Influence of Solvent on Reaction Path to Synthesis of Methyl N-Phenyl Carbamate from Aniline, CO2and Methanol*
AN Hualiang (安华良), ZHANG Lili (张丽丽), YUAN Baoguo (苑保国), ZHAO Xinqiang (赵新强)** and WANG Yanji (王延吉)Hebei Provincial Key Lab of Green Chemical Technology and Efficient Energy Saving, Hebei University of Technology, Tianjin 300130, China
Influence of Solvent on Reaction Path to Synthesis of Methyl N-Phenyl Carbamate from Aniline, CO2and Methanol*
AN Hualiang (安华良), ZHANG Lili (张丽丽), YUAN Baoguo (苑保国), ZHAO Xinqiang (赵新强)** and WANG Yanji (王延吉)
Hebei Provincial Key Lab of Green Chemical Technology and Efficient Energy Saving, Hebei University of Technology, Tianjin 300130, China
Methyl N-phenyl carbamate (MPC), an important organic chemical, can be synthesized from aniline, CO2and methanol. Catalyst Cu-Fe/ZrO2-SiO2was first prepared and its catalytic performance for MPC synthesis was evaluated. Then the influence of solvent on the reaction path of MPC synthesis was investigated. It is found that the reaction intermediate is different with acetonitrile or methanol as a solvent. With acetonitrile as a solvent, the synthesis of MPC follows the reaction path with diphenyl urea as the intermediate, while with methanol as a solvent the reaction occurs via the reaction path with dimethyl carbonate as the intermediate. The catalytic mechanism of cooperative catalysis comprising metal sites, Lewis acid sites and Lewis base sites is proposed according to different reaction intermediates.
reaction path, methyl N-phenyl carbamate, CO2, aniline, methanol, Cu-Fe/ZrO2-SiO2catalyst
1 INTRODUCTION
As an important organic chemical, methyl N-phenyl carbamate (MPC) not only has found wide applications in the fields of pesticide, pharmaceutical, and organic synthesis, but also is one of the crucial intermediates for non-phosgene production of diphenylmethane diisocyanate (MDI). The clean synthesis routes to MPC include oxidative carbonylation of aniline [1], reductive carbonylation of nitro benzene [2], alcoholysis of aromatic urea [3], urea alcoholysis of aniline [4] and methoxycarboxylation of aniline [5, 6]. In the oxidative carbonylation process, there exist some problems such as harsh reaction conditions and potential safety hazard. Only one-third of CO is used efficiently and the separation of CO from CO2will increase the operation cost in the reductive carbonylation route. The alcoholysis of aromatic urea uses costly diphenylurea or phenylurea as raw material while the urea alcoholysis of aniline poses the problem of pipe blockage by sublimated urea or its decomposition product. The methoxycarboxylation of aniline utilizes expensive dimethyl carbonate (DMC) as raw material and generates an azeotrope of DMC and methanol, which is difficult to separate. Therefore a new synthesis route to MPC is expected.
CO2is a major greenhouse gas and the reduction of its emission has become a worldwide hot topic. The chemical utilization of CO2can transform the greenhouse gas into value-added products and has received much more attention. The synthesis of MPC from aniline, CO2and methanol is one of the examples for utilization of CO2.
The synthesis of carbamates starting from amines, CO2and alcohol is an environmentally benign route. Most of the researches, however, focused on the synthesis of aliphatic carbamates starting from aliphatic amines, CO2and alcohol [7, 8]. The studies on the synthesis of aromatic carbamates from aromatic amines, CO2and alcohol are few. Honda et al utilized a heterogeneous CeO2to catalyze the one-pot synthesis of methyl benzylcarbamate from benzylamine, CO2and methanol. The selectivity of methyl benzylcarbamate reached 92% at 99% benzylamine conversion. However, the conversion of aniline and selectivity of MPC were only 3% and 56%, respectively, for the reaction of aniline, CO2and methanol [9]. Yao synthesized MPC from aniline, CO2and methanol using bicyclic amidine catalyst and obtained an aniline conversion of 7.1% and a MPC yield of 1.8% [10]. The p-π hyperconjugation of the lone electron pair in the nitrogen atom of amino groups with the aromatic rings lessens the electron cloud density of nitrogen atom in amino groups, resulting in the low reaction activity.
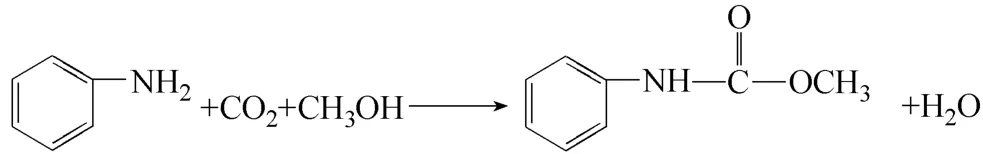
In the present work, Cu-Fe/ZrO2-SiO2catalyst is prepared and its catalytic performance for MPC synthesis with aniline, CO2and methanol is evaluated. Then the influence of acetonitrile and methanol solvent on the reaction path to MPC synthesis is investigated and the catalysis mechanism of Cu-Fe/ZrO2-SiO2catalyst is proposed. The equation for MPC synthesis is as follows.
2 EXPERIMENTAL
2.1 Catalyst preparation
Composite support ZrO2-SiO2was prepared by grinding the mixture of ZrO2and SiO2with a mass ratio of 1︰1 and then calcined at 400 °C for 4 h in a muffle. Taking the sample with a molar ratio of Cu to Fe=3︰1 and the loading of Cu and Fe=10% (by mass) based on the metal oxides as an example, the preparation of Cu-Fe/ZrO2-SiO2was as follows. A mixture of 0.910 g cupric nitrate and 0.507 g ferric nitrate was dissolved in 8.8 ml water to form the impregnation solution. Then 4 g ZrO2-SiO2was incipiently impregnated with the impregnation solution and aged at room temperature for 24 h. Cu-Fe/ZrO2-SiO2sample was attained by undergoing successive processes: dried at 110 °C for 10 h, calcined at 450 °C for 6 h, and reduced in the atmosphere of gas mixture (2HV︰2NV=1︰4) at 300 °C for 3 h.
2.2 Analysis of nitrogen adsorption-desorption
The textural parameters of the catalyst were obtained from nitrogen adsorption-desorption isotherm at 77 K, determined by Micromeritics ASAP 2020 Surface Area and Porosity Analyzer, after degassing at 473 K and 1.3 Pa for 4 h. Multipoint Brunauer-Emmett-Teller (BET) analysis method was used to calculate the surface area. The pore volume and pore size were determined by the Barrett-Joyner-Halenda (BJH) method.
2.3 Activity test
The reaction of aniline, CO2and methanol to MPC was carried out in a 100 ml Hastelloy alloy autoclave. A typical procedure was as follows: 20 g aniline, 16 g acetotrile, 8 g methanol and 2 g Cu-Fe/ ZrO2-SiO2catalyst were introduced into the autoclave successively. After being purged three times with CO2, the autoclave was pressured to 1.0 MPa with CO2. Then the mixture was heated to 160 °C and kept for 7 h. After the completion of reaction, the catalyst was removed by filtration and the filtrate was analyzed by high performance liquid chromatography (HPLC) and gas chromatography (GC).
2.4 Product analyses
The products were quantitatively analyzed on a Waters HPLC equipped with two Waters 1525 pumps, a Turner C18 column (φ4.6mm×150mm) and a Waters 2998 Photodiode Array Detector operated at 232 nm. The flow rate of the mobile phase was 1 ml·min−1with a volumetric ratio of methanol/water=70/30. The intermediate DMC and substrate methanol were quantitatively analyzed on a 3420 model GC equipped with a hydrogen Flame Ionization Detector (FID) operated at 220 °C and a polyethyleneglycol-20000 column, whose temperature was program-controlled as follows: initial temperature of 80 °C for 1 min, increasing to 150 °C at a rate of 6 °C·min−1and holding for 1 min, and then rising to 220 °C at a rate of 10 °C·min−1and holding for 5 min. Nitrogen was used as a carrier gas.
The conversion of aniline is defined as the ratio of the number of moles of aniline converted in the reaction to the total moles of aniline initially added. The yield of MPC is defined as the ratio of the number of moles of MPC produced in practice to the number of moles of MPC generated in theory. The selectivity for MPC was calculated from the relationship between the conversion and the yield.
3 RESULTS AND DISCUSSION
3.1 Nitrogen adsorption-desorption
Nitrogen adsorption-desorption isotherm of Cu-Fe/ZrO2-SiO2is shown in Fig. 1. The catalyst presents Type IV isotherm according to the International Union of Pure and Applied Chemistry (IUPAC) classification, with typical hysteresis loop of mesoporous materials. The BET specific surface area, pore volume, and average pore size of the catalyst are 140.9 m2·g−1, 0.73 cm3·g−1and 17.4 nm, respectively.

Figure 1 Nitrogen adsorption-desorption isotherm of Cu-Fe/ZrO2-SiO2at 77 K (STP: standard temperature and pressure)
3.2 Catalytic performance of Cu-Fe/ZrO2-SiO2
The catalytic behavior of Cu-Fe/ZrO2-SiO2was evaluated in the reaction of MPC synthesis from aniline, CO2and methanol, and Cu-Fe/ZrO2-SiO2showed pretty good catalytic performance. Under the conditions of reaction temperature of 160 °C, reaction time of 7 h, initial pressure of CO2=1 MPa, mass ratio of the catalyst to the sum of aniline and methanol=1︰14, and volumetric ratio of acetotrile to aniline to methanol= 2︰2︰1, aniline conversion, MPC yield and selectivity were 11.1%, 1.2% and 11.1% respectively.
3.3 Influence of solvent on the reaction path to MPC
The influence of the two solvents, acetonitrile and methanol, on the MPC synthesis over Cu-Fe/ ZrO2-SiO2catalyst was investigated and the results are listed in Table 1. The yield (YDPU) and selectivity (SDPU) of diphenyl urea (DPU) were 6.0% and 93.9%, respectively, for the reaction of aniline with CO2in acetonitrile solvent (first line). With methanol in the reaction system, aniline conversion (Xaniline) increased but the yield and selectivity of DPU decreased (third line), forming a certain amount of MPC. This suggests that the reaction of aniline, CO2and methanol to MPC in acetonitrile solvent may follow the reaction path with DPU as the intermediate. Cu-Fe/ZrO2-SiO2showed good catalytic performance for the synthesis of DMC from methanol and CO2in acetonitrile solvent (second line). Methanol conversion (Xmethanol) was 12.4% and DMC yield (YDMC) attained 12.2%. This is attributed to the breakage of thermodynamic restriction of the reaction due to the role of both solvent and dehydrant of acetonitrile [11]. With aniline in the reaction system, no DMC formed while DPU and MPC were produced (third line). It demonstrates that the formation of DPU from aniline and CO2is more favorable compared with the reaction of methanol with CO2to DMC in the presence of acetonitrile solvent, in accordance with Yao’s thermodynamic analysis [10]. The above results show that the reaction of aniline, CO2and methanol to MPC in acetonitrile solvent is along the reaction path with DPU as the intermediate. With methanol used as the substitute for acetonitrile (forth line), the MPC yield (YMPC) is very similar to that in acetonitrile solvent, but a little DPU and a certain amount of DMC formed, suggesting that the reaction of aniline and CO2to DPU is difficult to proceed while DMC can form via methanol and CO2in the presence of methanol solvent. It can be inferred that MPC in this reaction system may be formed by the reaction of aniline and DMC, i.e. the MPC synthesis from aniline, CO2and methanol occurs via the reaction path with DMC as the intermediate in the presence of methanol solvent.

Table 1 Influence of solvent on MPC synthesis①
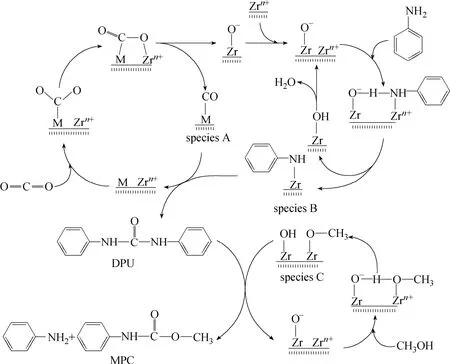
Figure 2 The catalytic mechanism for MPC synthesis in the presence of acetonitrile solvent
2013-09-29, accepted 2013-12-31.
* Supported by the National Natural Science Foundation of China (20976035), the Natural Science Foundation of Tianjin City (12JCYBJC12800) and the Key Basic Research Project of Applied Basic Research Plan of Hebei Province (12965642D).
** To whom correspondence should be addressed. E-mail: zhaoxq@hebut.edu.cn
 Chinese Journal of Chemical Engineering2014年5期
Chinese Journal of Chemical Engineering2014年5期
- Chinese Journal of Chemical Engineering的其它文章
- Soft Sensor Model Derived from Wiener Model Structure: Modeling and Identification*
- Kinetics of Forward Extraction of Boric Acid from Salt Lake Brine by 2-Ethyl-1,3-hexanediol in Toluene Using Single Drop Technique*
- Effect of Adsorbent Diameter on the Performance of Adsorption Refrigeration*
- High-Thermal Conductive Coating Used on Metal Heat Exchanger*
- A Facile Route for Synthesis of LiFePO4/C Cathode Material with Nano-sized Primary Particles*
- Filtering Surface Water with a Polyurethane-based Hollow Fiber Membrane: Effects of Operating Pressure on Membrane Fouling*
