Research on Thermodynamic Properties of Polybrominated Diphenylamine by Neural Network
Xi-hua DuWen-chang ZhuangXiao-qin ShiChang-jun Feng
School of Chemistry and Chemical Engineering,Xuzhou Institute of Technology,Xuzhou 221111,China
Research on Thermodynamic Properties of Polybrominated Diphenylamine by Neural Network
Xi-hua Du∗,Wen-chang Zhuang,Xiao-qin Shi,Chang-jun Feng
School of Chemistry and Chemical Engineering,Xuzhou Institute of Technology,Xuzhou 221111,China
Based on the location of bromine substituents and conjugation matrix,a new substituent position index0Xnot only was defned,but also molecular shape indexesKmand electronegativity distance vectorsMmof diphenylamine and 209 kinds of polybrominated diphenylamine (PBDPA)molecules were calculated.Then the quantitative structure-property relationships (QSPR)among the thermodynamic properties of 210 organic pollutants and0X,K3,M29,M36were founded by Leaps-and-Bounds regression.Using the four structural parameters as input neurons of the artifcial neural network,three satisfactory QSPR models with network structures of 4∶21∶1,4∶24∶1,and 4∶24∶1 respectively,were achieved by the back-propagation algorithm.The total correlation coefcientsRwere 0.9999,0.9997,and 0.9995 respectively and the standard errorsSwere 1.036,1.469,and 1.510 respectively.The relative mean deviation between the predicted value and the experimental value ofSo−,∆fHo−and∆fGo−were 0.11%,0.34%and 0.24%respectively,which indicated that the QSPR models had good stability and superior predictive ability.The results showed that there were good nonlinear correlations between the thermodynamic properties of PBDPAs and the four structural parameters.Thus,it was concluded that the ANN models established by the new substituent position index were fully applicable to predict properties of PBDPAs.
Polybrominated diphenylamine,Neural networks,Molecular shape index,Electronegativity distance vector,Substituent position index,Thermodynamic properties
I.INTRODUCTION
Due to good stability,diphenylamine has been widely applied to produce antioxidants,gunpowder stabilizers, intermediates of dyes and pesticide.However,its longterm exposure to the human body will damage to the nervous,cardiovascular system and blood system,also have teratogenic efect,even induce severe bladder cancer[1−5].Polybrominated diphenylamines(PBDPAs) with typical characteristics of diphenylamine are mainly used as chemical intermediates of fame retardants,rodenticide,herbicides,fungicides,and other products[6, 7].Because of the lack of their environmental risk assessment,the research on PBDPAs environmental toxicity is particularly important and urgent.Thermodynamic properties of PBDPAs,closely relating to their other physical and chemical properties,their formation and distribution in the environment,will be benefcial to investigate environmental toxicological efects of PBDPAs,but there are few researches reported[8].
As an easy and efective means to obtain data of the compounds properties,the quantitative structureproperty relationships(QSPR)method has been widely employed in the studies of chemistry,environment, food,life,and so on[9−14].In the work,combined with the artifcial neural network(ANN)which can found models with high prediction accuracy[15−19], QSPR were used to calculate the thermodynamic properties of PBDPA.On the basis of the previous work [20,21],a new substituent position indexmXconsidering the location of bromine substituents on PBDPAs was defned,which refected the location of bromine substituents on PBDPAs and contained characteristic information such as atomic electronegativity,molecular space structure.Then molecular shape indexesKmand electronegativity distance vectorsMmwere also calculated.Through multiple regression analysis between these indexes and thermodynamic parameters,standard entropy(So−),standard heat of formation(∆fHo−),and standard free energy(∆fGo−),several optimal structural parameters were selected as input neurons of ANN using the back-propagation(BP)algorithm.According to diferent network structures,the satisfactory prediction models for thermodynamic properties of PBDPAs were established,results were highly consistent with the values in Ref.[8].

TABLE I The Results of0X,Km,Mmcorresponding to∆fGo-by Leaps-and-Bounds regression.
II.METHODOLOGY
A.Data of PBDPAs’thermodynamic properties
Based on the position and number of bromine substituents on the benzene ring,there exist maybe 209 kinds of isomers of PBDPA.The numbering system for C atoms in diphenylamine is shown in Fig.1.
The experimental data of three thermodynamic parameters,So−,∆fHo−and∆fGo−were obtained from Ref.[8].
B.New substituent position indexmX
Through studying the relationships between molecular structures of 209 kinds of PBDPAs or diphenylamine and their thermodynamic properties which were listed in Ref.[8],it can be concluded that the thermodynamic properties were closely related to the position and number of bromine substituents on the benzene ring.Taking the nitrogen atom as the starting point denoted by 0, the atomic characteristic value of atomiwas defned asδi∶
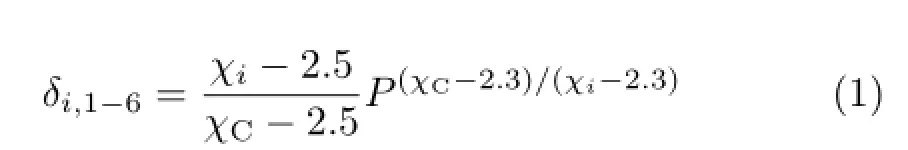
whereχiwas the electronegativity of atomi,χCdenoted the electronegativity of the carbon atom andPrepresented the serial number of bromine substituents on the benzene ring of PBDPA.When there were two or more substituents locating at position 1′−6′,their contribution to the molecule was diferent from the same substituents locating at position 1−6.After optimization,the matricesδiof atoms locating at position 1′−6′was modifed as follows∶
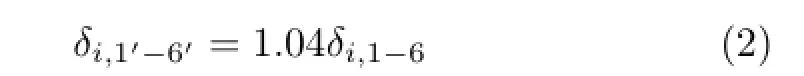
Therefore,matricesδiof diphenylamine are also presented in Fig.1.
Using Eqs.(1)and(2),δBrof Br atoms locating at position 2−6 and 2′−6′were calculated and their results are∶δBr2=11.962,δBr3=13.948,δBr4=15.554,δBr5=16.926,δBr6=18.136,δBr2′=12.440,δBr3′=14.506,δBr4′=16.176,δBr5′=17.603,δBr6′=18.861.Based on the above results,the substituent position indexmXwas defned∶
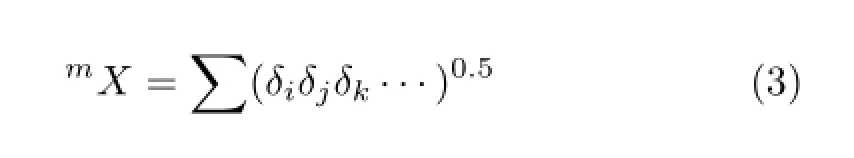
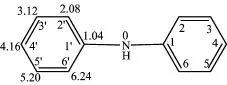
FIG.1 Numbering system for C atoms and matrices in diphenylamine.
wherejrepresents the non-hydrogen atom directly attached to atomi,kindicates the non-hydrogen atom directly attached to atomj,and so forth.In this work, only the 0-order index was calculated as0X∶

C.Establishing indices and selecting variables
On the basis of molecular conjugation matrix[22], molecular shape indexKmwas defned by Kier and it was used to characterize molecular shape[23,24]. The electronegativity distance vectorMmobtained by a certain formula[25]was employed to simulate molecular interactions among diferent non-hydrogen atoms. In this work,Km,MmandmXwere applied as independent variables.Meanwhile three thermodynamic parameters of PBDPAs,So−,∆fHo−and∆fGo−,were used as dependent variables.By Leaps-and-Bounds regression(LBR),the variables were extracted to carry out statistical regression analysis,such as the regression analysis of∆fGo−presented in Table I.
In Table I,R,Radj2,FandSwere correlation coefcient,the coefcient of determination,Fischer test value and the standard error,respectively.To be noted,the value of FIT(Kubinyi function)[26],was introduced and calculated as follows∶

whereyrepresents the sample size of compounds andbis the number of variables.The bigger the value of FIT was,the more stable the model would be,as well as the higher the predictive ability of the model would be.From the results in Table I,the best regression results with PBDPAs’thermodynamic properties were achieved by selecting substituent position index0X,molecular shape indexK3,electronegativity distance vectorsM29andM36.
III.RESULTS AND DISCUSSION
A.Multiple regression model
According to the thermodynamic properties of PBDPAs reported in Ref.[8],the correlations between thermodynamic properties and four kinds of variables including0X,K3,M29,M36have been analyzed.Multiple regression equations are shown as follows∶
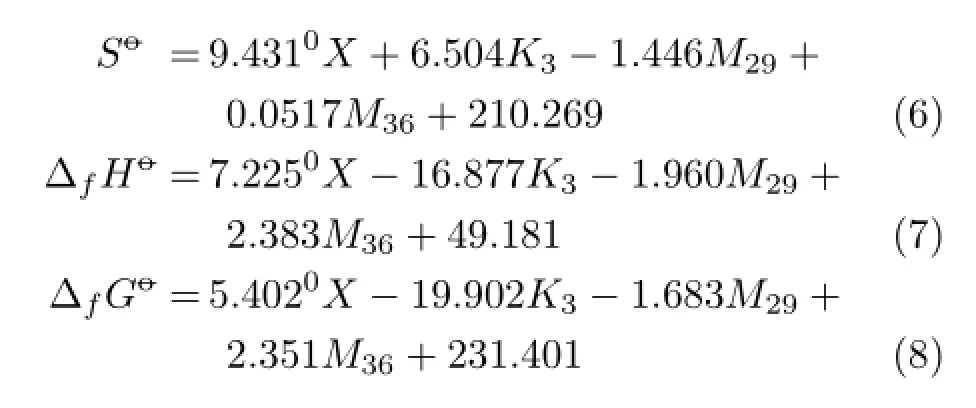
The correlation coefcientR,represented standard errorS,and the Fischer test valueFfor Eqs.(6)−(8)are shown in Table II.
From Table II,all the correlation coefcients of the multiple regression equations are bigger than 0.99, which indicates that the QSPR models were satisfactory.
B.Neural network model
The four parameters0X,K3,M29andM36were selected as input neurons while the thermodynamic properties of PBDPAs were used as output neurons.The optimal number of hidden layer unitH,was calculated according to the rules proposed by Xuet al.[27]∶2.2>ρ(=N/M)≥1.4,whereNwas the sample size andMdenoted the total weight of the network which was obtained by the following equation∶

whereI,HandQwere the numbers of variables in the input layer,the hidden layer,and the output layer, respectively.In this work,N=210 andI=4 so that the value ofHwas an integer arranging from 16 to 24. After repeated testing,the optimal results ofSo−were achieved whenH=21,whileH=24 to obtain those of∆fHo−and∆fGo−.In order to test the stability and predictive ability of the model,the sample data of each property have been divided into three groups,viz.training set,test set and validation set,to establish various neural network models as shown in Table III.
The correlation coefcients of the three sets were almost equal to the corresponding total correlation coefcients,which indicated that the models were quite stable and there was no overftting or overtraining phenomenon.The predictive results of thermodynamic properties by the neural network model are presented in Table IV.Comparing the results in Table II with those in Table III,it showed that the standard errors of the neural network model were signifcantly less than those obtained by the multiple regression method.In the meantime,the correlation coefcients of the neural network model were all close to 1.Our predictive results highly agreed with those reported in Ref.[8].Moreover, our neural network model with four variables had better correlation coefcients and smaller standard errors than the model in the literature[8].Hence,our model had stronger predictive ability.
For isomers of the same group,the smaller∆fGo−was,the more stable the molecule was,and vice versa [28].∆fGo−of the molecule with bromine substituents located in the same benzene ring and at the adjacent position of nitrogen atom,would be bigger due to the larger interactions between bromine atom and nitrogen atom which would increase molecular instability.However,when bromine substituents located in the diferent benzene ring and at the meta-position of nitrogen atom or at the meta-position of each other,∆fGo−would be smaller because of the smaller interactions between heteroatoms with large spacing which would enhance molecular stability.Furthermore,molecular volume of PBDPA as well as the values ofSo−,∆fHo−and∆fGo−, increased with the number of bromine substituents.As shown in Table III,it was confrmed that0Xcombined withK3,M29,andM36revealed essential infuence factors of PBDPA’s thermodynamic properties.The mean relative errors between predictive results obtained by neural network models and the data reported in the literature were only between 0.1%−0.3%,which indicated the predictive ability of neural network model was better than that of the multiple regression model.

TABLE II QSPR between characteristic parameters andSo-,∆fHo-,∆fGo-of PBDPAs.
IV.CONCLUSION
Substituent position indexmXcan refect molecular stability.As shown in Table IV,∆fGo−of the molecule with bromine substituents located at position 2 and 6 would be bigger,however it would be smaller when bromine substituents located at position 3(3′)and 5(5′). Thus,mXhad good correlation with∆fGo−.
mXcontained molecular spatial structure information.The molecular volume of PBDPAs would be bigger when the number of bromine substituents increased, meanwhile those thermodynamic data would also increase.These change rules can be fully refected by0X.
The neural network method was superior to the mul-
tiple regression method.Using the former method, there were good nonlinear correlations rather than simple linear relationships between those indices and thermodynamic properties,which showed the predictive ability of neural network model was better than that of the multiple regression model.Moreover,the former also had fne stability which was refected by FIT values.

TABLE IV The predicted values of the thermodynamic properties of PBDPA includingSo-(in J/(mol·K)),∆fHo-(in kJ/mol),and∆fGo-(in kJ/mol).
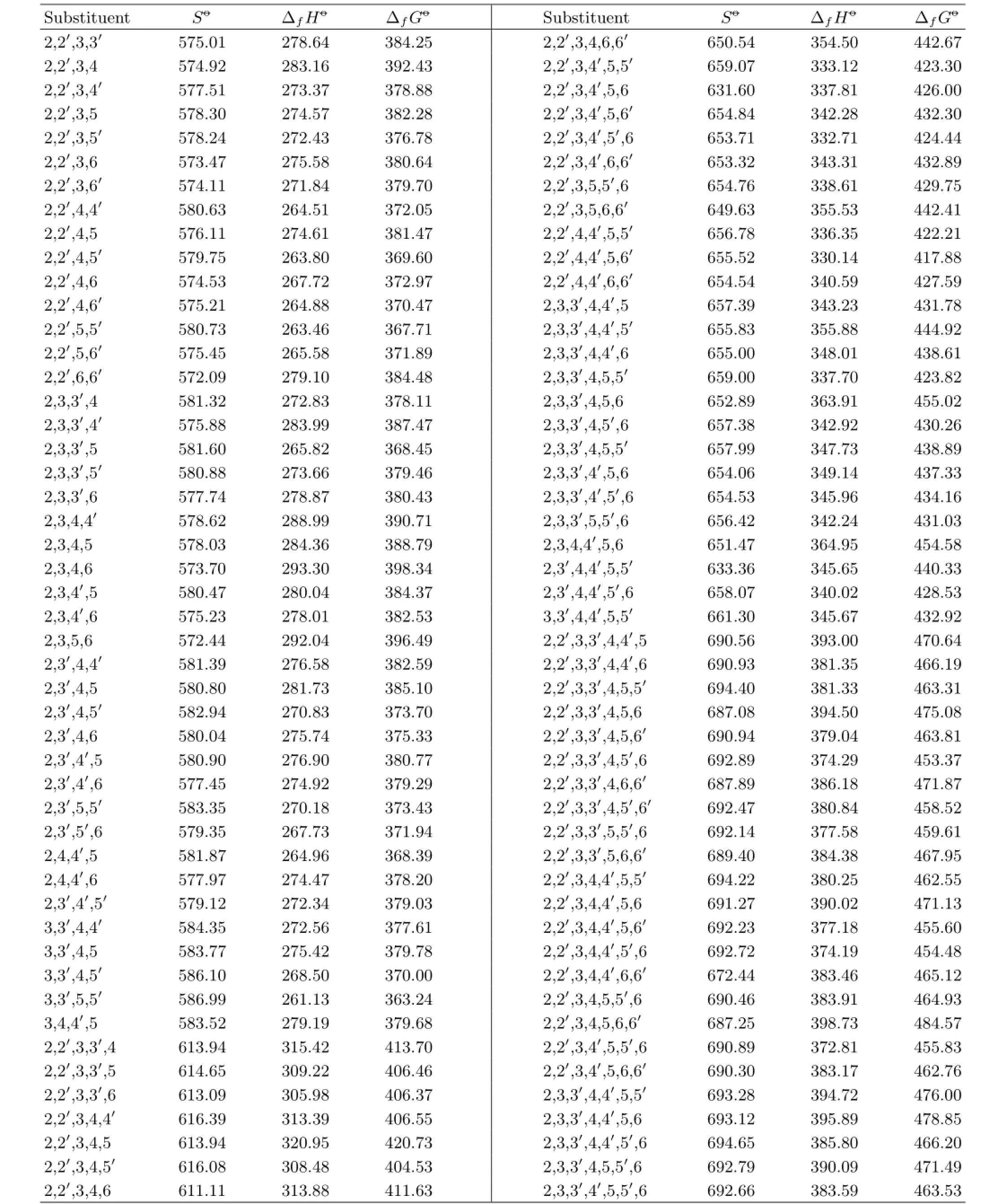
Table IV continued.
V.ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China(No.21472071), the Natural Science Foundation of Jiangsu Province(No.09KJD150012)and Special Funding of Xuzhou City Key Laboratory of Green Technology (No.SYS2012009).
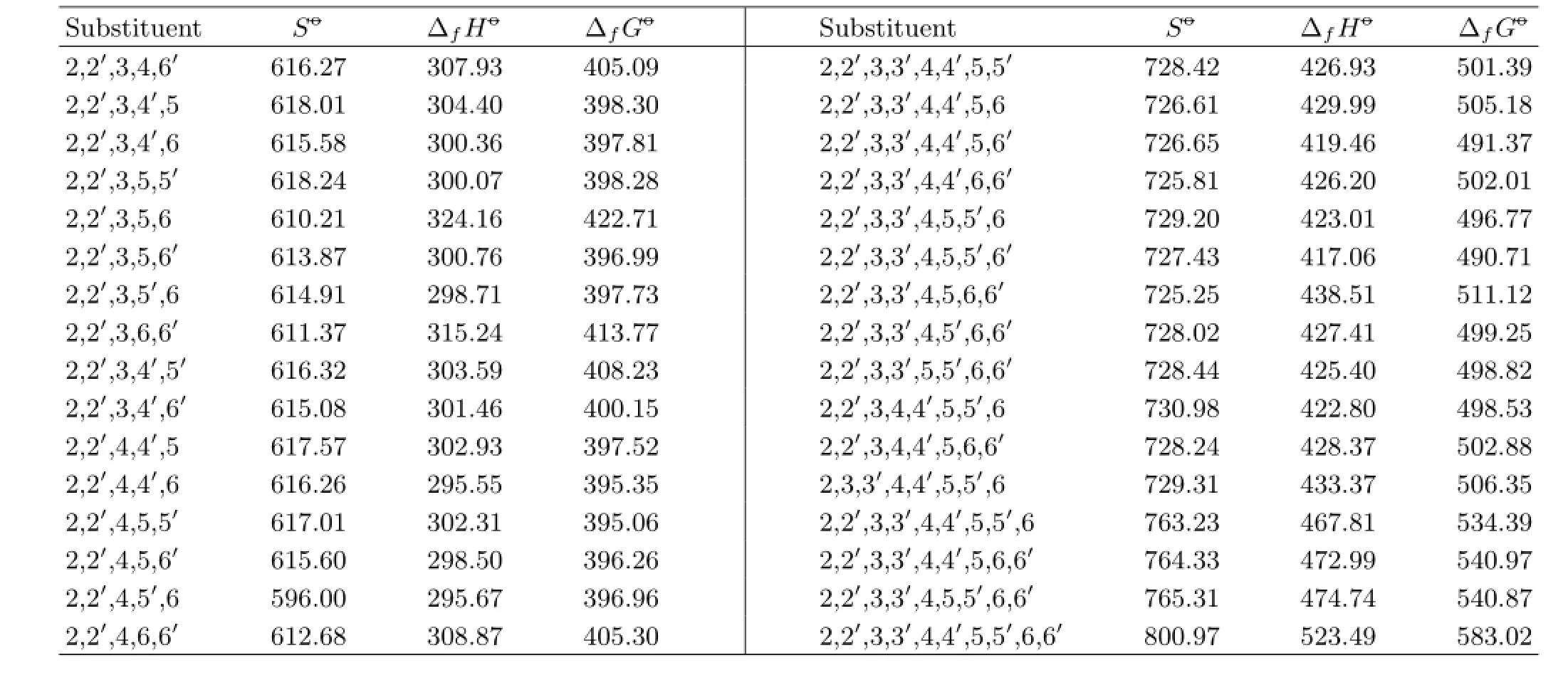
Table IV continued.
[1]A.Muslim,R.J.amal,T.Abdiryim,T.Awut,and I. Nurulla,J.Funct.Polym.20,198(2007).
[2]A.Farokhcheh and N.Alizadeh,LWT-Food Sci.Technol.54,6(2013).
[3]G.Ke and J.Hazard.Mater.146,249(2007).
[4]S.X.Li,D.Wei,M.Naiki,Z.W.Cai,X.R.Xue,H. B.Li,and Y.Jiang.J.Hazard.Mater.164,26(2009).
[5]A.Santovito,P.Cervella,and M.Delpero,Mutat.Res.747,135(2012).
[6]X.X.Wang,Y.L.Wang,C.L.Wang,X.Y.Wang,and G.L.Meng,J.Xiamen Univ.38,317(1999).
[7]S.A.Dong,G.Z.Zhang,and Y.X.Ou,Fine Chem.13,27(1996).
[8]C.Wang,Z.Y.Fang,Z.Y.Wang,and F.Y.Wang, Acta Chim.Sin.67,2319(2009).
[9]B.Wan and L.H.Guo,Environ.Chem.30,143(2011).
[10]G.Fayet,D.Jacquemin,V.Wathelet,E.A.Perp`ete,P. Rotureau,and C.Adamo,J.Mol.Graphics Modell.28,465(2010).
[11]H.Y.Xu,J.W.Zou,G.X.Hu,and W.Wang,Chemosphere.80,665(2010).
[12]H.Y.Wang,A.Q.Zhang,C.Sun,and L.S.Wang, Res.Environ.Sci.22,421(2009).
[13]D.A.Saldana,L.Starck,P.Mougin,B.Rousseau,L. Pidol,N.Jeuland,and B.Creton,Energy Fuels25, 3900(2011).
[14]C.Bertinetto,C.Duce,A.Micheli,R.Solaro,A. Starita,and M.R.Tin´e,J.Mol.Graphics Modell.27, 797(2009).
[15]F.J.Prado-Prado,X.Garc´ıa-Mera,and H.Gonz´alez-D´ıaz,Bioorgan.Med.Chem.18,2225(2010).
[16]Y.Chetouani,Stoch.Env.Res.Risk A22,339(2008).
[17]N.Hattab and M.Motelica-Heino,J.Geochem.Explor.136,14(2014).
[18]M.Goodarzi,M.P.Freitas,and N.Ghasemi,Eur.J. Med.Chem.45,3911(2010).
[19]K.Roy and P.P.Roy,Eur.J.Med.Chem.44,2913 (2009).
[20]X.H.Du,J.Chem.Ind.Eng.61,3059(2010).
[21]X.H.Du,J.Chem.Ind.Eng.58,2432(2007).
[22]L.B.Kier and L.H.Hall,Molecular Connectivity in Structure-Activity Analysis,England:Research Studies Press,69,(1986).
[23]X.H.Du,Y.Chen,and W.Yue,Food Sci.31,357 (2010).
[24]Y.H.Zhang,Z.N.Xia,L.T.Qin,and S.S.Liu,J. Mol.Graphics Modell.29,214(2010).
[25]S.S.Liu,Y.Liu,D.Q.Yin,X.D.Wang,and L.S. Wang,J.Sep.Sci.29,296(2006).
[26]L.Saiz-Urra,M.P.Gonzaez,and M.Teijeira,Bioorgan. Med.Chem.15,3565(2007).
[27]L.Xu and X.G.Shao,Methods of Chemometrics,Beijing:Science Press,441(2004).
[28]J.Yu,X.C.Zhang,Z.Y.Wang,and X.L.Zeng,Acta Chim.Sin.64,1961(2006).
10.1063/1674-0068/28/cjcp1406109
∗Author to whom correspondence should be addressed.E-mail:12dxh@sina.com,dxh@xzit.edu.cn,Tel.:+86-13852436068,FAX:+86-516-85608300
(Dated:Received on June 22,2014;Accepted on January 10,2015)
 CHINESE JOURNAL OF CHEMICAL PHYSICS2015年1期
CHINESE JOURNAL OF CHEMICAL PHYSICS2015年1期
- CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Nanosecond Rapid Crystallization of Water Induced by Quartz Glass under Dynamic Compression
- Benzene and Toluene Levels Measured with DOAS During Vehicular Restrictions in Beijing
- Antimicrobial Expanded Polytetrafuoroethylene Film Prepared byγ-ray Radiation Induced Grafting of Poly(acrylic acid)
- Aromatic Compounds Production from Sorbitol by Aqueous Catalytic Reforming
- Synthesis of Hierarchically Porous CaFe2O4/Carbon Fiber Hybrids and Microwave Induced Catalytic Activity
- Infuence of Triarylamine and Indoline as Donor on Photovoltaic Performance of Dye-Sensitized Solar Cells Employing Cobalt Redox Shuttle
