Synthesis, Crystal Structure and Neuroprotection of Methyl 2-((4-(2-(2-Methylphenoxy)acetyl)-piperazin-1-yl)-methyl)benzoate①
ZHONG Yn WANG Yo WANG Jing XU Yi LI Ping WU Bin② (Shool of Chemistry nd Chemil Engineering, Southest University, Nnjing 211189, Chin) (Shool of Phrmy, Nnjing Medil University, Nnjing 211166, Chin) (Shool of Bsi Medil Sienes, Nnjing Medil University, Nnjing 210029, Chin)
1 INTRODUCTION
In China, stroke is the second cause for mortality in all diseases. Particularly, ischemic strokes account for 60~80% of these strokes. With the rapid increase of aging population in China, stroke has become a critical cause for the health of the old,and is becoming a major burden facing the government and health providers[1-6]. Previous work reported by our group suggested that cinnamide scaffold often affords neuroprotective compounds(Fig. 1)[7]. To further elucidate the structure-activity relationship, we designed and synthesized a novel series of phenoxyacetamide derivatives using-OCH2CO- connecting bridge as the surrogate replacing -CH=CHCO- moiety of cinnamide according to the bioisosterism approach[8-11].
In this paper, we are delighted to report the synthesis, crystal structure and neuroprotective effects of the title compound methyl 2-((4-(2-(2-methylphenoxy)acetyl)piperazin-1-yl)methyl)benzoate for the first time, which is a new compound of phenoxyacetamide derivatives.
2 EXPERIMENTAL
2.1 Materials and apparatuses
All reagents were obtained from commercial sources and used without further purification. Fetal bovine serum (FBS) was obtained from HyClone(Logan, UT, USA). Horse serum (HS), penicillin and streptomycin were obtained from Gibco BRL(Div. of Invitrogen, Gaithers-burg, MD, USA).Melting points were measured on an Electrothermal WRR-401 digital apparatus and uncorrected. The1H and13C NMR spectra were measured with a Bruker ACF-300 MHz spectrometer and the chemical shifts are expressed in ppm relative to TMS. High resolution mass spectra of the compound were recorded on a MALDI Micro MX instrument.
2.2 Synthesis
The title compound was prepared by the method outlined in Scheme 1. A mixture of 2-methylphenol(1, 49 mmol), ethyl chloroacetate (58.8 mmol), and potassium carbonate (49 mmol) in acetonitrile (30 mL) was heated under reflux for 5 h. After completion of the reaction, the reaction mixture was filtered, the filtrate was evaporated, and the residue was distilled under reduced pressure (102~106 ℃/1 mmHg) to afford 2 as a pale yellow oil. The amide 3 was synthesized according to the general procedure reported by Chou et al[12].
Methyl 2-methylbenzoate (4, 70.0 mmol), N-bromosuccinimide (NBS, 70.0 mmol), and azobisisobutyronitrile (AIBN, 7.0 mmol) in carbon tetrachloride (108 mL) were heated to reflux under irradiation conditions for 1 h. Cyclohexane (108 mL) was added to the mixture and the reaction was stirred for 10 min. After removal of precipitate by filtration, the filtrate was concentrated and recrystallized in ethanol to obtain compound 5 as a white solid[13,14].
Under a nitrogen atmosphere, 1-(piperazin-1-yl)-2-(2-methylphenoxy)ethanone (3, 17.0 mmol),methyl 2-(bromomethyl)benzoate (5, 17.0 mmol),and triethylamine (2.4 mL, 17.0 mmol) in acetonitrile (90 mL) were heated to reflux for 20 h.The reaction mixture was filtered, and the filtrate was concentrated and chromatographed on a silica gel column (ethyl acetate/hexane (2:1)) to give the title compound 6 as a white solid in a yield of 72.5%.m.p.: 136.4~138.5 ℃.1H NMR (CDCl3, 300 MHz)δ: 2.23 (s, 3H, PhCH3), 2.39~2.42 (m, 4H,N(CH2)2), 3.54~3.61 (m, 4H, CON(CH2)2), 3.77 (s,2H, PhCH2), 3.87 (s, 3H, COOCH3), 4.67 (s, 2H,OCH2), 6.81~7.73 (m, 8H, Ar-H);13C NMR(CDCl3, 75 MHz) δ: 16.23, 42.24, 45.52, 51.96,52.62, 53.10, 60.23, 67.96, 111.03, 121.27, 126.63,126.95, 127.19, 129.84, 129.88, 130.90, 131.14,131.48, 138.78, 155.95, 166.56; HRMS (ESI, m/z):Calcd. for C22H27N2O4[M + H]+383.1971. Found:383.1975.
Single crystals suitable for X-ray diffraction were obtained by slow evaporation from an ethyl acetate/hexane (4:1) solution of the title compound at room temperature.
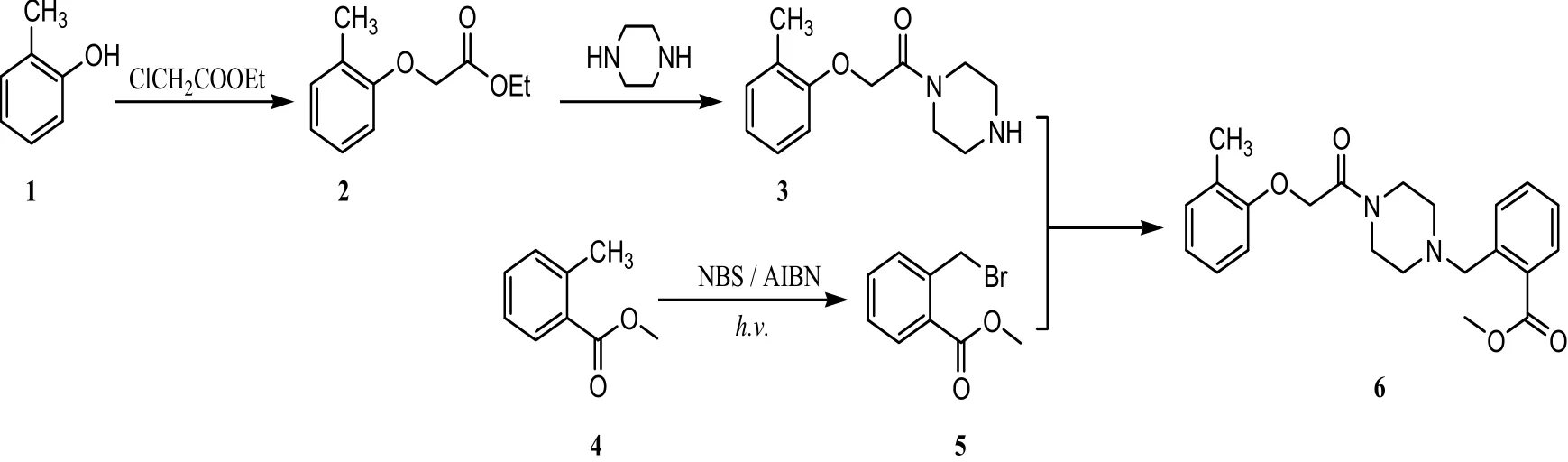
Scheme 1. Synthesis of the title phenoxyacetamide compound 6
2.3 X-ray crystal structure determination
Suitable crystals were obtained by slow evaporation from an ethyl acetate and hexane solution at room temperature. A colorless crystal of the title compound (0.30mm × 0.20mm × 0.10mm) was selected and mounted on the top of a glass fiber. The single-crystal X-ray data were collected on an Enraf-Nonius CAD4/PC four-circle diffractometer.All diffraction measurements were performed at room temperature (293 K) using graphite-mono-chromated MoKα radiation (λ = 0.71073 Å).Reflection data were recorded in the rotation mode using the ω scan mode. Unit cell parameters were determined from least-squares refinement of setting angels with θ falling in the range of 1.97≤θ≤25.41º (0≤h≤13, 0≤k≤16, –16≤l≤15). The structure was solved by direct methods with SHELXS-97 and refined against F2by full-matrix least-squares method on the positional and anisotropic temperature parameters of non-hydrogen atoms,or equivalently corresponding to 254 crystallographic parameters, using SHELXL-97[15]. All H atoms were positioned geometrically and treated using a riding model by fixing the bond lengths at 0.93 CH atoms. A totle of 3836 reflections were collected, of which 3642 were independent (Rint=0.0292) and 2343 were observed with I > 2σ(I). The final refinement gave R = 0.0602, wR = 0.1533 (w =1/[σ2(Fo2) + (0.1000P)2+ 0.4200P], where P = (Fo2+ 2Fc2)/3), S = 1.002, (Δρ)max= 0.182, (Δρ)min=–0.250 e/Å3and (Δ/σ)max< 0.001.
2.4 Neuroprotective effects
In order to study the potential neuroprotective activity of the title compound, a preliminary screening was performed investigating neuroprotection on impairment induced by glutamine (Glu) in PC12 cells, as evaluated by MTT assay[16-18].
PC12 cells obtained from New Drug Research Center of China Pharmaceutical University (Nanjing,China) were grown in Dulbecco’s modified Eagle’s medium (DMEM) supplemented 10% FBS (heatinactivated), 5% HS (heat-inactivated), 100 IU/mL penicillin and 100 µg/mL streptomycin in a humidified incubator at 37 ℃ and 5% CO2. PC12 cells were seeded in 96-well plates at a density of 1 ×105/mL. Prior to treatments, PC12 cells were rinsed by DMEM for three times (5 min each), and then covered with serum-free DMEM in which Glu (10 mmol/L) or Glu (10 mmol/L) with compound 6 (0.1,1, 10 μmol/L) or Edaravone (90 μmol/L) was added.After incubation in a CO2incubator for 24 h, PC12 cells were subsequently tested by MTT assay.
Tetrazolium (10 µL/well; 5 mg/mL of stock in PBS) salt was added for 4 h prior to the completion of incubation periods. Thereafter, the reaction mixture was carefully taken out and 150 µL of dimethyl sulfoxide (DMSO) solution was added to each well to achieve solubilisation of the formazan crystals formed in viable cells. The absorbance was read at 490 nm on an ELISA plate reader, with DMSO as a blank. All assays were performed in triplicate and repeated thrice. The degree of protection induced by the test compound was calculated using the following equation: protection (%) = ((C –B)/(A – B)) × 100, where A is the viability of control cultures, B is the viability of the cultures treated with Glu, and C is the viability of the cultures treated with Glu and the test compound[19,20].
3 RESULTS AND DISCUSSION
The1H NMR,13C NMR, H RMS and X-ray diffraction data for the product are in good agreement with the structure of the title compound.The selected bond lengths, bond angles and torsion angles are given in Tables 1 and 2, respectively. The molecular structure and packing diagram of the title compound 6 are shown in Figs. 2 and 3.
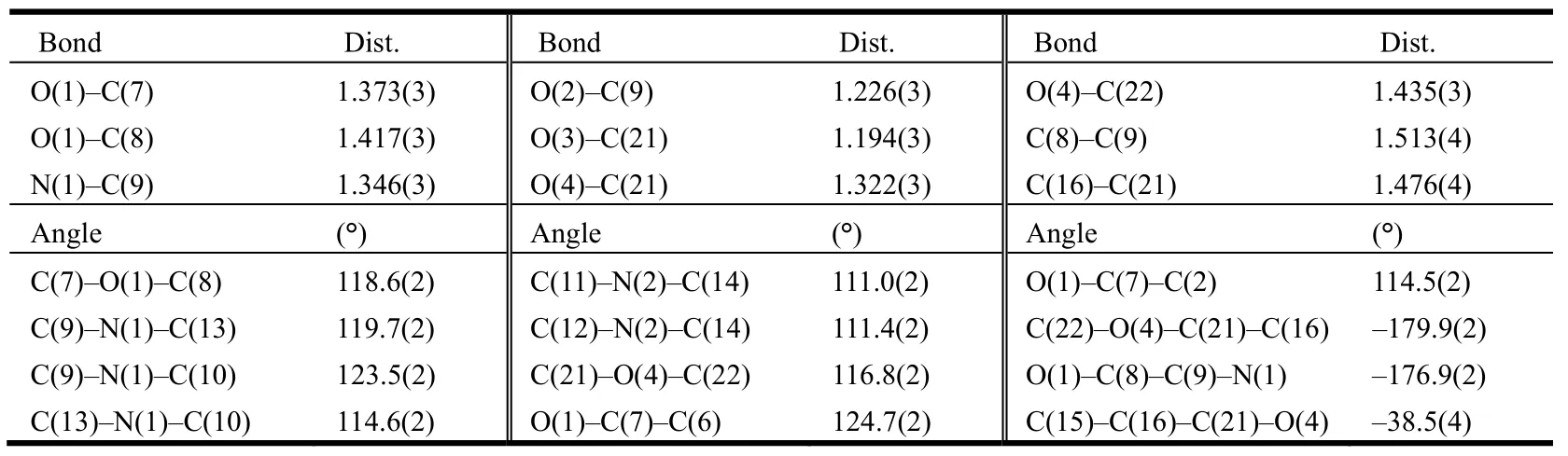
Table 1. Selected Bond Lengths (Å) and Bond Angles (°)/Torsion Angles (°) of Compound 6
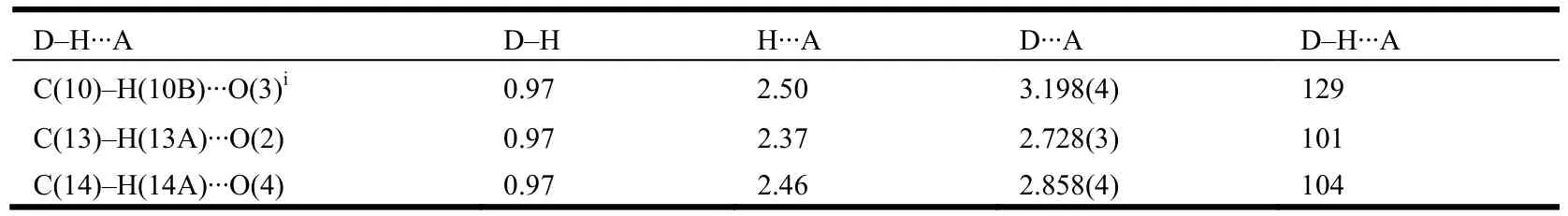
Table 2. Hydrogen Bond Lengths (Å) and Bond Angles (°)
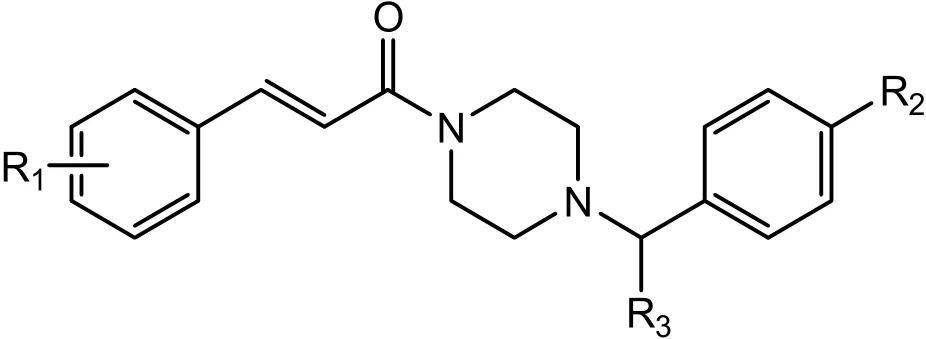
Fig. 1. Structure of novel cinnamide deravatives
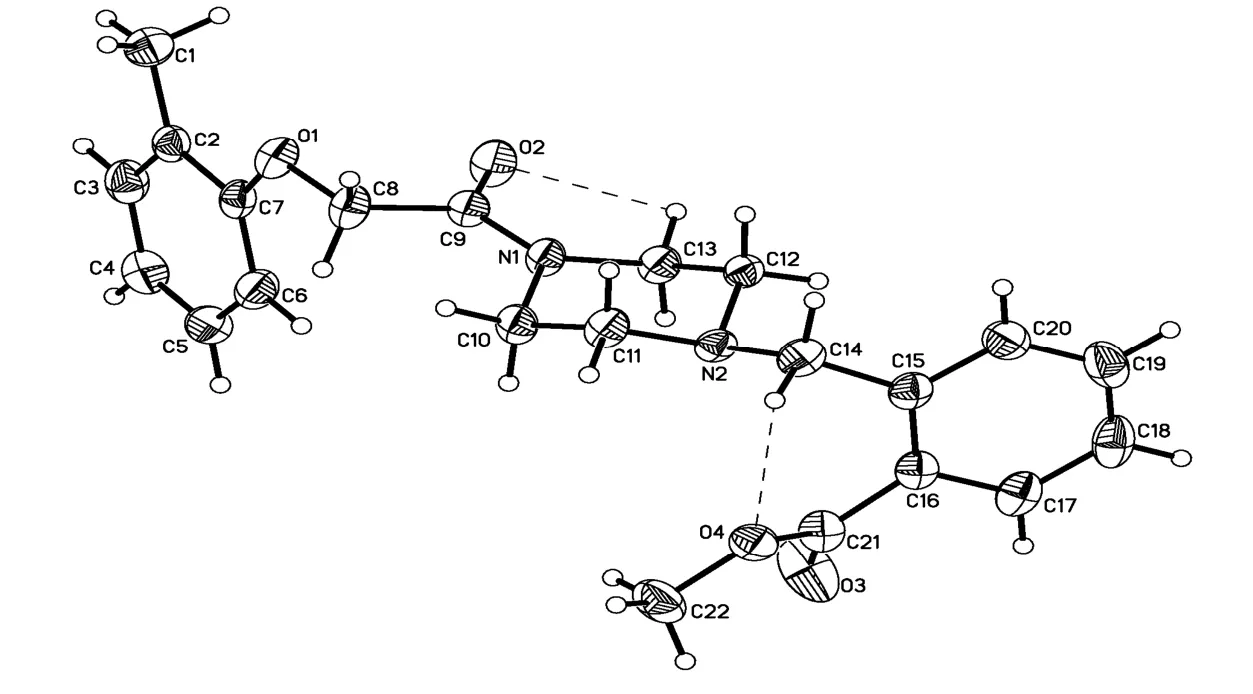
Fig. 2. X-ray structure of the title compound showing the atom-numbering scheme and symmetry-independent C–H··O (dashed line). Displacement ellipsoids are draw at the 70% probability level
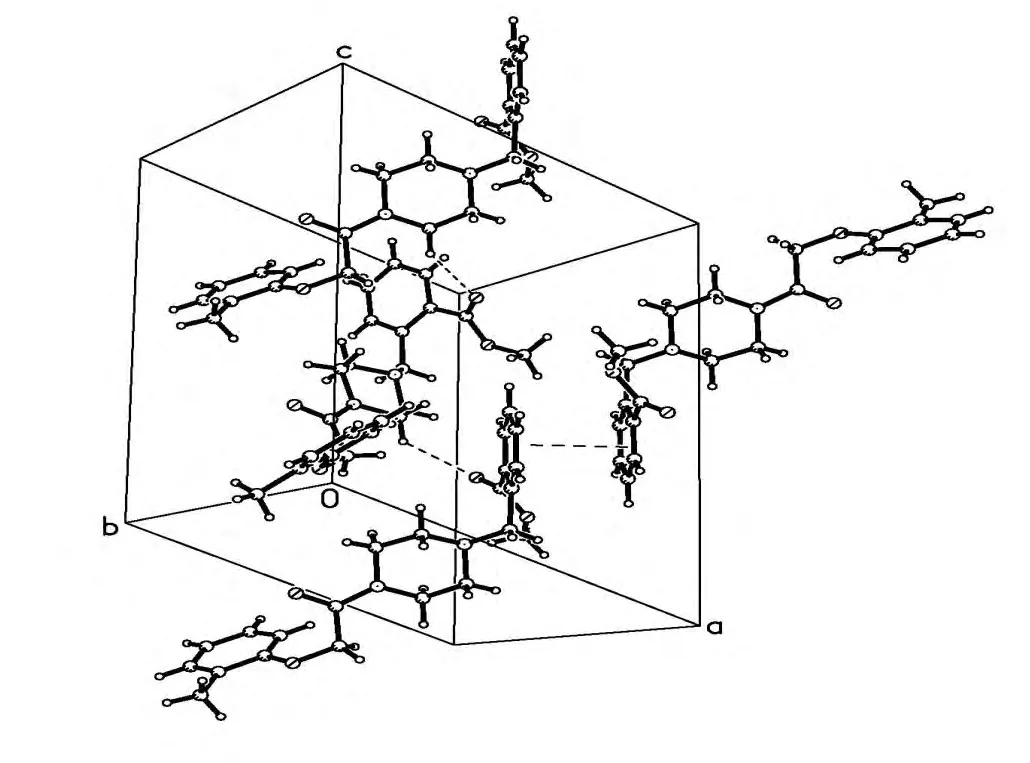
Fig. 3. Part of the crystal structure of the title compound showing intermolecular hydrogen bonds and π-π stacking in the benzene rings. The centroid-centroid distance is of 3.957 Å
It is noteworthy that in the molecule of the title compound, the N(1)–C(9) bond (1.346(3) Å) is remarkably shorter than the typical C(sp2)–N (1.426 Å)[21], but close to the C=N double bond (1.33 Å). In the carboxylate moiety, the O(4)–C(21) bond distance of 1.322(3) Å exhibits the single bond character and O(3)–C(21) bond distance of 1.194(3)is showing the value of double bond character.
The piperazine ring has a chair conformation with the N(1)–COCH2O- and N(2)-benzyl groups in the equatorial positions. The N(1)–C(9) bond itself lies in the plane of the carbonyl group (N(1)–C(9)–C(8)–O(1)–176.9(2)°). The -OCH2CO- moiety exhibits non-coplanar conformation with the main piperazine plane (formed by atoms C(10), C(11),C(12), and C(13)) and the -OCH2CO- group inclined approximately by 13.61(13)°. In crystals, the COOMe group is planar and twisted relative to the benzene ring approximately by 38° (C(15)–C(16)–C(21)–O(4) = –38.5(4)°). The two benzene rings are not coplanar with a dihedral angle of 67.88(15)°between them.
Two intramolecular hydrogen bonding interactions are observed in the title compound (Table 2).An intramolecular hydrogen bridge C–H··O is observed between C(14)/H(14A) and carboxylate oxygen O(4) with the non-bonding C(14)··O(4)distance to be 2.858 Å, forming a six-membered ring with C(21)/C(16)/C(15)/C(14)/H(14A)··O(4).Another intramolecular hydrogen bridge is observed between C(13)/H(13A) and carbonyl oxygen with the non-bonding distance of 2.728 Å, forming a five-membered ring with C(9)/N(1)/C(13)/ H(13A)··O(2). An intermolecular hydrogen interaction is found between C(10)··O(3) (C(10)··O(3) 3.198(4)Å), as shown in Table 2. In the bent structure of 6,the dimmer in a head-to-head type is created by C=O(3)··H–C(10) hydrogen bonds. π-π face-toface stacking interactions between the phenyl rings(C(15), C(16), C(17), C(18), C(19) and C(20),symmetry code: –x, –y, –z) can also be observed.The centroid-centroid distan- ce between the phenyl rings of neighbouring mole- cules is 3.957(2) Å,and the dihedral angle is 0.00°. The face-to-face π-π stacking interactions link the molecules into a three-dimensional network. These interactions stabilize the crystal packing of compound 6, the crystal packing of which is shown in Fig. 3.
As shown in Table 3, remarkably, the tile compound shows potent neuroprotective effects in a dose-dependent manner (protection: 43.0% at 10µM, 33.6% at 1 µM and 21.8% at 0.1 µM), which demonstrates that this compound can attenuate Glu neurotoxicity.
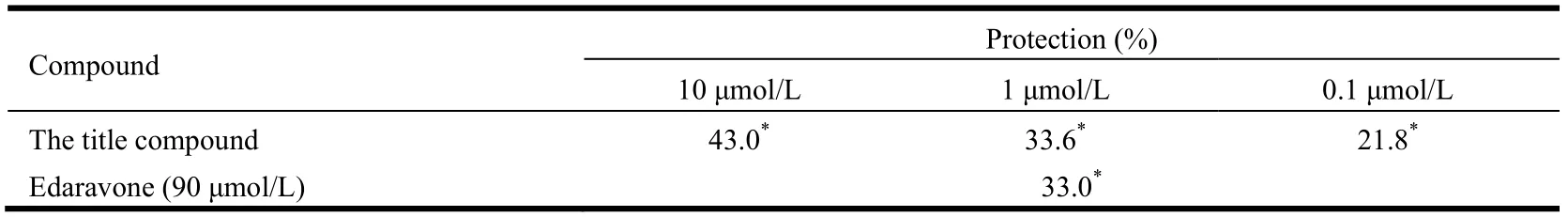
Table 3. Neuroprotective Effects of Compound 6 against Glutamate-induced Neurotoxicity in PC12 Cells
(1) Jia, Q.; Liu, L. P.; Wang, Y. J. Risk factors and prevention of stroke in the Chinese population. J. Stroke Cerebrovasc. Dis. 2011, 20, 395–400.
(2) Zhang, Z. J.; Peng, D. T.; Zhu, H. Y.; Wang, X. M. Experimental evidence of Ginkgo biloba extract EGB as a neuroprotective agent in ischemia stroke rats. Brain Res. Bull. 2012, 87, 193–198.
(3) Wang, D. R.; Hao, Z. L.; Tao, W. D.; Kong, F. Y.; Zhang, S. H.; Wu, B.; Lin, S.; Liu, M. Acute ischemic stroke in the very elderly Chinese: risk factors, hospital management and one-year outcome. Clin. Neurol. Neurosurg. 2011, 113, 442–446.
(4) Liu, M.; Wu, B.; Wang, W. Z.; Lee, L. M.; Zhang, S. H.; Kong, L. Z. Stroke in China: epidemiology, prevention, and management strategies.Lancet Neurol. 2007, 6, 456–464.
(5) He, L.; Chen, X. Y.; Zhou, M. K.; Zhang, D. P.; Yang, J.; Yang, M.; Zhou, D. Radix/rhizoma notoginseng extract (Sanchitongtshu) for ischemic stroke: a randomized controlled study. Phytomedicine 2011, 18, 437–442.
(6) Liu, X. D.; Lv, Y. L.; Wang, B.; Zhao, G.; Yan, Y. P.; Xu, D. Z. Prediction of functional outcome of ischemic stroke patiants in northwest China.Clin. Neurol. Neurosurg. 2007, 109, 571–577.
(7) Wu, B.; Zhou, L.; Cai, H. H. Synthesis and neuroprotective properties of novel cinnamide derivatives. Chin. Chem. Lett. 2008, 19, 1163–1166.
(8) Sheng, C. Q.; Che, X. Y.; Wang, W. Y.; Wang, S. Z.; Cao, Y. B.; Miao, Z. Y.; Yao, J. Z.; Zhang, W. N. Design and synthesis of novel triazole antifungal deravatives by structure-based bioisosterism. Eur. J. Med. Chem. 2011, 46, 5276–5282.
(9) Wang, H. F.; Byun, Y. J.; Barinka, C.; Pullambhatla, M.; Bhang, H. E.; Fox, J. J.; Lubkowski, J.; Mease, R. C.; Pomper, M. G. Bioisosterism of urea-based GCPⅡ inhibitors: synthesis and structure-activity relationship studies. Bioorg. Med. Chem. Lett. 2010, 20, 392–397.
(10) McNulty, J.; Nielsen, A. J.; Brown, C. E.; DiFrancesco, B. R.; Vurgun, N.; Nair, J. J.; Crankshaw, D. J.; Holloway, A. C. Investigation of aryl halides as ketone bioisosteres: refinement of potent and selective inhibitors of human cytochrome P450 19A1 (aromatase). Bioorg. Med. Chem. Lett.2013, 23, 6060–6063.
(11) Biraboneye, A. C.; Madonna, S.; Maher, P.; Kraus, J. L. Neuroprotective effects of N-alkyl-1,2,4-oxadiazolodine-3,5-diones and their corresponding synthetic intermediates N-alkylhydroxylamines and N-1-alkyl-3-carbonyl-1-hydroxyureas against in vitro celebral ischemia.ChemMedChem. 2010, 5, 79–85.
(12) Chou, W. C.; Tan, C. W.; Chen, S. F.; Ku, H. One-pot neat reactions of carboxylic esters and alkylenediamines for efficient preparation of N-acylalkylenediamines. J. Org. Chem. 1998, 63, 10015–10017.
(13) Sahin, A.; Cakmak, O.; Demirtas, I.; Okten, S.; Tutar, A. Efficient and selective synthesis of quinoline deravatives. Tetrahedron 2008, 64,10068–10074.
(14) Šterk, D.; Časar, Z.; Jukič, M.; Košmrlj, J. Concise and highly efficient approach to three key pyrimidine precursors for rosuvastatin synthesis.Tetrahedron 2012, 68, 2155–2160.
(15) Sheldrick, G. M. SHELXL97 and SHELXS97. University of Göttingen: Göttingen, Germany 1997.
(16) Ma, S. W.; Liu, H. X.; Jiao, H. Y.; Wang, L. Y.; Chen, L. Y.; Liang, J.; Zhao, M.; Zhang, X. T. Neuroprotective effect of ginkgolide K on gluamateinduced cytotoxicity in PC12 cells via inhibition of ROS generation and Ca2+influx. NeuroToxicology 2012, 33, 59–69.
(17) Park, S. J.; Nam, K. W.; Lee, H. J.; Cho, E. Y.; Koo, U.; Mar, W. Neuroprotective effects of an alkaloid-free ethyl acetate extract from the root of Sophora flavescens Ait. against focal cerebral ischemia in rats. Phytomedicine 2009, 16, 1042–1051.
(18) Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63.
(19) Terauchi, T.; Doko, T.; Yonaga, M.; Kajiwara, A.; Niidome, T.; Taguchi, R.; Kume, T.; Akaike, A.; Sugimoto, H. Synthesis and neuroprotective effects of serofendic acid analogues. Bioorg. Med. Chem. Lett. 2006, 16, 5080–5083.
(20) Akaike, A.; Tamura, Y.; Sato, Y.; Yokota, T. Protective affects of a vitamin B12 analog methylcobalamin against glutamate cytotoxicity in cultured cortical neurons. Eur. J. Pharmacol. 1993, 241, 1–6.
(21) Bartell, L. S.; Roth, E. A.; Hollowed, C. D. Electron-diffraction study of the structures of C2H4and C2D4. J. Chem. Phys. 1965, 42, 2683–2686.

