Real-Time Observation on Water-Soluble Ions of PM2.5 in Beijing under the Influences of Different Air Masses in Summer
State Key Laboratory of Atmospheric Boundary Layer Physics and Atmospheric Chemistry (LAPC), Institute of Atmospheric Physics,Chinese Academy of Sciences, Beijing 100029, China
1 Introduction
Atmospheric particles are of major concern because of their effects on climate, the environment, and human health (Schwartz, 1996, Petit et al., 1999, Watson, 2002,Tan et al., 2009). Water-soluble inorganic ions are important components of atmospheric fine particles (PM2.5)(Wang et al., 2005b) and play key roles in the formation of acid rain and haze pollution in cities (Schwartz, 1996,Wang et al., 2012). To obtain the concentration levels of water-soluble ions in the atmosphere, offline methods have been applied in previous studies that generally use deionized water to extract the water-soluble components on sample membranes before quantitative analysis conducted through chemical methods (Pathak and Chan,2005, Wang et al., 2005a). However, filter samples must be performed manually, and the sampling frequency is low. Subsequent chemical analysis processes also require additional times and materials, which restrict its application in rapid or sudden pollution incidents. More importantly, the filter sampling process is very difficult for 24-h continuous sampling, therefore, it failed to obtain the diurnal variations of water soluble ions. To overcome the limitations of manual filter sampling, automatic online observation instruments have been developed.
Using the principles of condensation growth and inertial impaction of atmospheric fine particles driven by airstream, the research group of Yuesi WANG, Institute of Atmospheric Physics, Chinese Academy of Sciences, independently developed the rapid collection of fine particles (RCFP) system in 2002 (Wen et al., 2006). In this method, fine particles are quickly contained in aqueous solution, which is transferred by a peristaltic pump and coupled with ion chromatography (IC) for quantitative analysis. The RCFP-IC integrated system enables fast,accurate, and continuous measure the concentrations of water-soluble inorganic ions in fine particles.
In this study, RCFP-IC was used to observe the concentrations of water-soluble inorganic ions in PM2.5in real-time and combined with backward trajectory analysis variation characteristics of water-soluble inorganic ions during 12–18 July 2010. Moreover, we used high temporal resolution of RCFP-IC to analyze the diurnal variations of eight water-soluble inorganic ions including,K+, Mg2+, Ca2+, Cl–,,, and. This study is important for deeper understanding the chemical composition of atmospheric fine particles in Beijing.
2 Methods and sampling
2.1 Sampling location and sampling time
Sampling points were located at the roof of the Chinese Ecosystem Research Network (CERN) Atmospheric subcenter building approximately 11 m above the ground.This location (39°58′27.6″N, 116°22′17.5″E) is between North 3rd Ring Road and North 4th Ring Road approximately 1 km from the 3rd Ring Road and 200 m from the Badaling. A highway runs north-south to the east of the sampling location, and Beitucheng West Road runs eastwest 50 m to the north.
Sampling time was 12–18 July 2010. The time resolution of RCFP-IC online observation was 15 min. Realtime measurement was conducted to determine atmospheric concentrations of nine water-soluble inorganic ions. Each ion yielded 672 valid datasets. To facilitate analysis, the 15-min values were averaged into an hour mean. Essentially, we analyzed and discussed 168 values per ion in this study.
2.2 Methods and instruments
The RCFP-IC system was used to run the automatic real-time online measurement of water-soluble inorganic ions concentrations in PM2.5. Atmospheric particles were processed through a series of annular denuders to remove acidic and basic gases before entering the system. ICS-90 ion chromatography of Dionex Corporation was used for IC analysis. IonPac AS14, 4 × 250 mm was used as the anion analysis column. The base eluent was 3.5 mmol L−1Na2CO3+1.0 mmol L−1NaHCO3. IonPac CS12A, 4 × 250 mm was used as the cation analysis column. The acid eluent was 20 mmol L−1methyl sulfonic acid.
2.3 RCFP-IC quality control
RCFP-IC quality control included a combination of external and internal standard methods for quantitative analysis of water-soluble ions and for flow calibration of liquid and gas, respectively. The internal standard was 100 × 10−6V V−1(1mL LiF 1mL deionized water) LiF solution. Additional Li+was applied to correct the gas and liquid flow generated by the suction and peristaltic pumps. To obtain detection limits, 1 g L−1ion standard solution was diluted to 1 mg L−1and was progressively diluted until the ion peak detected by IC was approximately three times higher than the noise peak. This dilution process was repeated six times and measured to calculate standard deviation. Three times of standard deviation corresponded to the detection limit of RCFP-IC. The detection limits of the nine water-soluble inorganic ions analyzed in this study were all below 0.3 μg m−3.
The background blank of the RCFP-IC system primarily includes trace ions on the inlet membrane. For the blank test, 1 mL deionized water with resistivity 18.2 MΩcm−1was added to replace samples. This process was repeated three times. During the experiment, the system blanks were lower than 1 μg m−3. These blanks were removed from all experimental data.
3 Results and discussion
3.1 Meteorological conditions during observation and air mass back trajectories
Table 1 shows the meteorological conditions during the observation period. The average daily temperature was 24°C–28°C during the observation period, and relative humidity was 69%–86%. Figure 1 shows air mass back trajectories during the observation period. That originated from southeast Bohai Bay during 12–15 July. Affected by air masses from the ocean, the average temperature during these four days was 25°C , and the relative humidity was 83%. This air mass also passed through the Tianjin area and might have acquired characteristics of Tianjin pollution. The air masses originating from the southwestern Hebei Province 16–18 July were affected by air masses from the land, and the average temperature during these four days was 28°C, which was higher than that during the previous four days. The relative humidity was 75%,which was lower than that during the previous four days.This air mass passed through Shijiazhuang and Baoding and might have acquired pollution characteristics of this region.
3.2 Concentration trends of water-soluble ions
Due to the influences of various air mass, the concentration changes of water-soluble inorganic ions in PM2.5differed. Under the control of the marine air mass, the water-soluble inorganic ions concentrations were relatively stable, and the standard deviations of concentrations were low, except for. Under the control of the continental air mass crosscurrent, the concentrations of water-soluble inorganic ions fluctuated dramatically, and the standard deviations were high (shown on Table 2).Whether total mass concentration of PM2.5(measured by RP1405DF produced by Rupprecht & Patashnik) or the water-soluble inorganic ions concentrations, part of PM2.5,had the same change trend, and they both were higher under the control of continental air mass than those under the control of the marine air mass (shown on the supplementary material).
In Fig. 2, the concentrations of,, andwere 16%, 46%, and 42% higher under the influence of the continental air mass than those of the marine air mass,respectively. A comparison of the various concentrations of the three ions under the control of two air masses revealed that the variations of average concentration ofwere more stable than those ofand. But the amplitude ofunder the control of the continental air mass was relatively intense. Using 17 July and 18 July as examples, the greatest change in concentration ofwas higher than 80 μg m−3within 12 h. This result occurred primarily because of the response ofconcentration to wet scavenging and its sensitivity to temperature change. Furthermore, the strong photochemical reaction in summer resulted in high concentrations ofand large amplitude.
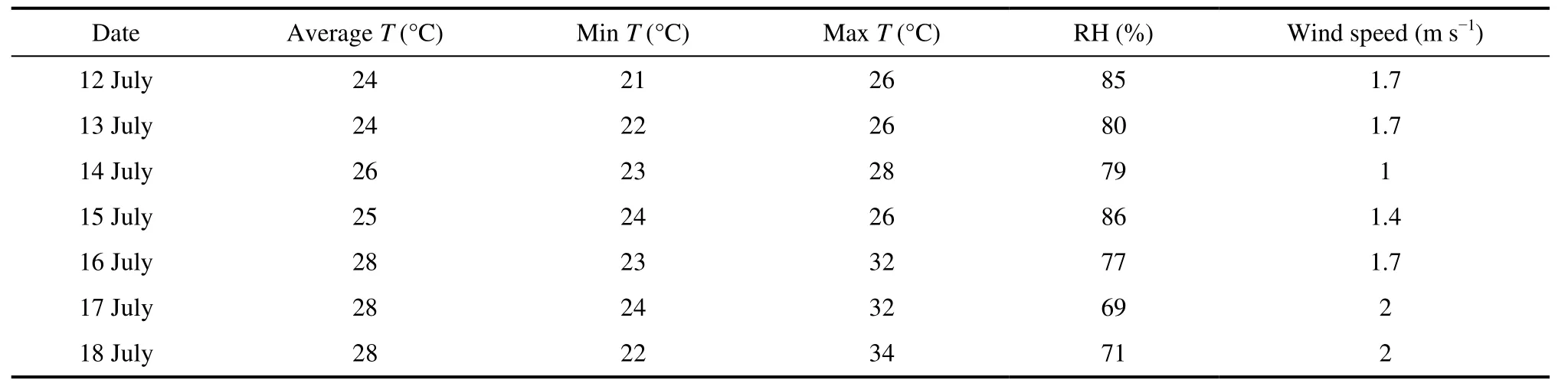
Table 1 Meteorological conditions during the observation period.
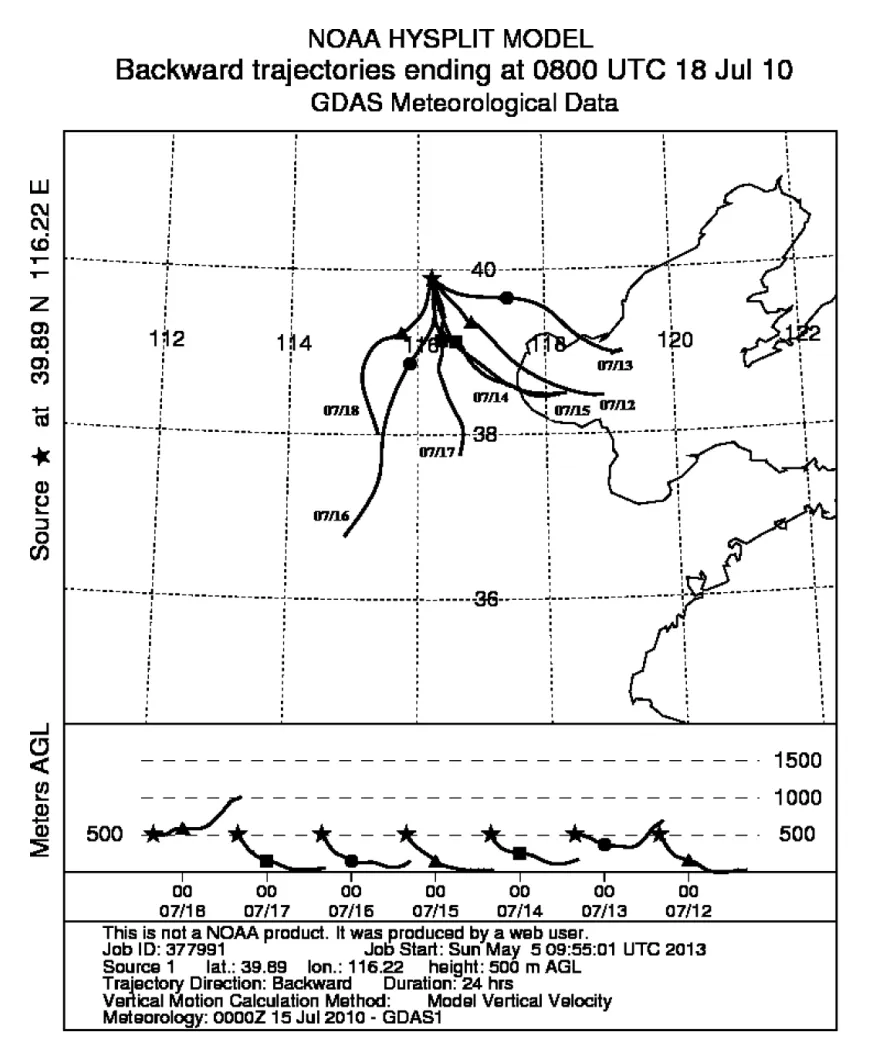
Figure 1 Air mass back trajectories recorded during the observation period.
3.2.2 Cl−, Na+, K+, Mg2+, and Ca2+
The changes of Cl−and Na+were not significant under the influence of either air mass. In particular, Na+showed almost no change. This result indicates that in summer in Beijing, the Na+in atmospheric fine particles does not originate from sea salt. K+appears mainly through biomass burning (Liu et al., 2000) and winter heating pollution (Mueller et al., 2006). During the period of observation, the concentration of K+under the control of the continental air mass was more than twice that under the control of the marine air mass, which implies that anthropogenic emission is the controlling factor of K+.
Under the control of the continental air mass, concentrations of Mg2+and Ca2+were higher than those under the control of the marine air mass. Mg2+and Ca2+mainly originated from soil and construction dust (Karageorgos and Rapsomanikis, 2007, Mkoma et al., 2009). The source characteristics resulted in high concentrations of these ions under the control of the continental air mass.From 15 to 16 July, precipitation led to significant decreases in Mg2+and Ca2+concentrations. In particular,these concentrations were less than 0.5 μg m−3in the morning of 16 July (shown on Fig. 3).
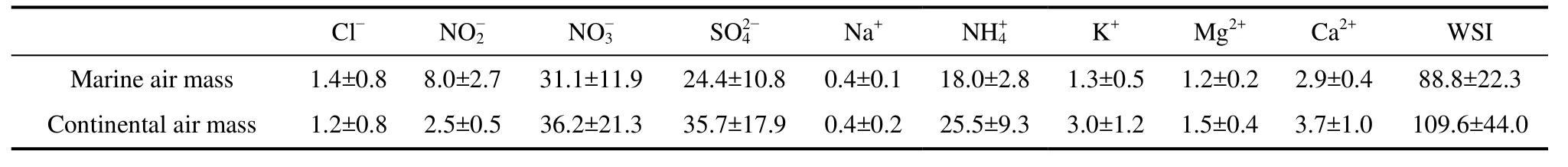
Table 2 Mass concentrations of water-soluble ions during observation period (units: μg m−3 ).
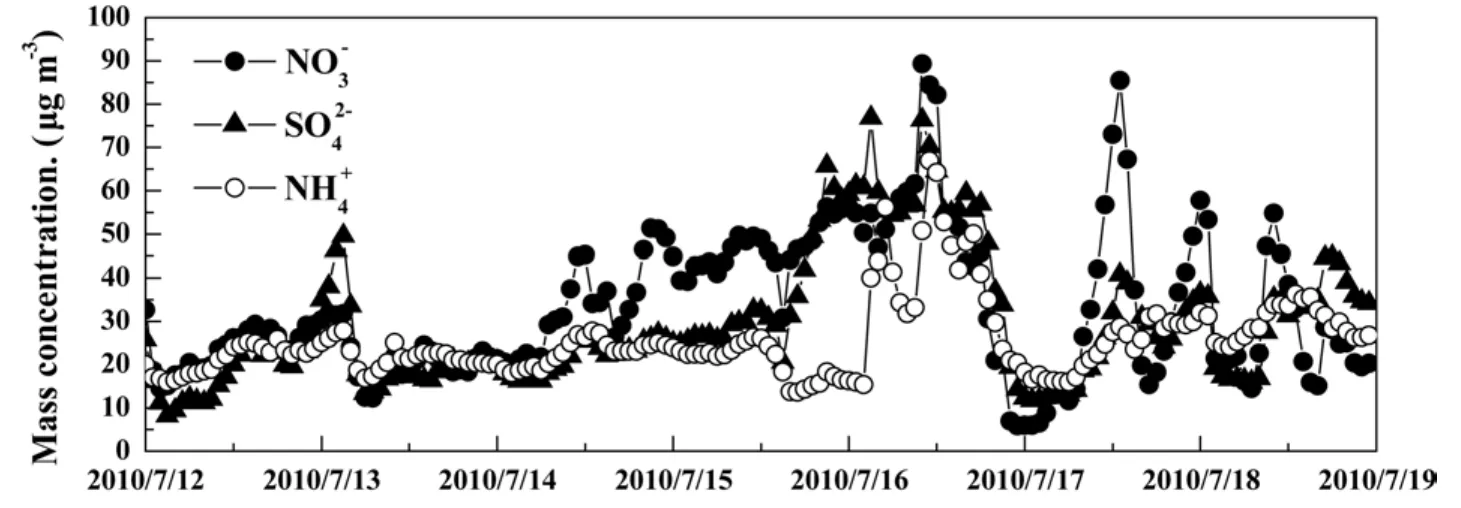
Figure 2 Time series of, , and during the observation period.
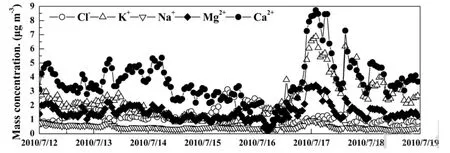
Figure 3 Time series of Cl−, Na+, K+, Mg2+, and Ca2+ during the observation period.
From Fig. 4 we can see the changes in characteristics ofdiffered from other ions during the observation period. Its concentration was significantly higher under the control of the marine air mass. Atmosphericoccurs primarily in the form of nitrite (HONO) (Harrison et al., 1996) due to the effect of NO2heterogeneous hydrolysis in the atmosphere (Indarto, 2012). Thus, water plays a significant role in HONO formation. Under the control of the marine air mass, atmospheric relative humidity was high and provided a wealth of reactants to HONO formation, and the relatively low temperatures inhibited nitrite conversion to gas. As a result, the concentration ofunder the control of marine air mass was 3.2 times than that under control of the continental air mass.
3.3 Diurnal variation of soluble ions
Eight water-soluble ions showed obvious diurnal variation in Fig. 5. In addition to, the concentrations of other ions showed diurnal variations which were low at night and high during the day. The concentrations of,Cl−, andbegan to increase at approximately 7:00 CST (China Standard Time). The source intensity increased when human activities began, resulting in these ions concentrations accumulation. The three ions reached peak levels before noon, and decreased in the afternoon because stronger light during these hours resulted in particle conversion to gases such as HNO3, HCl, and NH3.When light weakened at sunset, gaseous substances transferred to particles, thereby increasing concentration.Our results on diurnal variations of, Cl−, andand concentrations levels were consistent with the findings of Wu et al. (2009) in Beijing in 2002.showed significant bimodal distribution with concentration accumulation during rush hours in morning and nightfall: the concentration reached its first peak before noon, and its second peak occurred between 17:00 and 20:00 CST. The effects of anthropogenic sources were more obvious than meteorological conditions withbecause of its stable chemical properties. Because the emission sources of Mg2+and Ca2+did not change significantly, their diurnal variations were controlled primarily by weather conditions. During the day, these ions ascended along with the rising boundary layer. At approximately 15:00 CST, the concentrations of these ions reached peak levels when the boundary layer reached maximum height, and then decreased when the boundary layer fell. The form of K+indicated that emission sources and weather conditions were factors together. Its peak appeared at approximately 13:00 CST. Becauseis strongly affected by light, a very clear “S” type of diurnal variation was observed. Its concentration accumulated overnight and reached peak before sunrise, underwent photolysis after sunrise, and reached its valley at sunset. After a day of consumption,the accumulation process resumed after sunset.
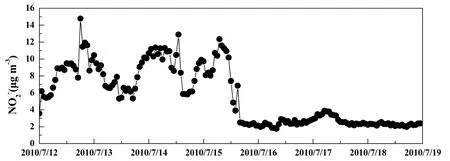
Figure 4 Time series of during the observation period.
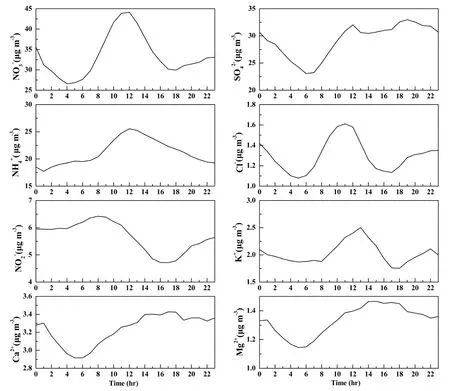
Figure 5 Diurnal variation of water-soluble ions during the observation period.
4 Summary
The concentrations of water-soluble ions in PM2.5in summer in Beijing differed according to air mass types.Concentrations of most water-soluble ions were higher with relatively acute fluctuation under the control of the continental air mass. However, the concentration ofwas significantly higher under the control of the marine air mass. Eight water-soluble ions showed obvious diurnal variation:, Cl−,, Mg2+, Ca2+, and K+showed the forms with a single peak., Cl−,reached peak levels before noon, while Mg2+, Ca2+, and K+reached peak in the afternoon.showed a significant bimodal distribution, andpresented a very clear “S” type of diurnal variation.
Acknowledgements.This study was funded by the National Natural Science Foundation of China (41175107 and 41275139).The authors thank Guangren LIU and Tianxue WEN for their fruitful help in their technique assistance.
Harrison, R. M., J. D. Peak, and G. M. Collins, 1996: Tropospheric cycle of nitrous acid, J. Geophys. Res., 101(D9), 14429–14439.
Indarto, A., 2012: Heterogeneous reactions of HONO formationfrom NO2and HNO3: A review, Res. Chem. Intermed., 38,1029–1041.
Karageorgos, E. T., and S. Rapsomanikis, 2007: Chemical characterization of the inorganic fraction of aerosols and mechanisms of the neutralization of atmospheric acidity in Athens, Greece, Atmos. Chem. Phys., 7(11), 3015–3033.
Liu, X. D., P. Van Espen, F. Adams, et al., 2000: Biomass burning in southern Africa: Individual particle characterization of atmospheric aerosols and savanna fire samples, J. Atmos. Chem.,36(2), 135–155.
Mkoma, S. L., W. Wang, and W. Maenhaut, 2009: Seasonal variation of water-soluble inorganic species in the coarse and fine atmospheric aerosols at Dar es Salaam, Tanzania, Nucl.Instrum. Methods Phys. Res., 267(17), 2897–2902.
Mueller, M., K.-J. Wolf, A. Smeda, et al., 2006: Release of K, Cl,and S species during co-combustion of coal and straw, Energy Fuels, 20(4), 1444–1449.
Pathak, R. K., and C. K. Chan, 2005: Inter-particle and gas-particle interactions in sampling artifacts of PM2.5in filter-based samplers, Atmos. Environ., 39(9), 1597–1607.
Petit, J. R., J. Jouzel, D. Raynaud, et al., 1999: Climate and atmospheric history of the past 420,000 years from the Vostok ice core, Antarctica, Nature, 399(6735), 429–436.
Schwartz, J., D. W. Dockery, and L. M. Neas, 1996: Is daily mortality associated specifically with fine particles? J. Air Waste Manage. Assoc., 46(10), 927–939.
Tan, J., J. Duan, K. He, et al., 2009: Chemical characteristics of PM2.5during a typical haze episode in Guangzhou, J. Environ.Sci., 21(6), 774–781.
Wang, Y., W. Yu, Y. Pan, et al., 2012: Acid neutralization of precipitation in Northern China, J. Air Waste Manage. Assoc.,62(2), 204–211.
Wang, Y., G. Zhuang, Y. Sun, et al., 2005a: Water-soluble part of the aerosol in the dust storm season—evidence of the mixing between mineral and pollution aerosols, Atmos. Environ., 39(37),7020–7029.
Wang, Y., G. Zhuang, A. Tang, et al., 2005b: The ion chemistry and the source of PM2.5aerosol in Beijing, Atmos. Environ., 39(21),3771–3784.
Watson, J. G., 2002: Visibility: Science and regulation, J. Air Waste Manage. Assoc., 52(6), 628–713.
Wen, T. X., Y. S. Wang, S. Y. Chang, et al., 2006: On-line measurement of water-soluble ions in ambient particles, Adv.Atmos. Sci., 23(4), 586–592.
Wu, Z., M. Hu, K. Shao, et al., 2009: Acidic gases, NH3and secondary inorganic ions in PM10during summertime in Beijing,China and their relation to air mass history, Chemosphere, 76(8),1028–1035.
 Atmospheric and Oceanic Science Letters2014年1期
Atmospheric and Oceanic Science Letters2014年1期
- Atmospheric and Oceanic Science Letters的其它文章
- Interdecadal Variability in Large and Small Warm Pools in Western Pacific and Their Association with Rainfall Anomalies
- Uncertainties in Quantitatively Estimating the Atmospheric Heat Source over the Tibetan Plateau
- Evaluation of Reanalysis Products with in situ GPS Sounding Observations in the Eastern Himalayas
- Sensitivity of Precipitation in Aqua-Planet Experiments with an AGCM
- Aerosol Direct Radiative Forcing over Shandong Peninsula in East Asia from 2004 to 2011
- Robustness of Precipitation Projections in China: Comparison between CMIP5 and CMIP3 Models
