Social organization of Shortridge’s capped langur (Trachypithecus shortridgei) at the Dulongjiang Valley in Yunnan, China
Ying-Chun LI, Feng LIU, Xiao-Yang HE, Chi MA, Jun SUN, Dong-Hui LI, Wen XIAO,, Liang-Wei CUI4,1Forestry Faculty, Southwest Forestry University, Kunming, Yunnan 6504, China
2Nujiang Administration Bureau, Gaoligongshan National Nature Reserve, Liuku, Yunnan 673100, China
3Institute of Eastern-Himalaya Biodiversity Research, Dali University, Dali, Yunnan 671003, China
4College of Life Sciences, Northwest University, Xi’an,710069, China
Social organization of Shortridge’s capped langur (Trachypithecus shortridgei) at the Dulongjiang Valley in Yunnan, China
Ying-Chun LI1,†, Feng LIU1,†, Xiao-Yang HE2, Chi MA3, Jun SUN2, Dong-Hui LI2, Wen XIAO3,*, Liang-Wei CUI1,4,*
1Forestry Faculty, Southwest Forestry University, Kunming, Yunnan 650224, China
2Nujiang Administration Bureau, Gaoligongshan National Nature Reserve, Liuku, Yunnan 673100, China
3Institute of Eastern-Himalaya Biodiversity Research, Dali University, Dali, Yunnan 671003, China
4College of Life Sciences, Northwest University, Xi’an,710069, China
Non-human primates often live in socially stable groups characterized by bonded relationships among individuals. Social organization can be used to evaluate living conditions and expansion potential. Bisexual group size, ratio of males to females and group composition are essential elements determining the type of social organization. Although the first report on Shortridge’s capped langurs (Trachypithecus shortridgei) was in the 1970s, until now, the species only inhabits forests of the Dulongjiang valley in northwest Yunnan, China, with c. 250-370 individuals in 19 populations. To understand its social organization, we collected data from five groups of Shortridge’s langurs at Silaluo in the Dulongjiang valley during August 2012-October 2013. Family groups consist of one adult male, 2–3 adult females and up to five young. Group size averaged 8 (7-9) individuals. The ratio of adult males to females (M/F) was 1:2.9, infants to adult females was (I/F) 1:2.2; and ratio of adults to immatures was 1:1.2, indicating the potential of a population increasing. Birth season was during March-July and the inter-birth interval was two years.
Trachypithecus shortridgei; Social organization; One-male, multi-female group; Multimale, multi-female group; Group size
INTRODUCTION
Social organization, including group size, sexual composition and bonded relationships among individuals (Kappeler & van Schaik, 2002), is the most basic characterization of non-human primate societies (Clutton-Brock & Harvey, 1977; Crook & Gartlan, 1966; Eisenberg et al, 1972). Three fundamental types of social organization can be categorized into solitary, pair-living and group-living speceis (Kappeler & van Schaik 2002). Solitary individuals typically forage alone (Boinski & Garber, 2000) and their activities are desynchronized with each other both spatially and temporally (Charles-Dominique, 1978). Except for orangutan, most solitary primate species are nocturnal (Kappeler & van Schaik, 2002). Pair-living species refer to couples of one adult male and one adult female (Kappeler, 1999), such as gibbons. Most primates live in bisexual groups (van Schaik & Kappeler, 1997), which are much more stable compared with other mammals, and consist of more than two adults. Group living primates displayed a diversity with respect to the size, sex ratio and temporal stabitliy of compositioin. Accordingly, polyandrous, polygynous and multi-male, multi-female groups (MMGs) have been diistinguished (Kappeler, 2000). Variation in the number of adult males is the most prominent feature of primate group composition (Hamilton & Bulger, 1992; Preuschoft & Paul, 2000; van Hooff, 2000), and can thus be categorized as one-male, multi-female groups (OMGs) or MMGs. The number of adult males in each bisexual group related to the predatory pressure (van Schaik & Hőrstermann, 1994), is positively associated with the number of adult females (Mitani et al, 1996) and temporal overlap of female receptive periods (Nunn, 1999).1
Group size is another vital feature of the social organization of group living primates, and may be influenced by birth rate, morality rate and the transfer of individuals (Kappeler & van Schaik, 2002). Significant variations can be found in colobine species, e.g., group size in Mentawai langurs (Presbytis potenziani) is less than four, whereas, golden snub-nosed monkeys (Rhinopithecus roxellana) can include approximately 400 individuals (Grueter, 2013; Newton & Dunbar, 1994). Social organization of langurs includes monogamy, such as found in Mentawai langurs (P. potenziani); matrilineal-harem, such as seen in Sumatran surilis (P. melalophos), maroon leaf monkeys (P. rubicunda), Nilgiri langurs (Trachypithecus johnii), Gee's golden langurs (T. geei), capped langurs (T. pileatus), dusky leaf monkeys (T. obscurus), proboscis monkeys (Nasalis larvatus), and Northern plains gray langurs (Semnopithecus entellus) at Abu and Jodhpur; matrilineal-multimale, such as observed in Northern plains gray langurs (S. entellus) at Orcha and Rajaji; and patrilineal-multimale, such as found in blackand-white colobus (Colobus polykomos), olive colobus (Procolobus verus) and western red colobus (P. badius) monkeys. However, the most typical social organizaiton is the one-male, multi-female unit (OMU). A large population can be composed of several OMUs, and one or several all-male units (AMUs) (Cui et al, 2008; Grueter, 2013; Kirkpatrick, 1996; Li et al, 2014; Newton & Dunbar, 1994; Qi et al, 2009, 2014).
Group size of Trachypithecus species can range from a dozen to approximately 100, and their social organization includes OMGs and MMGs (Koenig & Borries, 2012; Fan et al, 2014). Bisexual groups of white-headed langurs (T. leucocephalus) are composed dominantly of OMGs, occasionaly of MMGs (9.1%, n=11), with 3-30 individuals in each group. Non-breeding individuals can live solitarily or in groups (Jin et al, 2009; Li & Rogers, 2004). Non-breeding groups are either AMUs or include males (82.6%) and juvenile females (17.4%) (Jin et al, 2009). A bisexual group usually lives in an OMG with 6-12 individuals in François' langurs (T. francoisi) (Hu et al, 2011; Zhou et al, 2009). Bisexual groups of Delacour's langurs (T. delacouri) mostly live mostly in OMG,s, and occasionally in two-male, multi-female groups (TMGs), with 5-30 individuals in each group (Harding, 2011). Golden langur (T. geei) groups, which inhabit the rubber forests of Assam in India, usually consist of 7-26 (17.3 on average) individuals. Among three observed groups, two were TMGs with 19 and 26 individuals, respectively, and one was an OMGs with only seven individuals (Medhi et al, 2004). Silvered langur (T. cristatus) groups consist of 7-40 individuals (Timmins et al, 2013), one OMG includes seven individuals (Boonratana, 1998). Small OMGs and occasionally MMGs have been found in capped langurs (T. pileatus) (Green, 1981; Mukherjee, 1978; Mukherjee et al, 1995; Stanford, 1991). Most Phayre's leaf monkeys (T. phayrei) form bisexual groups (Bose, 2003; Koenig & Borries, 2012; Mukherjee, 1982) or AMUs (Mukherjee, 1982). Each group consists of 6-33 (Koenig & Borries, 2012) up to 45 individuals (Zheng, 1993), including 1-5 adult males and 3-12 adult females. Large groups of Indochinese gray langurs (Trachypithecus crepusculus) (60-100 individuals, n=6) were observed at Wuliangshan Mountain (Fan et al, 2014). Bisexual groups of Phayre's leaf monkeys include OMGs (48.4%), TMGs (24.9%) and three-male, multi-female groups (15.9%) (Koenig & Borries, 2012). The diverse social organization of Trachypithecus species are adaptations to diverse habitat environments (including natural and social environments) and are also the result of both environmental pressure and phylogeny.
Shortridge's capped langurs (Trachypithecus shortridgei) (Wroughton, 1915) are now considered as a separate species from capped langurs (T. pileatus) (Groves, 2001) and to only distribute in the Dulongjiang valley, northwest Yunnan, China (Cui et al, 2015) and northeastern Myanmar (Groves, 2001; Htun et al, 2008; Pocock, 1939). T. shortridgei is categorized as Endangered on the IUCN Red List (Htun et al, 2008) and is listed in CITES Appendix I (CITES, 2014). In China it is a Category I protected species under Chinese animal conservation laws, and in Myanmar it is protected under the national Wildlife Protection Law (Htun et al, 2008).. In India, capped langur (Trachypithecus pileatus) groups usually consist of 8-11 individuals (Solanki, 2007, 2008), usually forming OMGs and occasionally TMGs (Mukherjee, 1978) or MMGs (Green, 1981; Mukherjee, 1978; Mukherje et al, 1995; Solanki, 2007; Stanford, 1991, 1987, 1988). Non-breeding individuals live solitarily or in AMGs (Choudhury, 1988; Green, 1981; Stanford, 1991, 1988).
Although Shortridge's langurs were first found in China since 1972, little is known about this species. Community interview indicated that they live in bisexual groups of 10-30 individuals, and only 19 groups with 250-370 individuals have been found in the Dulongjiang region of Yunnan, China (Cui et al, 2015). To date, knowledge on their social organization remains very little. In this study, we aim to clarify some basic information of T. shotridgei: (1) whether its bisexual group size is 10-30 or approximately 10 indicdiuals similar to that of T. pileatus; (2) whether its social organization is OMG or MMG; (3) the number of adult females in bisexual group and (4) the age-sexal composition of bisexual group and its dynamic trends.
MATERIALS AND METHODS
Study area
The study area is located in the Dulongjiang region, Gongshan County, northwest Yunnan, China, and neighbors with Chayu in Tibet to the north and with Kachin in Myanmar to the south and west. East and west of Dulongjiang River are the west side of Gaoligongshan Mountain and the east side of the Dandanglika Mountains, respectively. The Gaoligongshan Mountain and Dandanglika Mountains are south-north oriented, descending from north to south and are featured with significant altitudinal differences (4 000 m maximum) and steepness. Under the influence of southwest monsoon and topographic features, the climate of the Drung River Basin is quite mild. Average yearly temperature was 14.5 °C during 2010-2012; the difference in average monthly temperature was 15.0 °C; the highest and lowest average temperature occurred in August (21.6 °C) and January (6.6 °C). The rain season is from February to October; average yearly precipitation was 2 745.1 mm, with two peak periods of precipitation from March to April and from June to September, respectively. The Drung region is characterized bydense forests and obvious vertical zonality in vegetation. The vegetation type from low to high altitude is monsoonal evergreen broad-leaved forests (1 200–1 500 m), montane moist evergreen broad-leaved forests (1 500–2 400 m), mixed broadleaf-conifer forests (2 400–2 800 m), cold temperate coniferous forests (2 800–3 000 m), frigid-temperate coniferous forest (3 000–3 700 m) and alpine scrubs and meadows (>3 700 m) (He & Li, 1996).

Figure 1 Average monthly temperature and precipitation in the Dulongjiang valley from January 2010 to December 2012

Table 1 Features of different age-sex classes of Trachypithecus shortridgei
Study subject
The study subjects included five groups (group A, B, C, D, E) of Shortridge’s langurs inhabiting the Silaluo region (N27o47′, E98o19′) in the Dulongjiang valley. Age-sex composition of the species was distinguished according to body size, body color and other morphological features (Table 1). Except for one adult female with a chopped tail (its length is approximately 10 cm), others were not individually differentiated.
Method
Although an observing location (1 500 m a.s.l.) with open view was found on the mountain facing the activity areas of the langurs, the steep terrain and dense forests inhibited our observation. Therefore, all observations were conducted along the road opposite the activity areas of the monkey, so we only observed activities of the groups in areas between the riverside (approximately 1 420 m) and the elevation of 1 700 m.
From August 2012 to September 2013 (except December 2013), surveys were conducted daily during 0700–1830 in Silaluo-Pukawang region of the Dulongjiang valley. When monkeys were sighted, we observed them in the distance of 60-800 m by binoculars (Olympus 10×42 EXWP I) or monoculars (Leica Televid 77, 8×42). Age–sex compositions of the groups were recorded when they were crossing areas with open view (such as naked rocks or forest gaps). A topographic map (1: 50 000) of the study area was subdivided into small squares (250 m×250 m) and the locations of groups were recorded on the map every 30 min.
Due to the steep terrain and dense forests, it was not possible to continuously track groups or recognize individuals, so the birth season was only roughly estimated based on information acquired from group observations and the number and morphological chacteristics of infants.
RESULTS
Bisexual groups of Shortridge’s langur at the Dulongjiang valley were composed of one-male, multi-female and their immatures. Average group size was 8 (7-9) individuals, including one adult male, 2-3 adult females and up to five offspring. The ratio of adult males to females (M/F) was 1:2.9; infants (I/F) to females was 1:2.2 and adults to immature individuals (Ad/Im) was 1:1.2. Although a possible solitary male was observed at Qinlangdang south (30 km straight distance) of our study area and 12 single male individuals were previously surveyed at Pianma, Lushui; neither a all-male group nor solitary males were observed in our study area. The increasing size of group D was directly resulted from the birth of infants in the group. However, as we were unable to recognize individuals, it was not possible to determine the underlying mechanisms of variations in the number of individuals (Table 2).
One infant in group D was born sometime between 9 March and 6 April 2013. According to its morphological characteristics, we assumed the infant to be two weeks old on 6 April. Another infant in group D was born sometime during 7 April-23 May, 2013. One infant in group E was approximately 1-2 weeks old when it was first sighted and it was assumed born sometime during 22-31July 2013. Therefore, we concluded that the brith season of Shortridge’s langurs in the Dulongjiang valley was from March to July.
In 2012, 11 females gave birth to six infants (birth rate=0.55), and three infants were produced by six females (birth rate=0.5) in 2013, which demonstrated an overall birth rate of 0.56 during the study period. From 2012 to 2013, three infants were produced by three females in group D. These results indicate that Shortridge’s langurs produce one infant every two years.
This study ran for 299 days (2 343.9 h), of which 69 days was used for observation and 63 days for scanning record (273 h). Thirty-nine habitat squares, totaling 2.44 km2, were utilized by the five groups. Although habitat overlap among the different groups was 33%, no direct competition was observed. We therefore assumed that the monkeys might avoid competition through using habitats at different time periods.
DISCUSSION
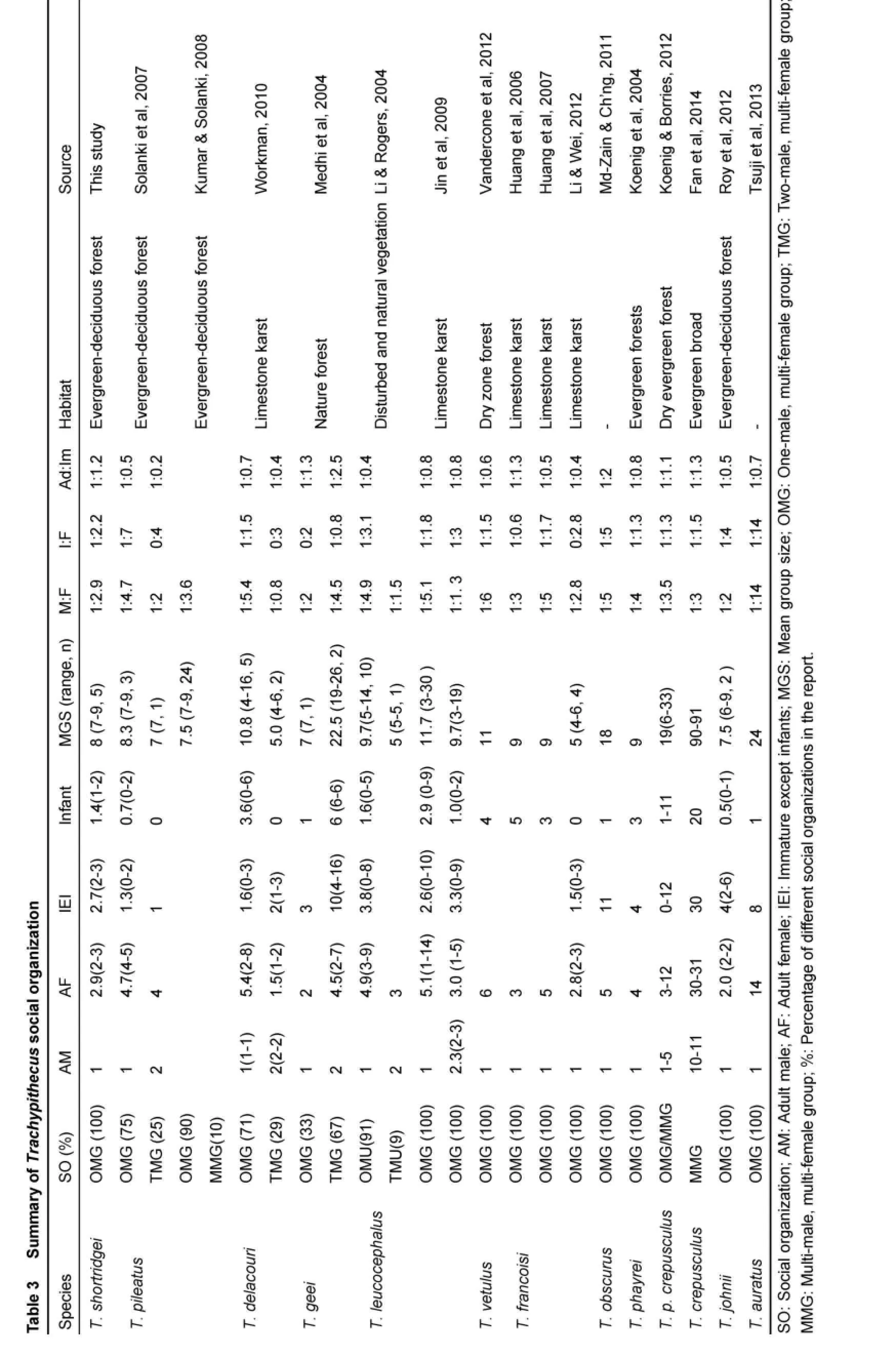
Group size in colobine varies from three to several hundred, living in small families or larger group (Newton & Dunbar, 1994). Shortridge’s langurs at the Dulongjiang valley usually live in small groups of 7-9 (8 on average) individuals, which were much smaller than those estimated in community interviews (10-30 individuals, Cui et al, 2015), but were comparable with group sizes (approximately 10 individuals) of capped langur (T. pileatus) (Kumar & Solanki, 2008; Solanki et al, 2007), Delacour's langur (T. delacouri) (Workman, 2010), whiteheaded langur (T. leucocephalus) (Jin et al, 2009), purple-faced langur (T. vetulus) (Vandercone et al, 2012), François' langur (T. francoisi) (Huang et al, 2006, 2007; Li & Wei, 2012) and Gee's golden langur (T. johnii) (Roy et al, 2012); whereas, smaller than those of other species, such as cantor’s dusky leaf monkey (T. obscurus halonifer) (Md-Zain & Ch’ng, 2011) and Javan lutung (T. auratus sondaicus) (Tsuji et al, 2013).
Group size is affected by the spatial and temporal distribution of food resources, predator pressures and foraging strategies of individuals. When predator pressure is low and food patches are small and/or abundant, group sizes are usually small; when food patches are large and food is high in variety but low in abundance, group sizes are usually large. When population densities are high, populations are limited by habitat quality (Newton & Dunbar, 1994). Fan et al (2014) found that large groups of Indochinese gray langurs (Trachypithecus crepusculus) were dependent on the high variety of food resources. The high overlap (33%) of habitats observed in the Shortridge’s langurs at the Dulongjiang valley indicated that habitat limitations may have confined population expansion. During our study, no natural enemies of the Shortridge’s langurs were found, suggesting low predator pressure, whereas, human activities (such as illegal hunting) may have disturbed the populations. The small Shortridge’s langur populations may be the result of all these interactions. However, the specific factors underlying population size can only be determined by systematic research in gradient environments (various habitat qualities and human disturbance).
Bisexual groups of many Trachypithecus species are OMGs, though MMGs are occasionally observed (Kirkpatrick, 2007; Newton & Dunbar, 1994). Bisexual groups in Shortridge’s langurs at the Dulongjiang valley usually included one male adult, 2-3 female adults and up to five immature offspring, which is quite similar with that of T. vetulus (Vandercone et al, 2012), T. francoisi (Huang et al, 2006, 2007; Li & Wei, 2012), T. obscurus (Md-Zain & Ch’ng, 2011), T. phayrei (Koenig et al, 2004), T. johnii (Roy et al, 2012) and T. auratus (Tsuji et al, 2013).
The number of male adults in a breeding group is irrelevant to population density, weather conditions and male mortality rate (Newton & Dunbar, 1994), but is correlated with predator pressure, number of females and reproduction synchronicity (Kappeler & van Schaik, 2002; Mitani et al, 1996; Nunn, 1999; van Schaik & Hőrstermann, 1994). For example, predator pressure (such as eagles) increased the numbers of adult males from one to two in breeding groups of howler monkeys and colobus monkeys, whereas, breeding groups of langurs that inhabit the same environment, but without predator pressure, are composed of OMG (van Schaik & Hőrstermann, 1994). The number of males is also affected by the number of females, which are restrained by food resources and population size (Andelman, 1986; Crockett & Eisenberg, 1987; Dunbar, 1988; Terborgh, 1986). The reproduction strategy of males depends on the number of estrous females that can be monopolized by males, which is correlated with both the number of adult females and their reproduction synchronicity (Emlen & Oring, 1977, Wrangham, 1980). Moreover, the percentage of OMGs is also influenced by the number of adult females and their reproduction synchronicity (Srivastava & Dunbar, 1996). Theoretically, one male HanumanLangur (Semnopithecus entellus) can monopolize up to 12 adult females, but once the number of females has exceeded this limitation, more than one male would be expected in a breeding group (Newton, 1988).

?
Extreme reproductive synchronicity (either too high or too low) decreases the ability of males to monopolize females, and only moderate reproduction synchronicity can enhance the monopolization (Emlen & Oring, 1977). Monopolizaiton ability of the male can also be enhanced by the concentrated distribution of females and its more non-feeding time (van Schaik & van Van Hoof, 1983). On average, only five adult females were observed in OMGs of white-headed langur, which may result from low overall numbers of adult females (Jin et al, 2009). OMGs of Shortridge’s langur at the Dulongjiang valley had only three adult females, whereas, the number of adult females in OMGs of other species of Trachypithecus are usually 4-5 (ranging from 2 to 14) (Table 3). Although the reproduction synchronicity of female Shortridge’s langurs cannot be precisely determined, the scattered birth pattern suggests low synchrony. According to our study, females basically moved within the view field of males and the feeding time of adult males was 5.9%. Therefore, the number of adult males was correlated with both low predator pressure and the small number of adult females.
Although OMGs of Shortridge’s langurs at the Dulongjiang valley contained only a few adult females, the ratio of adults to immature individuals (1:1.2) indicated their increasing potential. The ratio of OMGs in genus of Trachypithecus is 0.5-2, but that of most of other species is less than 1:1 (Solanki et al, 2007; Workman, 2010; Jin et al, 2009; Vandercone et al, 2012; Koenig et al, 2004; Koenig & Borries 2012; Roy et al, 2012; Tsuji et al, 2013), suggesting a significant trend of decreasing population. In the future, factors influencing population increase, such as human disturbance, habitat quality and inter-specific competition, should be explored.
Bisexual groups of genus of Trachypithecus are composed typically of OMGs and occasionally of MMGs. For example, MMGs in T. leucocephalus and T. pileatus account for 9-10% and 10-25%, respectively; and TMGs in T. delacouri and T. geei account for 29% and 67%, respectively (Table 3). The number of adult males in MMGs is mostly two and occasionally three (Koenig & Borries, 2012) and theri adult females are less than those in OMGs (T. Pileatus: Solanki et al, 2008; T. delacouri: Workman, 2010; T. leucocephalus: Li & Rogers, 2004; Jin et al, 2009), but the number of adult females in MMGs and OMGs of white-headed langurs (T. Leucocephalus) exhibit no differences. These phenomena indicate that MMG is not a strategy of males to monopolize more females (Jin et al, 2009). The occurrence of MMGs in white-headed langurs is the result of male replacement (Jin et al, 2009; Li & Rogers, 2004), which finally changes into OMGs or non-breeding groups (Jin et al, 2009). So the formation of MMGs in species of colobus may be agegraded or is only a temporary phenomenon during the process of male replacement (Jin et al, 2009; Sterck & Hooff, 2000).
Other than bisexual groups, there are also non-breeding groups and solitary males. Non-breeding groups mainly consist of males and occasionally females, suggesting that males play vital roles in sex dispersal (Jin et al, 2009; Kirkpatrick, 2007; Newton & Dunbar, 1994). In our study, no all-male group or a solitary male were observed, although 12 solitary males were previously reported in Fugong and Lushui counties near Myanmar. A similar phenomenon was also reported in species of colobus (Jin et al, 2009; Kirkpatrick et al, 1998; Newton & Dunbar, 1994). Allmale groups were reported in white-headed langurs, but no in Shortridge’s cpped langurs, which are important for promoting genetic communication among different populations and in avoiding inbreeding. However, the reason why all-male groups were not observed in our study is worth future exploration, for instance, wether they were poached or immigrated into other groups.
Under the influence of the warm and humid current brought by the southeast monsoon, the Dulongjiang valley experiences high precipitation, and has small yearly temperature variations (≤15 °C), with an average yearly temperature of 14.5 °C. The vegetation consists predominately of monsoon evergreen broad-leaved forests, warm temperate evergreen broad-leaved forest and broad-leaved deciduous forest, which provides many kinds of plant speices and abundant food resources for wild animals. Shortridge’s langurs at the Dulongjiang valley have a long birth period (March to July) and scattered birth pattern. At the Pakhui Wildlife Sanctuary in India, the average highest and lowest yearly temperature is 28 °C and 19 °C, respectively, and the yearly average precipitation is 2 040 mm. Its optimal weather results in various vegetations, dominated by tropical ever-green and semi-evengreen broad-leaved forests and semitropical forest (Champion & Seth, 1968). Although local capped langur (T. pileatus) exhibits a birth peak, the birth pattern is very scattered (December to April next year) (Solanki et al, 2007). The scattered birth patterns in these two primates are likely adaptations to abundant food and mild seasonal changes in weather conditions and food resources. Conversely, blank-and-white snub-nosed monkeys inhabit temperate zones at high altitude with low temperatures, resulting in food shortage and severely seasonal changes in weather and food resources, and thus strict birth seasonality is found in their birth patterns. To ensure successful reproduction and increase fitness, these two primate species use different birth patterns to cope with specific environmental pressures.
In summary, Shortridge’s langurs at the Dulongjiang valley are composed of one-male, multi-female groups without allmale groups, but with solitary males, which usually keeped distances from the breeding groups. There were, on average, eight individuals in each group and large habitat overlap between groups. The number of adult females in bisexual group was usually 2-3, smaller than the number in most species of Trachypithecus, which suggests a possible correlation between small number of adult females and limited suitable habitat and illegal hunting. Age structure of Shortridge’s langurs indicated a trend of increasing potential. In the future, suitable habitat and threatening factors regarding Shortridge’s langurs should be explored, and their population densities should be more precisely evaluated, from which feasible protection suggestions can be established.
ACKNOWLEDGEMENTS
Special thanks are given to the Nujiang Administrative Bureau of Gaoligongshan Nature Reserve for their support, Ding SQ and Meng XJ for their field assistance, and two anonymous reviewers for their useful suggestions.
Andelman SJ. 1986. Ecological and social determinants of cercopithecine mating systems. In: Rubenstein DI, Wrangham RW. Ecological Aspects of Social Evolution: Birds and Mammals. Princeton: Princeton University Press, 201-216.
Boinski S, Garber PA. 2000. On the Move: How and Why Animals Travel in Groups. Chicago: University of Chicago Press.
Boonratana R. 1998. Wildlife survey training at Dong Hua Sao and Phou Xiang Thong National Biodiversity Conservation Areas, Lao RDR. IUCN/LSFP, Vientiane.
Bose J. 2003. ‘Search for a Spectacle’: A conservation Survey of Phayre’s Leaf Monkey (Trachypithecus phayrei) in Assam and Mizoram. Wildlife Trust of India.
Charles-Dominique P. 1978. Solitary and gregarious prosimians: evolution of social structures in primates. In: Chivers DJ, Joysey KA. Recent Advances in Primatology, vol. 3. Evolution. London: Academic, 139-149.
Champion SHG, Seth SK. 1968. A Revised Survey of the Forest Types of India. Delhi: Manager of Publications.
Choudhury A. 1988. Phayre’s leaf monkey (Trachypithecus phayrei) in Cachar. Journal of Bombay Natural History Society, 85: 485-492.
Clutton-Brock TH, Harvey PH. 1977. Primate ecology and social organization. Journal of Zoology, 183(1): 1-39.
CITES. 2014. Convention on International Trade in Endangered Species of Wild Fauna and Flora. Appendices I, II and III. Http://www.cites. org/eng/app/appendices.php [accessed 20 November 2014].
Crockett CM. Eisenberg JF. 1987. Howlers: variation in group size and demography. In: Smutes BB, Cheney DL, Seyfarth RM, Wrangham RW, Stuhsaker TT. Primate Societies. Chicago: University of Chicago Press, 54-68. Crook JH, Gartlan JS. 1966. Evolution of primate societies. Nature, 210(5042): 1200-1203.
Cui LW, Huo S, Zhong T, Xiang ZF, Xiao W, Quan RC. 2008. Social organization of black-and-white snub-nosed monkeys (Rhinopithecus bieti) at Deqin, China. American Journal of Primatology, 70(2): 169-174.
Cui LW, Li YC, Li JF, He XY, Ma C, Scott MB, Li DH, Sun J, Sun WM, Xiao W. 2015. Distribution and conservation status of Shortridge’s capped langurs (Trachypithecus shortridgei) in Yunnan, China. Oryx, (in press).
Dunbar RIM. 1988. Primate Social Systems. Beckenham: Croom Helm.
Eisenberg JF, Muckenhirn NA, Rudran R. 1972. The relation between ecology and social structure in primates. Science, 176(4037): 863-874.
Emlen ST, Oring, LW. 1977. Ecology, sexual selection, and the evolution of mating systems. Science, 197(4300): 215-223.
Fan PF, Garber P, Ma C, Ren GP, Liu CM, Chen XY, Yang JX. 2014. High dietary diversity supports large group size in Indo-Chinese gray langurs in Wuliangshan, Yunnan, China. American Journal of Primatology, doi: 10.1002/ajp.22361.
Green KM. 1981. Preliminary observations on the ecology and behavior of the capped langur, Presbytis pileatus, in the Madhupur Forest of Bangladesh. International Journal of Primatology, 2(2): 131-151.
Groves CP. 2001. Primate Taxonomy. Washington and London: Smithsonian Institution Press.
Grueter CC. 2013. The Biology of the Snub-nosed Monkeys, Douc Langurs, Proboscis Monkeys, and Simakobus. New York: Nova Science Publishers, Inc.
Hamilton W, Bulger J. 1992. Facultative expression of behavioral differences between one-male and multimale savanna baboon groups. American Journal of Primatology, 28(1): 61-71.
Harding LE. 2011. Trachypithecus delacouvi (Primates: Cercopithecidae). Mammalian Species, 43(1): 118-128.
He DM, Li H. 1996. The Synthetical reserch of Dulong river and Dulong nationality. In: He DM, Li H. The Synthetical reserch of Dulong river and Dulong nationality. Kunming. Kunming: Yunnan Science and Technology Press, 1-24.
Htun S, Long YC, Richardson M. 2008. Trachypithecus shortridgei. In: IUCN 2014. The IUCN Red List of Threatened Species, Version 2014. 3 [online]. Available: Http: www.iucnredlist.org [Accessed 4 May 2015].
Hu G, Dong X, Luo HZ, Su XW, Li DY, Zhou CQ. 2011. The distribution and population dynamics of François’ langur over the past two decades in Guizhou, China and threats to its survival. Acta Theriologica Sinica, 31(3): 306-311. (in Chinese)
Huang CM, Zhou QH, Li YB, Cai XW, Wei FW. 2006. Activity rhythm and diurnal time budget of François langur (Trachypithecus françoisi) in Guangxi, China. Acta Theriologica Sinica, 26(4): 380-386. (in Chinese)
Huang ZH, Zhou QH, Li YB, Wei SM, Wei H, Huang SM. 2007. Daily activity pattern and time budget of François langur Trachypithecus françoisi in Longgang Nature Reserve, China. Acta Zoologica Sinica, 53(4): 589-599. (in Chinese)
Jin T, Wang DZ, Zhao Q, Yin LJ, Qin DG, Ren WZ, Pan WZ. 2009. Social organization of white-headed langurs (Trachypithecus leucocephalus) in the Nongguan Karst Hills, Guangxi, China. American Journal of Primatology, 71(3): 206-213.
Kappeler PM. 1999. Convergence and nonconvergence in primate social systems. In: Fleagle JG, Janson CH, Reed KA. Primate Communities. Cambridge: Cambridge University Press, 158-170.
Kappeler PM. 2000. Primate males: History and theory. In: Kappeler PM. Primate Males. Cambridge: Cambridge University Press, 3-7.
Kappeler PM, van Schaik CP. 2002. Evolution of primate social systems. International Journal of Primatology, 23(4): 707-740.
Kirkpatrick RC. 1996. Ecology and Behavior of the Yunnan Snub-Nosed Langur Rhinopithecus bieti (Colobinae). Ph. D. dissertation. University of California, Davis.
Kirkpatrick PC. 2007. The Asian colobines: diversity among leaf-eating monkeys. In: Campbell CJ, Fuentes A, MacKinnon KC, Panger M, Bearder SK. Primates in Perspective. New York: Oxford University Press, 186-200. Kirkpatrick RC, Long YC, Zhong T, Xiao L. 1998. Social organization and range use in the Yunnan snub-nosed monkey Rhinopithecus bieti. International Journal of Primatology, 19(1): 13-51.
Koenig A, Borries C. 2012. Social organization and male residence pattern in Phayre’s leaf monkeys. In: Kappeler PM, Watts DP. Long-Term Field Studies of Primates. Berlin: Springer, 215-236.
Koenig A, Larney E, Lu A, Borries C. 2004. Agonistic behavior and dominance relationships in female Phayre’s leaf monkeys: preliminary results. American Journal of Primatology, 64(3): 351-357.
Kumar A, Solanki GS. 2008. Population status and conservation of capped langurs (Trachypithecus pileatus) in and around Pakke Wildlife Sanctuary, Arunachal Pradesh, India. Primate Conservation, 23: 97-105.
Li GS, Chen YX, Sun WM, Wang XW, Huang ZP, Li YP, Xiang ZF, Ding W, Xiao W, Li M. 2014. Preliminary observation of population status and social organization of Rhinopithecus strykeri in Pianma Town, Nujiang County, China. Acta Theriologica Sinica, 34(4): 323-328. (in Chinese)
Li YB, Wei ZY. 2012. Survey on distdbufion and populafion of Trachypithecus francoisi in Nongdeng, Fusui of Guangxi. Journal of Anhui Agricultural Sciences, 40(26): 12952-12953, 12956. (in Chinese)
Li ZY, Rogers E. 2004. Social organization of white-headed langurs Trachypithecus leucocephalus in Fusui, China. Folia Primatologica, 75(2): 97-100.
Md-Zain BM, Ch’ng CE. 2011. The activity patterns of a group of cantor’s dusky leaf monkeys (Trachypithecus obscurus halonifer). International Journal of Zoological Research, 7(1): 59-61.
Medhi R, Chetry D, Bhattacharjee PC, Patiri BN. 2004. Status of Trachypithecus geei in a rubber plantation in Western Assam, India. International Journal of Primatology, 25(6): 1331-1337.
Mitani JC, Gros-Louis J, Manson JH. 1996. Number of males in primate groups: Comparative tests of competing hypotheses. American Journal of Primatology, 38(4): 315-332.
Mukherjee RP. 1978. Further observations on the golden langur (Presbytis geei KHAJURIA, 1956) with a note to capped langur (Presbytis pileatus BLYTH, 1843) of Assam. Primates, 19(4): 737-747.
Mukherjee RP. 1982. Survey of non-human primates of Tripura. Zoological Journal of the Linnean Society, 34: 70-81.
Mukherjee RP, Chaudhuri S, Murmu A. 1995. Population survey of South-Asian non-human primates in and around Darjeeling. Primate Report, 41: 23-32.
Newton PN. 1988. The variable social organization of Hanuman langurs (Presbytis entellus), infanticide, and the monopolization of females. International Journal of Primatology, 9(1): 59-77.
Newton PN, Dunbar RIM. 1994. Colobine monkey society. In: Davies AG, Oates JF. Colobine Monkeys: Their Ecology, Behaviour, and Evolution. Cambridge: Cambridge University Press, 311-346. Nunn CL. 1999. The number of males in primate social groups: A comparative test of the socioecological model. Behavioral Ecology and Sociobiology, 46(1): 1-13.
Pocock RI. 1939. The Fauna of British India, Including Ceylon and Burma. Mammalia 1: Primates and Carnivora (in part), Families Felidae and Viverridae. London: Taylor & Francis.
Preuschoft S, Paul A. 2000. Dominance, egalitarianism, and stalemate: An experimental approach to male-male competition in Barbary macaques. In: Kappeler PM. Primate Males: Causes and Consequences of Variation in Group Composition. Cambridge: Cambridge University Press, 205-216.
Qi XG, Garber PA, Ji WH, Huang ZP, Huang H, Zhuang P, Guo ST, Wang XW, He G, Zhang P, Li BG. 2014. Satellite telemetry and social modeling offer new insights into the origin of primate multilevel societies. Nature Communications, 5: Article number: 5296 doi: 10.1038/ncomms6296.
Qi XG, Li BG, Garber PA, Ji WH, Watanabe K. 2009. Social dynamics of the golden snub-nosed monkey (Rhinopithecus roxellana): female transfer and one-male unit succession. American Journal of Primatology, 71(8): 670-679. Roy D, Ashokkumar MA, Desai AA. 2012. Foraging ecology of Nilgiri Langur (Trachypithecus johnii) in Parimbikulam Tiger Reserve, Kerala, India. Asian Journal of Conservation Biology, 1(2): 92-102.
Solanki GS, Kumar A, Sharma BK. 2007. Reproductive Strategies of Trachypithecus pileatus in Arunachal Pradesh, India. International Journal of Primatology, 28(5): 1075-1083.
Solanki GS, Kumar A, Sharma BK. 2008. Winter food selection and diet composition of capped langur (Trachypithecus pileatus) in Arunachal Pradesh, India. Tropical Ecology, 49(2): 157-166.
Srivastava A, Dunbar RIM. 1996. The mating system of Hanuman langurs: a problem in optimal foraging. Behavioral Ecology and Sociobiology, 39(4): 219-226.
Stanford, CB. 1987. Ecology of the capped langur (Presbytis pileata) in Bangladesh. American Journal of Primatology, 12(3): 373.
Stanford, CB. 1988. Ecology of the capped langur and Phayre’s leaf monkey in Bangladesh. Primate conservation, (9): 125-128.
Stanford CB. 1991. Social dynamics of intergroup encounters in the capped langur (Presbytis pileata). American Journal of Primatology, 25(1): 35-47.
Sterck EHM, Hooff JARAM. 2000. The number of males in langur groups: monopolizability of females or demographic processes. In: Kappeler PM. Primate Males: Causes and Consequences of Variation in Group Composition. Cambridge: Cambridge University Press, 120-129.
Terborgh J. 1986. The social systems of new world primates: adaptionist’s view. In: Else JG, Lee PC. Primate Ecology and Conservation. Cambridge University Press, 199-211.
Timmins RJ, Steinmetz R, Poulsen MK, Evans TD, Duckworth JW, Boonratana R. 2013. The Indochinese silvered leaf monkey Trachypithecus germaini (Sensu lato) in Lao PDR. Primate Conservation, 26: 75-87.
Tsuji A, Widayati KM, Hadi I, Suryobroto B, Watanabe K. 2013. Identification of individual adult female Javan lutungs (Trachypithecus auratus sondaicus) by using patterns of dark pigmentation in the pubic area. Primates, 54(1): 27-31.
Vandercone RP, Dinadh C, Wijethunga G, Ranawana K, Rasmussen DT. 2012. Dietary Diversity and Food Selection in Hanuman Langurs (Semnopithecus entellus) and Purple-Faced Langurs (Trachypithecus vetulus) in the Kaludiyapokuna Forest Reserve in the Dry Zone of Sri Lanka. International Journal of Primatology, 33(6): 1382-1405.
van Hooff JARAM. 2000. Relationships among non-human primate males: A deductive framework. In: Kappeler PM. Primate Males: Causes and Consequences of Variation in Group Composition. Cambridge: Cambridge University Press, 183-191.
van Schaik CP, van Hooff JARAM. 1983. On the ultimate causes of primate social systems. Behaviour, 85(1): 91-117.
van Schaik CP, Hőrstermann M. 1994. Predation risk and the number of adult males in a primate group: a comparative test. Behavioral Ecology and Sociobiology, 35(4): 261-272.
van Schaik CP, Kappeler PM. 1997. Infanticide risk and the evolution of male–female association in primates. Proceedings of the Royal Society of London. Series B: Biological Sciences, 264(1388): 1687-1694.
Workman C. 2010. The Foraging Ecology of the Delacour’s Langur (Trachypithecus delacouri) in Van Long Nature Reserve, Vietnam. Ph. D. Dissertation, Duke University.
Wrangham RW. 1980. An ecological model of female-bonded primate groups. Behaviour, 75(3): 262-300.
Wroughton RC. 1915. Bombay Natural History Society’s Mammal Survey of India, Burma and Ceylon. Report No. 16. Dry Zone, central Burma and Mt. Popa. Journal of the Bombay Natural History Society. 23: 460-480
Zheng XJ. 1993. A primary study on the ecology of Presbytis phayrei shanicus. In: Ye ZZ. Langur Biology. Kunming: Yunnan Science and Technology Press, 52-69. (in Chinese)
Zhou QH, Huang CM, Li M, Wei FW. 2009. Sleeping site use by Trachypithecus francoisi at Nonggang Nature Reserve, China. International Journal of Primatology, 30(2): 353-365.
Received: 15 December 2014; Accepted: 05 March 2015
Foundation items: This study was supported by the Yunnan Green Environmental Development Fund, the Central Financial Assistance Fund, the National Natural Science Foundation of China (31160422, 30960084), the Program for New Century Excellent Talents in University (NCET-12-1079), the China Postdoctoral Science Foundation (2013M542379) and the Key Subject of Wildlife Conservation and Utilization in Yunnan Province
*Corresponding authors, E-mails: gcuilw@gmail.com; xiaowen.dali@ gmail.com
†Authors contributed equally to this work
- Zoological Research的其它文章
- A reflection on the significant findings published in Zoological Research over the past 35 years
- New observations - with older ones reviewed - on mass migrations in millipedes based on a recent outbreak on Hachijojima (Izu Islands) of the polydesmid diplopod (Chamberlinius hualienensis, Wang 1956): Nothing appears to make much sense
- Molecular characterization of an IL-1β gene from the large yellow croaker (Larimichthys crocea) and its effect on fish defense against Vibrio alginolyticus infection
- Selective recruitment of host factors by HSV-1 replication centers
- Establishment of HIV-1 model cell line GHOST(3) with stable DRiP78 and NHERF1 knockdown
- Autophagy prevents autophagic cell death in Tetrahymena in response to oxidative stress

