Subglottic Secretion Drainage for Preventing Ventilator Associated Pneumonia:A Meta-analysis
Rong Wang,Xiang Zhen,Bao-Yi Yang,Xue-Zhen Guo,Xue Zeng,Chun-Yan Deng
Department of Intensive Care Unit,Taihe Hospital,Hubei University of Medicine,Shiyan,Hubei 442000,China
Original article
Subglottic Secretion Drainage for Preventing Ventilator Associated Pneumonia:A Meta-analysis
Rong Wang,Xiang Zhen*1,Bao-Yi Yang**,1,Xue-Zhen Guo,Xue Zeng,Chun-Yan Deng
Department of Intensive Care Unit,Taihe Hospital,Hubei University of Medicine,Shiyan,Hubei 442000,China
A R T I C L E I N F O
Article history:
6 February 2015
Accepted 20 March 2015
Published 20 September 2015
Intensive care unit
Mechanical ventilation
Ventilator associated pneumonia
Meta-analysis
Objective:Ventilator associated pneumonia(VAP)has been shown to be associated with significant morbidity and mortality(Chastre and Fagon,2002;klompas,2007)among mechanically ventilated patients in the intensive care unit(ICU),with the incidence ranging from 9%to 27%;crude mortality ranges from 25%to 50%.1-3A meta-analysis of published studies was undertaken to combine information regarding the effect of subglottic secretion drainage(SSD)on the incidence of ventilated associated pneumonia in adult ICU patients.
Methods:Reports of studies on SSD were identified by searching the PUBMED,EMBASE,and COCHRANCE LIBRARY databases(December 30,2010).Randomized trials of SSD compared to usual care in adult mechanically ventilated ICU patients were included in this meta-analysis.
Results:Ten RCTs with 2,314 patients were identified.SSD significantly reduced the incidence of VAP[relative risk(RR)=0.52,95%confidence interval(CI):0.42-0.64,P<0.000 01].When SSD was compared with the control groups,the overall RR for ICU mortality was 1.00(95%CI,0.84-1.19)and for hospital mortality was 0.95(95%CI,0.80-1.13).Overall,the subglottic drainage effect on the days of mechanical ventilation was-1.52 days(95%CI,-2.94 to-0.11)and on the ICU length of stay(LOS)was-0.81days(95%CI,-2.33 to-0.7).
Conclusions:In this meta-analysis,when an endotracheal tube(ETT)with SSD was compared with an ETT without SSD,there was a highly significant reduction in the VAP rate of approximately 50%.Time on mechanical ventilation(MV)and the ICU LOS may be reduced,but no reduction in ICU or hospital mortality has been observed in published trials.
©2015Shanxi Medical Periodical Press.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Patients who require invasive mechanical ventilation(MV)are at risk for ventilator-associated pneumonia(VAP).4It has been shown to be associated with significant morbidity and mortality5,6among mechanically ventilated patients in the intensive care unit(ICU).It also has been proved to be associated with prolonged durations of MV,ICU stay and hospital stay,in addition to increased healthcare costs.7,8For all of these reasons,the prevention of VAP has been important in ICU clinician research.
Colonization of the upper respiratory tract(oropharaynx and trachea)secretions with potentially pathogenic organisms has been recognized as being a key factor in the pathogenesis of VAP.5,6These secretions have been shown to pool above the cuff of endotracheal tubes(ETT)prior to entering the lower respiratory tract as micro-aspirations.9Therefore,subglottic secretion drainage(SSD)with a specialized ETT that incorporates a suction port above the cuff has been proposed and tested as a method to attempt to reduce the incidence of VAP in ICU patients.10
Since the introduction of SSD into medical practice,many studies have examined the efficacy and effectiveness of SSD in preventing VAP.11It is necessary to update the studies to provide more solid evidence and to minimize the potential bias that is caused by limited publications.Thus,we performed an updated meta-analysis to investigate the results of published SSD trials and the effect of SSD on the incidence of VAP,ICU and hospital mortality,ICU length of stay(LOS),and duration of MV.
2.Materials and Methods
2.1.Search strategy
We followed the PRISMA guideline for reporting our meta-analysis.12Related articles that were published in English or Chinese were identified and selected by searching PUBMED,EMBASE,CINAHL and the Cochrane Central Register of Controlled Trials(December 31,2013).All of the bibliographies were also identified in the reference lists.Search strategies were adapted for all databases,and the following search terms were used:(1)glottis or suction or drainage and respiration,and(2)artificial or ventilation or intubation,and(3)mechanical,and(4)pneumonia.
2.2.Study selection criteria
All of the potential studies were retrieved and reviewed.We included studies if they:(1)were randomized controlled studies(RCTs),(2)studied adult critically ill patients who required invasive MV,(3)included ETTs with SSD in an experimental group compared to standard ETTs in a control group,and(4)reported on the incidence of VAP as defined by the investigators. Pregnant or lactating women and patients who were children were excluded.
2.3.Data extraction
Two reviewers independently extracted the following data:first author's surname,year of publication,setting of trials,number of participants in studies,method of subglottic drainage(intermittent or continuous and frequency),and definition of VAP and other interventions.The quality of trials was assessed with the method recommended by the Cochrane Collaboration for assessing the bias risk.13
2.4.Data synthesis and analysis
All of the data that were extracted were entered in the freeware program Review Manager(RevMan)Version 5.2(Copenhagen:The Nordic Cochrane Centre,The Cochrane Collaboration,2012).Differences were expressed as relative risks(RRs)with 95%confidence intervals(95%CIs)for dichotomous outcomes.Binary outcomes(i.e.,VAP and mortality)are reported as Mantel-Haenszel style risk ratios(RRs),whereas continuous outcomes are reported as inverse variance weighted mean differences.A fixed-effect model was used in cases of homogeneity(P value of χ2test>0.10 and I2≤50%),and a random-effects model was used in cases of significant heterogeneity(P value of χ2test<0.10 and I2>50%).Publication bias was assessed by the inspection of funnel plots,and asymmetry was assessed using the regression test suggested by Egger(a P-value<0.1 was considered to be evidence of funnel plot asymmetry).14
3.Results
From our search strategy,a total of 146 potential studies were selected for secondary review after excludingnon-randomized trials and review articles;twelve published randomized trials were included in the analysis,15,26but 2 studies15,16were excluded because the interventions might have influenced the effectiveness of SSD in reducing VAP and were therefore ineligible.We reviewed and abstracted data from each study prior to discussing the results(Table 1 and Table 2).
The included studies,with a randomized total of 2 314 patients,are summarized in Table 1.The main inclusion criterion was predicated on the duration of expected MV in 9 of the 10 studies:one study21had an expected duration of MV>24 h,four studies18,20,25,26had an expected duration of MV>48 h,and four studies17,22-24had an expected duration of MV>72 h.In all of the studies,ETTs with SSD were placed at the time of original intubation.The definitions of VAP always included radiologic criteria(new or persistent infiltrates on a chest radiograph)along with clinical and microbiological criteria.27The methods used to drain the subglottic secretion ranged from intermittent aspiration with a syringe to continuous suction(Table 1).
The pooled analyses across studies are graphically expressed in Fig.1 to 5.The vertical line in the centre indicates that the estimated effects are the same for both the intervention and control groups and is often called the line of no difference.The values on the left of the line favour SSD,and those on the right favour the control. The diamond on the lower aspect of the graph near the horizontal line represents pooled values.28
In the meta-analysis for the primary outcome,the overall risk ratio(RR)for VAP was 0.52(95%CI:0.42-0.64;P<0.000 01),with no heterogeneity(I2=0%)(Fig.1).It showed that SSD significantly reduced the incidence of VAP.Nine of these ten studies reported on mortality(either ICU or hospital);seven of these ten reported on ICU LOS.There was no effect on mortality in either the ICU(RR,1.00;95%CI,0.84-1.19;P= 0.91)or the hospital(RR,0.95;95%CI,0.80-1.13;P=0.98)(Figs.2 and 3,respectively).There was a significant reduction of 1.52 days in the duration of ICU LOS(95%CI,-2.94 to-0.11;P=0.03),although there was significant heterogeneity(I2=77%)When the study by Kollef and colleagues20was removed,I2=4% with z=5.83,P<0.000 01,which indicated adequate homogeneity for pooling the study results.The mean ICU stay length was 2.29 days shorter than that in the group that received subglottic drainage(Fig.4).Six studies reporting on the MV duration were able to be aggregated,and in these,MV duration was reduced(0.81 days;95%CI,-2.33-0.7;P=0.29),although there was significant heterogeneity(I2=94%)(Fig.5).
Moreover,two studies reported antibiotic utilization. Bouza et al.18reported a reduction in the number of defined daily doses of antibiotics with SSD(1 213 vs.1 932;P<0.001),whereas Lacherade et al.20did not find any difference in antibiotic utilization.Additionally,the re-intubation rates were also available in three studies:Kollef et al.19reported re-intubation rates of five out of 160(3.1%)patients in the SSD group and six out of 183(3.3%)patients in the control group,Bouza et al.18reported that re-intubation rates were 12 out of 331(3. 6%)patients in the SSD group and 14 out of 359(3.9%)patients in the control group,and Lacherade et al.20reported re-intubation rates of 21 out of 169(1. 2%)patients in the SSD group and 20 out of 164(1.2%)in the control group.
4.Discussion
The goal of this study was to examine the effectiveness of subglottic secretion aspiration in reducing the occurrence of VAP.In this meta-analysis,when an ETT with SSD was compared with an ETT without SSD,there was a highly significant reduction in the VAP rate of approximately 50%(RR=0.52,95%CI 0.42-0.64)and a reduction in the number of MV patients.No reduction in the risk of ICU or hospital mortality was observed in our study,although this may not be surprising given that adequately treated VAP may demonstrate little or no attributable mortality.27Although only a slight reduction in duration of MV(summary estimate nearly-1 days,95%CI,-2.33 to 0.7)and ICU LOS(about-1.5 days,95%CI,-2.94 to-0.11)was observed,significant variation existed among studies that were included in the meta-analysis. Although the number of RCTs increased to 10 and the number of randomized patients was over 2 300,our findings are similar to those of a previous smaller meta-analysis that found a 50%reduction in VAP,but no benefit in reducing ICU or hospital mortality;the number of ventilation days were also estimated to be reduced by approximately 2 days when the intervention and control groups were compared.28Muscedere and colleagues29also published an updated meta-analysis;the results of this more recent meta-analysis are also similar to ours.

Table 1Characteristics of the included trials.

Table 2Summary of the results of the included trials.
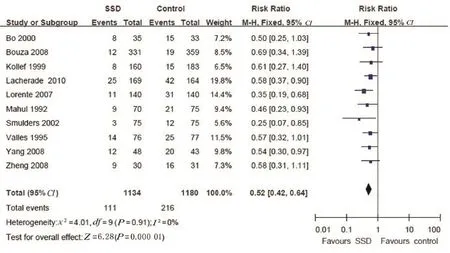
Fig.1.Rate of ventilator-associated pneumonia between groups with a subglottic secretion and without subglottic secretion.

Fig.2.Intensive care unit mortality compared between patients receiving endotracheal tubes with a subglottic secretion drainage and standard endotracheal tubes.

Fig.3.Hospital mortality compared between patients receiving endotracheal tubes with a subglottic secretion drainage and standard endotracheal tubes.

Fig.4.Intensive care unit length of stay compared between patients receiving endotracheal tubes with a subglottic secretion drainage and standard endotracheal tubes.IV.

Fig.5.Duration of mechanical ventilation compared between patients receiving endotracheal tubes with a subglottic secretion drainage and standard endotracheal tubes.
In addition,some complications that are attributed to SSD in the process of MV were also reported.In this meta-analysis,only three trials17-19mentioned the complications related to SSD;two17,18of them used continuous SSD,and no complications were observed.One19trial with intermittent SSD proved that the incidence of post-extubation events did not differ significantly,but safety concerns regarding SSD still existed.30In the study by Berra and colleagues,31all 30 sheep undergoing continuous SSD during the MV period suffered from different degrees of tracheal mucosal injury.Girou15also reported that approximately 40%of the patients with continuous SSD developed laryngeal oedema after extubation.Therefore,safety issues associated with SSD should be observed in a more systematic evaluation in the future.
In our meta-analysis,six studies17-19,24-26used continuous suction,and the other four studies22-25used intermittent suction.It has been reported that both intermittent and continuous SSD significantly reduced the incidence of VAP11;earlier studies were more likely to use intermittent SSD,whereas more recent studies utilized continuous aspiration.32However,no trial was conducted directly to compare the two methods and whether intermittent SSD was more effective than continuous SSD was unclear.11Thus,more RCTs that are powered to address this issue are warranted in further studies.
Because no efforts were made to locate either studies with negative results or unpublished studies,this metaanalysis may be at risk of publication bias.33Another potential limitation of this study may be the significant heterogeneity between study populations and methods of SSD,definitions of VAP,and other concurrent measures,which contributes to differing RRs across the trials.Additionally,the benefit of the drainage of subglottic secretions in preventing VAP appears to increase as the underlying risk increases in the underlying ICU population.
5.Conclusions
Our meta-analysis suggested that SSD reduced the incidence of VAP,shortened ventilation duration,and shortened the length of ICU stay.However,SSD did not improve ICU or hospital mortality,even though SSD has been suggested or recommended by several guidelines to prevent VAP.4,5,34,35Further research on methods to implement ETTs with SSD into practice and the safety issues of SSD is required.
Conflicts of interest
All contributing authors declare no conflicts of interest.
References
1.Rello J,Ollendorf DA,Oster G,et al.Epidemiology and outcomes of ventilator-associated pneumonia in a large US database.Chest.2002;122:2115-2121.
2.Tablan OC,Anderson LJ,Besser R,Bridges C,Hajjeh R. Guidelines for preventing health-care-associated pneumonia,2003:recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee.MMWR Recomm Rep.2004;53:1-36.
3.American Thoracic Society;Infectious Diseases Society of A-merica.Guidelines for the management of adults with hospitalacquired, ventilator-associated, andhealthcare-associated pneumonia.Am J Respir Crit Care Med.2005;171:388-416.
4.Vincent JL,Rello J,Marshall J,et al.International study of the prevalence and outcomes of infection in intensive care units.J Am Med Assoc.2009;302:2323-2329.
5.Chastre J,Fagon JY.Ventilator-associated pneumonia.Am J Respir Crit Care Med.2002;165:867-903.
6.Klompas M.Does this patient have ventilator-associated pneumonia?J Am Med Assoc.2007;297:1583-1593.
7.Safdar N,Dezfulian C,Collard HR,et al.Clinical and economic consequences of ventilator-associated pneumonia:a systematic review.Crit Care Med.2005;33:2184-2193.
8.Muscedere JG,Martin CM,Heyland DK.The impact of ventilator-associated pneumonia on the Canadian health care system.J Crit Care.2008;23:5-10.
9.Greene R,Thompson S,Jantsch HS,et al.Detection of pooled secretions above endotracheal-tube cuffs:value of plain radiographs in sheep cadavers and patients.Am J Roentgenol. 1994;163:1333-1337.
10.Dezfulian C,Shojania K,Collard HR,Kim HM,Matthay MA,Saint S.Subglottic secretion drainage for preventing ventilator-associated pneumonia:a meta-analysis.Am J Med.2005;118:11-18.
11.Wang F,Bo LL,Tang L,et al.Subglottic secretion drainage for preventing ventilator-associated pneumonia:an updated meta-analysis of randomized controlled trials.J Trauma Acute Care Surg.2012;72:1276-1285.
12.Liberati A,Altman DG,Tetzlaff J,et al.The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions:explanation and elaboration.Ann Intern Med.2009;151:w65-94.
13.Cochrane Collaboration,Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0;2011.[updatedMarch 2011].http://www.cochrane-handbook.org.
14.Egger M,Davey Smith G,Schneider M,Minder C.Bias in meta-analysis detected by a simple,graphical test.Br Med J. 1997;315:629-634.
15.Girou E,Buu-Hoi A,Stephan F,et al.Airway colonisation in long-term mechanically ventilated patients.Effect of semirecumbent position and continuous subglottic suctioning.Intensive Care Med.2004;30:225-233.
16.Liu QH,He LX,Hu BJ,et al.Comprehensive prevention and pathogenesis of ventilator associated pneumonia in elderly patients:a prospective,randomized,case-control clinical trial.Chin J Intern Med.2006;45:717-720.
17.Bo H,He L,Qu J.Influence of the subglottic secretion drainage on the morbidity of ventilator associated pneumonia in mechanically ventilated patients.Chin J Tuber Respir Dis. 2000;23:472-474.
18.Bouza E,Perez MJ,Munoz P,Rincon C,Barrio JM,Hortal J.Continuous aspiration of subglottic secretions in the prevention of ventilator-associated pneumonia in the postoperative period of major heart surgery.Chest.2008;134:938-946.
19.Kollef MH,Skubas NJ,Sundt TM.A randomized clinical trial of continuous aspiration of subglottic secretions in cardiac surgery patients.Chest.1999;116:1339-1346.
20.Lacherade JC,De Jonghe B,Guezennec P,et al.Intermittent subglottic secretion drainage and ventilator-associated pneumonia:a multicenter trial.Am J Respir Crit Care Med. 2010;182:910-917.
21.Lorente L,Lecuona M,Jimenez A,Mora ML,Sierra A.Influence of an endotracheal tube with polyurethane cuff and subglottic secretion drainage on pneumonia.Am J Respir Crit Care Med.2007;176:1079-1083.
22.Mahul P,Auboyer C,Jospe R,et al.Prevention of nosocomial pneumonia in intubated patients:respective role of mechanical subglottic secretions drainage and stress ulcer prophylaxis.Intensive Care Med.1992;18:20-25.
23.Smulders K,vander Hoeven H,Weers-Pothoff I,Vandenbroucke-Grauls C.A randomized clinical trial of intermittent subglottic secretion drainage in patients receiving mechanical ventilation.Chest.2002;121:858-862.
24.Valles J,Artigas A,Rello J,et al.Continuous aspiration of subglottic secretions in preventing ventilator-associated pneumonia.Ann Intern Med.1995;122:179-186.
25.Yang CS,Qiu HB,Zhu YP,Huang YZ,Xu XT,Gao L. Effect of continuous aspiration of subglottic secretions on the prevention of ventilator-associated pneumonia in mechanically ventilated patients:a prospective,randomized,controlled clinical trial.Chin J Intern Med.2008;47:625-629.
26.Zheng RQ,Lin H,Shao J,Chen QH,Lu NF,Yu JQ.A clinical study of subglottic secretion drainage for prevention of ventilation associated pneumonia.Chin Crit Care Med.2008;20:338-340.
27.Muscedere JG,Day A,Heyland DK:Mortality,attributable mortality,and time to clinical events as end points for clinical trials of ventilator-associated pneumonia and hospital-acquired pneumonia.Clin Infect Dis.2010;51:S120-S125.
28.Montoni V,Ioannides J,Cook D,Guyatt CG.Fixed-effects and random-effects models.In:Guyatt GH,Rennie D,Meade MO,Cook D,eds.Users’Guide to the Medical Literature:a Manual for Evidence-based Clinical Practice.2nd ed.New York,NY:Mc-Graw Hill;2008.
29.Muscedere J,Rewa O,McKechnie K,Jiang X,Laporta D,Heyland DK.Subglot-tic secretion drainage for the prevention of ventilator-associated pneumonia:a systematic review and meta-analysis.Crit Care Med.2011;39:1985-1991.
30.Deem S,Treggiari MM.New endotracheal tubes designed to prevent ventilator-associated pneumonia:do they make a difference?Respir Care.2010;55:1046-1055.
31.Berra L,De Marchi L,Panigada M,Yu ZX,Baccarelli A,Kolobow T.Evaluation of continuous aspiration of subglottic secretion in an in vivo study.Crit Care Med.2004;32:2071-2078.
32.Leasure AR,Stirlen J,Lu SH.Prevention of ventilator-associated pneumonia through aspiration of subglottic secretions:a systematic review and meta-analysis.Dimens Crit Care Nurs. 2012;31:102-117.
33.Montori VM,Ioannides J,Guyatt GH.Chapter 20.1.Reporting bias.In:Guyatt GH,Rennie D,Meade MO,Cook DJ,eds.Users’Guide to the Medical Literature:a Manual for Evidence-Based Clinical Practice.2nd ed.New York,NY: McGraw-Hill;2008.
34.Dodek P,Keenan S,Cook D,et al.Evidence-based clinical practice guideline for the prevention of ventilator-associated pneumonia.Ann Intern Med.2004;141:305-313.
35.Muscedere J,Dodek P,Keenan S,et al.Comprehensive evidence-based clinical practice guidelines for ventilator-associated pneumonia:diagnosis and treatment.J Crit Care.2008;23:138-147.
7 December 2014
in revised form
.
E-mail address:syzx481@163.com(X.Zhen).
.
E-mail address:yangbaoyigodfred@163.com(B.-Y.Yang).
1Authors Xiang Zhen and Bao-Yi Yang contributed equally to this study.
Peer review under responsibility of Shanxi Medical Periodical Press.
http://dx.doi.org/10.1016/j.cnre.2015.03.001
2095-7718/©2015 Shanxi Medical Periodical Press.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
- Frontiers of Nursing的其它文章
- The Outcomes and Influencing Factors of Telecare Managing Patients with Type 2 Diabetes
- Progress in Traditional Chinese and Western Medicine Treatments and Nursing Care of Knee Osteoarthritis☆
- Study on the Relationships between Nurses'Job Burnout and Subjective Well-being
- Practice and Evaluation:Management of Acupuncture Needle Disinfection and Sterilization
- Effect of Feeding Management on Aspiration Pneumonia in Elderly Patients with Dysphagia☆
- Active Learning Improves Nursing Student Clinical Performance in an Academic Institution in Macao☆

