Surface-enhanced infrared absorption spectroscopic studies in gold nanoparticles aqueous solution
LING Jing,JIN Bao-kang
(College of Chemistry and Chemical Engineering,Anhui University,Hefei 230039,China)
0 Introduction
Molecules adsor bed on metal nano fil m (metal island fil ms)exhibit 10-100 ti mes more intense infrared absorption than would be expected without the metal.The effect is known as surfaceenhanced infrared absor ption(SEIRA)[1-5].The pheno menon,in which p-nitr obenzoic acid (PNBA)was deposited on vapor-deposited silver,was first reported by Hartstein in 1980[6].SEIRAS is a highly sensitive detection technology which can pr ovide t he vibration inf or mation of biol ogical macromolecules and the change of molecular structure[7].In recent years,lots of research groups[8-9]devoted many eff orts on t he pheno menon of surface-enhanced infrared.As we know,a lar ge nu mber of met hods on surface-enhanced infrared are based on the attenuated total reflection set-ups ATR[10],whereas,literat ure have also been reported surface-enhanced infrared exter nal reflection spectr oscopy[11].A novel resonant mechanis m[12],invol ving t he interference of a br oadband plas mon with the narrowband vibration fro m molecules,is presented by Cornelius to demonstrate experi mentally t he enor mous enhancement of t he gold nanowires.Moreover,t he enhancement of adsor ption of gold and silver nanoparticles on polyelectrolyte layers and growth of polyelectrolyte multilayers by in situ ATR-IR has been reported[13].But all of these methods use roughened metal substrate deposited on t he surface as t he wor king electrode[14-15], and t he operation of electrodepositing metals fil m (electr oless deposition or vacuu m evaporation technique)is co mplicated and has low reproducibility.
Here,t he aut hors reported t he pheno menon of SEIRA based on adsor ption of molecular on t he surface of gol d nanoparticles(GNP),which is dispersed in aqueous sol ution.The results show t hat SEIRAS can be extended to investigate the electrochemical process of molecules adsorbed on nanoparticles in sol ution.A wider range of electrode materials can be used as wor king electr ode and the method is si mple and reproducible.
1 Experi mental
Dopamine(≥99%,HPLC),ascorbic acid(≥99%,HPLC)and benzoquinone(≥99%,HPLC)were purchased respectively from Sigma-Aldrich.[Co(phen)3(Cl O4)3]·3 H2O,was synthesized as that reported in the literature[16].The GNP were prepared as the method reported in the literature[17-18].The GNP was centrif uged ten minutes at 1 000 r p m,and then poured the super natant fluid,at last the phosphate buff er sol ution was added into sedi ment to prepare t he specified concentration.
An electrochemistry workstation CHI 630E (Chen Hua Instru ments Co.,Shanghai,China)is used f or electr ochemical measurements.A Pt electr ode(3 mm in dia meter)was empl oyed as t he wor king electrode.The electrode was polished using alu mina powder(3μm in diameter)to obtain a mirror finish and then clean in an ultrasonic bath with ethanol solution and ultra pure water respectively.The Pt wire and Ag/Ag Cl were empl oyed as t he counter and ref erence electr ode,respectively.The thin-layer electrolytic cell was made by ourselves as a spectroelectrochemical cell[19].
In sit u FT-IR spectroelectr ochemistr y experi ments were carried out si multaneously wit h t he electrochemistry experi ments.Rapid scan ti me-resolved spectroscopic measurements were perfor med on a Nicolet Nexus 870 spectr o meter equipped wit h a specular reflectance accessory(SMART i TR)and a Hg Cd Te/A(MCT/A)detector cooled with liquid nitrogen.The experi ments were carried out in a homemade reflection-absorption spectroelectrochemical cell;the sampling interval is 0.7-2.48 s,and t he spectral resol ution is 16 c m-1.The experi mental results were dealt wit h Gra ms/3D soft ware.
2 Results and discussion
The DA electr ochemical oxidation is conducted by in sit u FTIR spectr oscopy electr oche mistry in the thin-layer cell.The CV under consecutive t wo scan and the corresponding 3Dspectra of 5 mmol·L-1DA in 6 n m GNP aqueous sol ution wit h 0.2 mol·L-1KCl and 0.1 mol·L-1PBS(p H=7.0)as the supporting electrolyte are shown in Fig.1.As seen fro m the Fig.1,the redox process of DA in the t hin-layer is irreversible,which is consistent wit h t he reported literat ure[19].The oxidation peak (a)was observed on the first scan,while there were three oxidation peaks(a)/(b)/(c)on the second positive scan.The CV behavior of DA obser ved in GNP aqueous sol ution was si milar to t hat observed in aqueous solution without GNP.The result i mplies that the GNP does not affect the electrochemical behavor of DA on platinu m electr ode.In t he 3 D infrared spectra(Fig.1B),t he down war d band at 1 637 c m-1was assigned to the C= C stretch of indole and the up ward band at 1 575 c m-1was assigned to t he C= C stretching of dopaminequinone[19].Si milarly,t he IR peaks at 1 164 c m-1and 1 092 c m-1were due to the C—O stretching.Tab.1 gives the corresponding infrared absor bance of peaks 1 164 c m-1and 1 092 c m-1obtained in GNP aqueous wit h different gol d nanoparticles diameter(6 n m,18 n m,24 n m,and 42 n m).

Fig.1 Thin-layer cyclic voltammogram(A)and corresponding 3D spectra(B)of 5 mmol·L-1 DA in 6 n m GNP aqueous solution(p H=7.0)on the Pt disk electrode containing 0.1 mol·L-1 PBS and 0.2 mol·L-1 KCl;reference electrode:Ag/AgCl;potential scan rate:5 mV·s-1;scanning laps:2

Tab.1 The Abs at 1 092 c m-1 and 1 164 c m-1 in different diameter of GNP aqueous
Fig.2 is the corresponding IR CVAs[20](infrared cyclic voltabsorpto metry)at peaks 1 164 c m-1and 1 092 c m-1,and t he DCVAs(derivative cyclic voltabsor pto metr y)at peak 1 092 c m-1.The surface-enhanced infrared phenomenon could be seen in the Tab.1 and the Fig.2.And the absorbance(Abs)of peaks shown was significantly lar ger wit h t he decrease of t he particle size of GNP.The s maller the particle size of GNP is,the larger the surface area is.So more enhanced infrared absor bance of peak coul d be observed.

Fig.2 The corresponding overlay CVAs(A)at 1 092 c m-1(downward)and 1 164 c m-1(upward),and DCVAs(B)at 1 092 c m-1 of 5 mmol·L-1 DA in different size GNP solution
In or der to st udy t he relationship bet ween t he concentration of DA and GNP and infrared enhancement factors.We reproduce the above ti me-resolved rapid scan infrared spectroscopy experi ment in different concentrations of 6 n m GNP.The result is shown in Fig.3.Wit h increasing the GNP concentrations,the more enhanced infrared absor bance can be observed.However,if the concentration of 6 n m GNP is more t han 1.67×10-4mol·L-1,t he IR enhancement factor decrease.This may be interpreted by the fact that GNP agglomeration easily occurs in aqueous sol ution with high concentration of GNP.Hence,t he total surf ace area of GNP beco mes s mall and t he amount of DA molecular adsorbed on GNP decrease.

Fig.3 The CVAs(A)at 1 092 c m-1(downwar d)and 1 164 c m-1(upwar d)and DCVAs(B)at 1 092 c m-1 of 5 mmol·L-1 DA in different concentrations of 6 n m GNP aqueous solution (p H=7.0)on the Pt disk electrode containing 0.1 mol·L-1 PBS and 0.2 mol·L-1 KCl;reference electr ode:Ag/AgCl;potential scan rate:5 mV·s-1
Fig.4 is t he relationship bet ween IR enhancement f actors and DA concentration.(Enhancement factor:EF=Abs with GNP/Abs without GNP).The results suggest that the lower the concentration of DA is,t he lar ger IR enhancement f actor can be obser ved.All DA molecules can be adsor bed on t he surface of GNP in low concentration of DA.However,when the concentration of DA is high,the DA molecules are difficult to be co mpletely adsor bed on t he surface of gol d nanoparticles,so excess DA molecules are f ound free in the solution.Only the adsor bed DA molecular can contribute to the surface-enhanced infrared pheno menon.

Fig.4 The relationship bet ween the IR enhancement factor and the concentration of different concentrations of DA (1,3,5,7 and 9 mmol·L-1)in 1.67×10-4 mol·L-1 6 n m GNP aqueous solution(Enhancement factor:EF=Abs with GNP/Abs without GNP)
Based on t he discussion above,we guess t hat t he surf ace-enhanced infrared here is caused by t he adsor ption of DA on GNP.There are t hree kinds interaction f orce bet ween DA and GNP.Firstl y,as the atoms which located on the surface of the nanoparticles have surface energy,the surface ener gy of GNP continues to increase as ato ms assembling on t he surface,so DA molecules can be adsor bed on t he surf ace of GNP.Secondly,t he amino gr oup of DA can be adsor ded on the surf ace of GNP.Thir dly,gold nanoparticles usually take negative char ge[18]and dopamine(DA)shoul d be positively char ged in a p H 7.0 phosphate buff er saline(PBS).So,electr ostatic f orces can contribute to t he adsor ption.
GNP dispersed in solution may be accu mulated at the electrode surface during experi ment,resulting in IR enhancement.We carried out a contr olled experi ment.Instead of polished Pt electrode,GNP modified Pt electr ode(Dr op-coated 20μL GNP gel on t he Pt electr ode,t hen dried in air)is used as wor king electr ode and IR spectroelectr ochemistr y of DA in aqueous wit hout GNP is conducted again.We do not observe the obvious infrared enhancement phenomenon.The result supports that infrared enhancement is due to the adsorption of dopamine molecules on the surface of gol d nanoparticles dispersed in sol ution.
To f urther explore t he SEIRA based on t he molecular.Si milarly,we conducted t he spectr oelectroche mistry experi ment wit h three other substances,ascor bic acid(AA),benzoquinone and cobalt-phenanthroline co mplex.The ascor bic acid molecular is negatively charged,benzoquinone molecular maintain electrical neutrality and cobalt-phenanthr oline co mplex is positively char ged.

Fig.5 Thin-layer CVs(A)of 10 mmol·L-1 AA in 1.67×10-4 mol·L-1 6 n m GNP aqueous solution and aqueous solution without GNP(p H=7.0)containing 0.1 mol·L-1 PBS and 0.2 mol·L-1 KCl;the corresponding 3Dspectra(B)in 1.67×10-4 mol·L-1 6 n m GNP aqueous solution
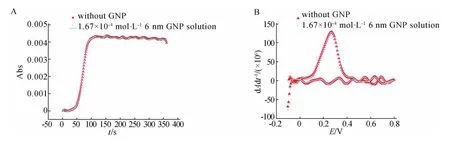
Fig.6 The corresponding stacking CVAs(A)and DCVAs(B)at 1 795 c m-1 of 10 mmol·L-1 AA in aqueous solution without GNP and in 1.67×10-4 mol·L-1 6 n m GNP aqueous solution
In the Fig.5 and Fig.6,we can find the CVs,CVAs and DCVAs diagrams of AA in aqueous sol ution wit hout GNP are t he same as t hose in 1.67×10-4mmol·L-16 n m GNP aqueous sol ution.

Fig.7 Thin-layer CVs(A)of 5 mmol·L-1 BQ in 1.67×10-4 mol·L-1 6 nm GNP aqueous solution and aqueous solution without GNP(p H=7.0)containing 0.1 mol·L-1 PBS and 0.2 mol·L-1 KCl;the corresponding 3Dspectra(B)in 1.67×10-4 mol·L-1 6 n m GNP aqueous solution

Fig.8 The corresponding stacking CVAs(A)and DCVAs(B)at 1 795 c m-1 of 5 mmol·L-1 BQ in aqueous solution without GNP and in 1.67×10-4 mol·L-1 6 n m GNP aqueous solution
In the Fig.7 and Fig.8,it can be found that the CVs,CVAs and DCVAs diagrams of BQ in aqueous sol ution wit hout GNP are si milar to t hose in 1.67×10-4mmol·L-16 n m GNP aqueous sol ution.
Fig.9A shows CV of 8 mmol·L-1[Co(phen)3(Cl O4)3]·3 H2O with and without 1.67×10-4mol·L-16 n m GNP.The corresponding 3 Dinfrared spectr u m is given in Fig.9B.The result shows t hat GNP does not aff ect t he electr oche mical behavior.In 3D spectra,t he IR absor ption peak at 1 419 c m-1could be attributed to the C—C stretching vibration.SEIRA can be observed clearly fro m CVAs and DCVAs(Fig.10)at peak 1 419 c m-1.However,we find t hat SEIRA don’t appear f or AA and BQ.The results suggest t hat the molecular wit h positive char ge can be adsor bed on GNP t horough electrostatic f orce and SEIRA spectra can be observed.

Fig.9 Thin-layer CVs(A)of 8 mmol·L-1[Co(phen)3(Cl O4)3]·3 H2 O in 1.67×10-4 mol·L-1 6 n m GNP aqueous solution and in aqueous solution without GNP(p H=7.0)containing 0.1 mol·L-1 PBS and 0.2 mol·L-1 KCl.The corresponding 3Dspectra of[Co(phen)3(Cl O4)3]·3 H2 O (B)in 1.67×10-4 mol·L-1 6 nm GNP

Fig.10 The corresponding stacking CVAs and DCVAs at 1 419 c m-1 of 8 mmol·L-1[Co(phen)3(Cl O4)3]·3 H2 O in aqueous solution without GNP(A)and GNP aqueous solution(B)
3 Conclusions
In this article,the SEIRA was f ound in the GNP aqueous sol ution,which is due to the electrostatic f orces bet ween t he positively and negatively char ged molecules.There is no requirement to prepare the ATR pris m and complex sample.In addition,we can carry out ti me-resolved rapid scan infrared spectr oscopy study of substances at low concentration via controlling t he surf ace char ge types of GNPs.As a result we establish a new method to investigate SEIRAS,which has the advantages of si mple,repr oducible and wider range of application.
[1]Lar mour I A,Graham D.Surface enhanced optical spectroscopies f or bioanalysis[J].Analyst,2011,136(19):3831-3853.
[2]Yue Y,Zhang L,Osawa M,et al.Surface-enhanced infrared spectroscopic study of a CO-covered Pt electrode in roo m-temperature ionic liquid[J].J Phys Chem Lett,2013,4(10):1582-1586.
[3]Wang J Y,Zhang H X,Jiang K,et al.Fro m HCOOH to CO at Pd electrodes:a surface-enhanced infrared spectroscopy study[J].J Am Chem Soc,2011,133(38):14876-14879.
[4]Ferri D,Bürgi T,Baiker A.Pt and Pt/Al2O3thin fil ms f or investigation of catalytic solid-liquid interfaces by ATR-IR spectroscopy:CO adsorption,H2-induced reconstruction and surface-enhanced absorption[J].J Phys Chem B,2001,105(16):3187-3195.
[5]Ye S,Fang L,Lu Y.Contribution of char ge-transfer effect to surface-enhanced IR for Ag@PPy nanoparticles[J].Phys Chem Chem Phys,2009,11(14):2480-2484.
[6]Hartstein A,Kirtley J,Tsang J.Enhancement of the infrared absor btion fro m molecular monolayers with thin metal overplays[J].Phys Rev Lett,1980,45(3):201-204.
[7]Siebert F,Hildebrandt P.Vibrational spectroscopy in life science[M].Weinhei m,Ger many:Wiley-VCH Verlag Gmb H & Co.KGa A,2008:99-153.
[8]Osawa M.Dynamic pr ocesses in electrochemical reactions studied by surface-enhanced infrared absor ption spectroscopy(SEIRAS)[J].Bulletin of the Chemical Society of Japan,1997,70(12):2861-2880.
[9]Lu G Q,Sun S G,Chen S P,et al.Novel properties of dispersed Pt and Pd thin layers supported on GC f or CO adsor ption studied using in situ MS-FTIR reflection spectr oscopy[J].Jour nal of Electroanalytical Chemistry,1997,421(1):19-23.
[10]Li J T,Zhou Z Y,Broad well I,et al.In-situ infrared spectroscopic st udies of electr ochemical energy conversion and storage[J].Acc Chem Res,2012,45(4):485-494.
[11]Nishikawa Y,Fuji wara K,Ataka K,et al.Surface-enhanced infrared exter nal reflection s pectroscopy at low reflective surfaces and its application to surface analysis of semiconductors,glasses,and poly mers[J].Anal Chem,1993,65(5):556-562.
[12]Neubrech F,Pucci A,Cor nelius T,et al.Resonant plasmonic and vibrational coupling in a tailored nanoantenna for infrared detection[J].Phys Rev Lett,2008,101(15):157403.
[13]Ghosh H,Bürgi T.Adsorption of gold and silver nanoparticles on polyelectrolyte layers and growt h of polyelectrolyte multilayers:an in situ ATR-IR study[J].The Journal of Physical Chemistry C,2013,117(50):26652-26658.
[14]Chen Y J,Sun S G,Chen S P,et al.Anomalous IR properties of nanostructured fil ms created by square wave potential on an array of Pt microelectrodes:an in sit u microscope FTIRS st udy of CO adsor ption[J].Lang muir,2004,20(23):9920-9925.
[15]Hofstead-Duffy A M,Chen D J,Tong Y J.An in situ attenuated total reflection-surface enhanced infrared absor ption spectroscopic study of enhanced met hanol electro-oxidation activity on car bon-supported Pt nanoparticles by poly(vinylpyrrolidone)of different molecular weights[J].Electrochi mica Acta,2012,82:543-549.
[16]Dollimore L S,Gillard R D.Optically active co-ordination compounds.Part XXXII.Potassium (+)tris-[L-cysteinesulphinato(2-)-SN]cobaltate(III):a versatile agent for resolution of 3+species[J].J Chem Soc Dalton Trans,1973(9):933-940.
[17]Br own K R,Fox A P,Natan M J.Mor phology-dependent electrochemistry of cytochro me c at Au colloidmodified Sn O2electrodes[J].J Am Chem Soc,1996,118(5):1154-1157.
[18]Grabar K C,Freeman R G,Hommer M B,et al.Preparation and characterization of Au colloid monolayers[J].Anal Chem,1995,67(4):735-743.
[19]Wang X Y,Jin B K,Lin X Q.In-situ FTIR spectroelectrochemical study of dopamine at a glassy carbon electrode in a neutral solution[J].Analytical Sciences,2002,18(8):931-933.
[20]Jin B K,Li L,Huang J L,et al.IR spectroelectrochemical cyclic voltabsorptometry and derivative cyclic voltabsor pto metry[J].Anal Chem,2009,81(11):4476-4481.
