, Campbell C (2011) Mortality from Parkinson’s disease and other causes among a workforce manufacturing paraquat: a retrospective cohort study. BMJ 1:e000283.
Wang XL, Zhang H, Liu R, Wang HM (2003) Protective effect of a protease inhibitor on acute lung injury after hepatic ischemia/reperfusion in the rat. Zhongguo Weizhongbing Jijiu Yixue 15:432-434.
Yamada K, Fukushima T (1993) Mechanism of cytotoxicity of paraquat. II. Organ specifcity of paraquat-stimulated lipid peroxidation in the inner membrane of mitochondria. ExpToxicol Pathol 45:375-380.
Yang WL, Sun AY (1998) Paraquat-induced cell death in PC12 cells. Neurochem Res 23:1387-1394.
Yasaka T, Ohya I, Matsumoto J, Shiramizu T, Sasaguri Y (1981) Acceleration of lipid peroxidation in human paraquat poisoning. Arch Intern Med 141:1169-1171.
Yasaka T, Okudaira K, Fujito H,Matsumoto J, Ohya I,Miyamoto Y (1986) Further studies of lipid peroxidation in human paraquat poisoning. Arch Intern Med 146:681-685.
Copyedited by Phillips A, Yajima W, Wang J, Qiu Y, Li CH, Song LP, Zhao M
*
10.4103/1673-5374.153698
http://www.nrronline.org/
Accepted: 2014-11-14
- 中国神经再生研究(英文版)的其它文章
- A new look at auranofn, dextromethorphan and rosiglitazone for reduction of glia-mediated infammation in neurodegenerative diseases
- what drives progressive motor defcits in patients with acute pontine infarction?
- Appearance of a neural bypass between injured cingulum and brainstem cholinergic nuclei of a patient with traumatic brain injury on follow-up diffusion tensor tractography images
- Compensatory recombination phenomena of neurological functions in central dysphagia patients
- Curcumin pretreatment and post-treatment both improve the antioxidative ability of neurons with oxygen-glucose deprivation
- Connecting the P300 to the diagnosis and prognosis of unconscious patients

