PVC Membrane Selective Electrode for Determination of Cadmium(II) Ion in Chocolate Samples
PVC Membrane Selective Electrode for Determination of Cadmium(II) Ion in Chocolate Samples
Sulekh Chandra1,*, Deepshikha Singh1,2and Anjana Sarkar2
1Department of Chemistry, Zakir Husain Delhi College, University of Delhi, New Delhi 110002, India
2School of Applied Sciences, Netaji Subhas Institute of Technology, University of Delhi, New Delhi 110075, India
Benzil bis(carbohydrazone) (BBC) is prepared and explored as new N N Schiff’s base, which plays the role of an excellent ion carrier in the construction of a Cd(II) ion membrane sensor. The tris(2-ethylhexyl) phosphate best performance corresponds to a membrane composition of 30% poly (vinyl chloride), 65% (TEHP), 3.5% BBC and 1.5% tetradodecyl-ammoniumtetrakis(4-chlorophenyl) borate (ETH 500). This sensor shows very good selectivity and sensitivity towards cadmium ion over a wide variety of cations, including alkali, alkaline earth, transition and heavy metal ions. The effect of membrane composition, selectivity, pH and influence of additive on the response properties of electrode were investigated. The response mechanism was discussed in the view of UV-spectroscopy. The electrode exhibits a Nernstian behavior (with slope of 29.7 mV per decade) over a very wide concentration range from 1.0×10−1to 1.0×10−8mol·L−1with a detection limit of 3.2×10−8mol·L−1. It shows relatively fast response time in whole concentration range (<8 s) and can be used for at least 10 weeks in the pH range of 2.0-9.0. The proposed sensor is successfully used for the determination of cadmium in different chocolate samples and as indicator electrode in titration with ethylene diamine tetraacetate (EDTA).
benzil bis(carbohydrazone), ionophore, Cd(II) ion membrane sensor, selectivity coefficient, additive
1 INTRODUCTION
Quick determination of minute quantities of ionic species by simple method has a great importance in analytical chemistry. Analytical methods based on potentiometric detection with ion-selective electrodes (ISEs) is considered as an advantageous alternative because they are eco-friendly techniques, provide easy construction and manipulation, present good selectivity in a wide concentration range of operatability and relatively low detection limit, show fast response and non-destructive analysis, and be used as very useful tools for clinical, chemical and environmental analysis [1-8].
In modern world, the pollution of heavy metal ions is becoming more and more severe and cadmium is one of them, which are characterized by strong toxicity and accumulation ability in living organisms. The main sources of cadmium are natural exposure from soils or earth crust with high content of it, and anthropogenic processes, such as combustion of coal and mineral oil, smelting, mining, alloy processing, and paint industries, are the major sources of lead and cadmium to the people in the vicinity of the industrial areas. Human uptake of cadmium takes place mainly through food. Foodstuffs that are rich in cadmium will greatly increase the cadmium concentration in human bodies. Examples are liver, mushrooms, shellfish, mussels, cocoa powder and dried seaweed. The provisional tolerable weekly intake of cadmium is currently 7 μg·kg−1body mass, but the recommendation is to limit cadmium intake as it offers no nutritional benefit. Typical maximum levels of cadmium in foodstuffs are currently between 0.05-0.2 mg·kg−1wet mass [9].
As a highly toxic element cadmium is responsible for several cases of ailment including fever, muscle ache and inflammation that can ultimately lead to respiratory and kidney damage [10, 11]. Medical researchers have revealed that cadmium may cause lung and prostate cancers [12]. Therefore, there is an urgent need to monitor the level of cadmium in the environment.
Even though Cd-ISE is not commonly found in the marketplace, some reports [13-22] lately indicate intense interest on its fabrications. However, their setbacks are mostly due to serious interference by cations such as Ag+, Hg2+, Cu2+, Zn2+and Pb2+.
Recently, there has been much focus on the construction of ISEs on the basis of chemical recognition principle because they can help to translate the chemistry of new substrate-binding systems into tools for recognizing selectively various target species in the presence of potentially interfering analytes. Based on above considerations, the aim of the present work is to devise a highly selective cadmium membrane electrode with good response characteristics. We attempt to develop selective cadmium sensors by using benzyl bis(carbohydrazone) (BBC) as sensing materials, which show high affinity for cadmium in poly(vinylchloride) (PVC) matrix and the membrane will turn out to be sufficiently selective and sensitive cadmium sensor. The electrodes are used in titration experiments and for the determination of cadmium ion concentrations in chocolate samples.
2 EXPERIMENTAL
2.1 Reagents and apparatus
Analytical reagent grade chemicals and doublydistilled water were used for preparing all aqueous solutions. Benzil, carbohydrazide, high molecular mass poly (vinylchloride) powder (PVC), dibutyl phthalate (DBP), dioctyl phthalate (DOP) and tetrahydrofuran (THF) were obtained from Sigma. Tetradodecylammoniumtetrakis(4-chlorophenyl) borate (ETH 500), tris(2-ethylhexyl)phosphate and 2-nitrophenyl octyl ether (NPOE) were obtained from Fluka. Salts of metal nitrates or chlorides and solvents such as methanol, ethanol, acetone, dioxane, nitric acid and hydrogen peroxide (all from Merck) were of the highest purity available and used without further purification. All the metal nitrate solutions were freshly prepared by accurate dilution from their stock solution of 0.1 mol·L−1, with distilled, de-ionized water.
The carriers or ionophore used in electrode preparation were synthesized in the laboratory and then characterized. The C, H, and N contents were analyzed on a Carlo-Erba 1106 elemental analyzer. Electronic spectra were recorded on Shimazdu UV mini-1240 spectrophotometer using dimethyl sulphoxide (DMSO) as a solvent. IR spectra (KBr) were recorded on a Perkin-Elmer Fourier transform infrared spectroscopy (FT-IR) spectrum BX-II spectrophotometer. The1H NMR spectrum was recorded with a model Bruker Advance DPX-300 spectrometer operating at 300 MHz using DMSO-d6as a solvent and tetramethylene silane (TMS) as an internal standard.
2.2 Synthesis of ionophore
An aqueous ethanolic solution of carbohydrazide (0.02 mol, 1.8 g) was treated with 1 ml glacial acetic acid followed by slow addition of a solution of benzil (0.01 mol, 2.1 g) in 20 ml ethanol. The resulting mixture was heated under reflux for about 12 h. On cooling overnight at 0 °C, white crystalline precipitate of ionophore (Fig. 1) was separated, which was filtered, washed with cold ethanol and dried under vacuum. Yield of 78%, melting point of 210 °C.
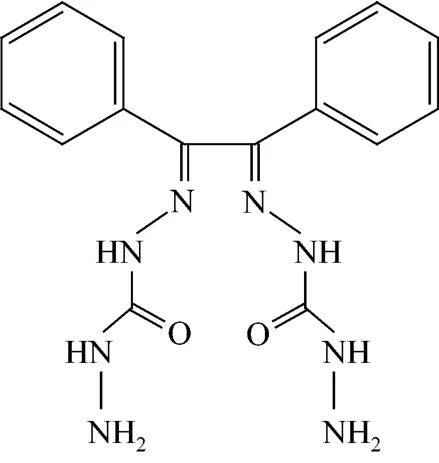
Figure 1 Benzilbis(carbohydrazone) (BBC)
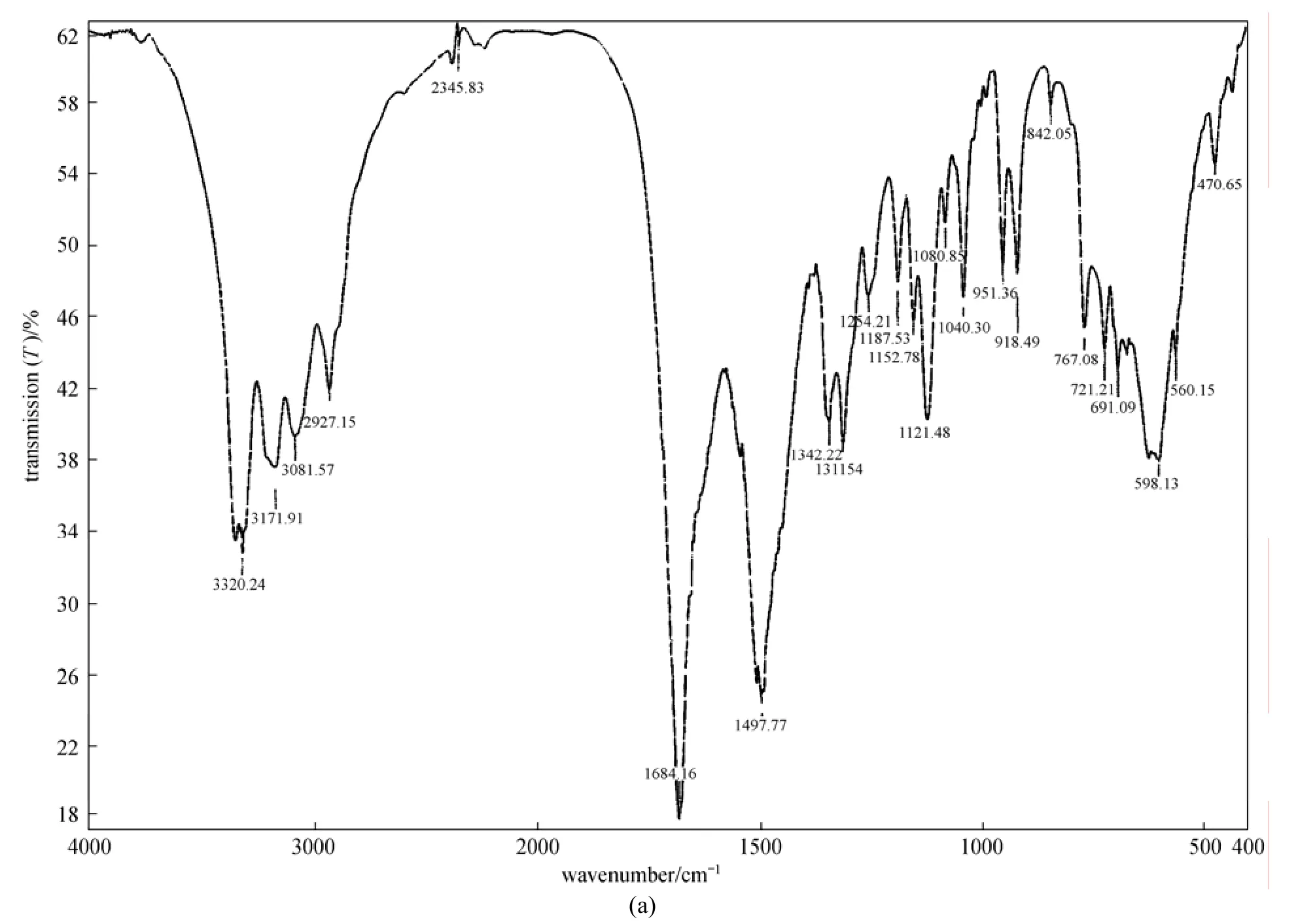
Figure 2 IR spectra (a), H1 NMR spectra (b) and mass spectra of ionophore BBC (c)

Figure 2 IR spectra (a), H1 NMR spectra (b) and mass spectra (c) of ionophore BBC
Elemental analysis: C16H18N8O2, found (calc.) C: 53.78 (54.23), H: 4.78 (5.08), N: 31.29 (31.64). IR bands (KBr cm−1); 3320 υ(NH), 3171 υ(OH), 1684 υ(C O), 1497 υ(C N), shown in Fig. 2 (a). H1NMR (300 MHz, DMSO-d6), δ; 4.0(s, br, 4H,NH2), 5.8(s, br, 2H,NH), 6.8-7.9(m, 10H,arH), 8.1(s, 2H,NH) shown in Fig. 2 (b). The electron impact mass spectrum of metal free ligand in Fig. 2 (c) confirms the proposed formula by showing a peak at 354 atomic mass unit (amu) corresponding to the [C16H18N8O2] and calculated atomic mass 354 amu. The series of peaks in the range, i.e. 16, 44, 59, 134, 110, 177, 248, 295, 354 and 355 amu etc., may be assigned to various fragments.2.3 Fabrication of electrodes
The membranes were fabricated as suggested by Craggs et al [23]. The PVC-based membranes were prepared by dissolving appropriate amounts of ionophore, anionic additive and plasticizers TEHP, DBP, o-NPOE, DOP and PVC in THF (~5 ml). The components were added in terms of mass percentages. The homogeneous mixture was obtained after complete dissolution of all components, poured into polyacrylate rings placed on a smooth glass plate, and concentrated by evaporating THF. The viscosity of the solution and solvent evaporation were carefully controlled to obtain membranes with reproducible characteristics and uniform thickness otherwise the response of membrane sensors presented a significant variation. The membranes of 0.4 mm thickness were removed carefully from the glass plate and glued to one end of a “Pyrex”glass tube. It is known that the sensitivity, linearity and selectivity for a given ionophore depends significantly on the membrane composition and nature of plasticizer used [24]. Thus the ratio of membrane ingredients, time of contact, concentration of equilibrating solution, etc. were optimized after a good deal of experimentation to provide membranes that generate reproducible and stable potentials. The membranes having only PVC as membrane ingredient were also prepared, which indicates that the potentials are not generated without the electroactive material in the membrane.
2.4 Equilibration of membrane and potential measurement
The time of contact and concentration of equilibrating solution were optimized so that the sensors generated stable and reproducible potential at relatively short response time.
The polymeric membrane electrode was equilibrated for about 2 days in 1.0×10−1mol·L−1Cd(NO3)2solution. The potentials were measured by varying the concentration of Cd(NO3)2in test solution in the range from 1×10−9mol·L−1to 1.0×10−1mol·L−1. Standard Cd(NO3)2solutions were obtained by gradual dilution of 10−1mol·L−1Cd(NO3)2solution.
The electromotive force (emf) measurements with the polymeric membrane electrode were carried out on digital potentiometer (Equiptronic Model no. EQ-609) at (25±1) °C using saturated calomel electrode (SCE) as reference electrode with the following cell assemblies:
Hg-Hg2Cl2, KCl (sat.) ||internal solution 1.0×10−1mol·L−1Cd2+|PVC membrane| test solution|| Hg2Cl2-Hg, KCl (sat.)
3 RESULTS AND DISCUSSION
3.1 Study of internal solution
In order to study the ion sensor response formalism, the influence of the concentration of internal solution on the potential response of the polymeric membrane electrode for Cd2+ion based on proposed ionophore were studied. The concentration was varied from 1.0×10−1mol·L−1to 1.0×10−4mol·L−1and the potential response of the sensor was observed. It was found that the most effective results in terms of slope and working concentration range for the smooth functioning of proposed sensor were obtained with internal solution of concentration 1.0×10−1mol·L−1.
3.2 Membrane study
Figure 3 shows the incorporation and distribution of ionophore in the PVC membranes evaluated with polarizing microscope. The ionophore distribution is visualized as long tubular structure throughout the membrane before the potentiometric study, and the membrane is saturated and the capturing sites are blocked after potentiometric study. Hence this membrane cannot be used for further study.
3.3 Response of PVC base membrane electrode to Cd2+ions
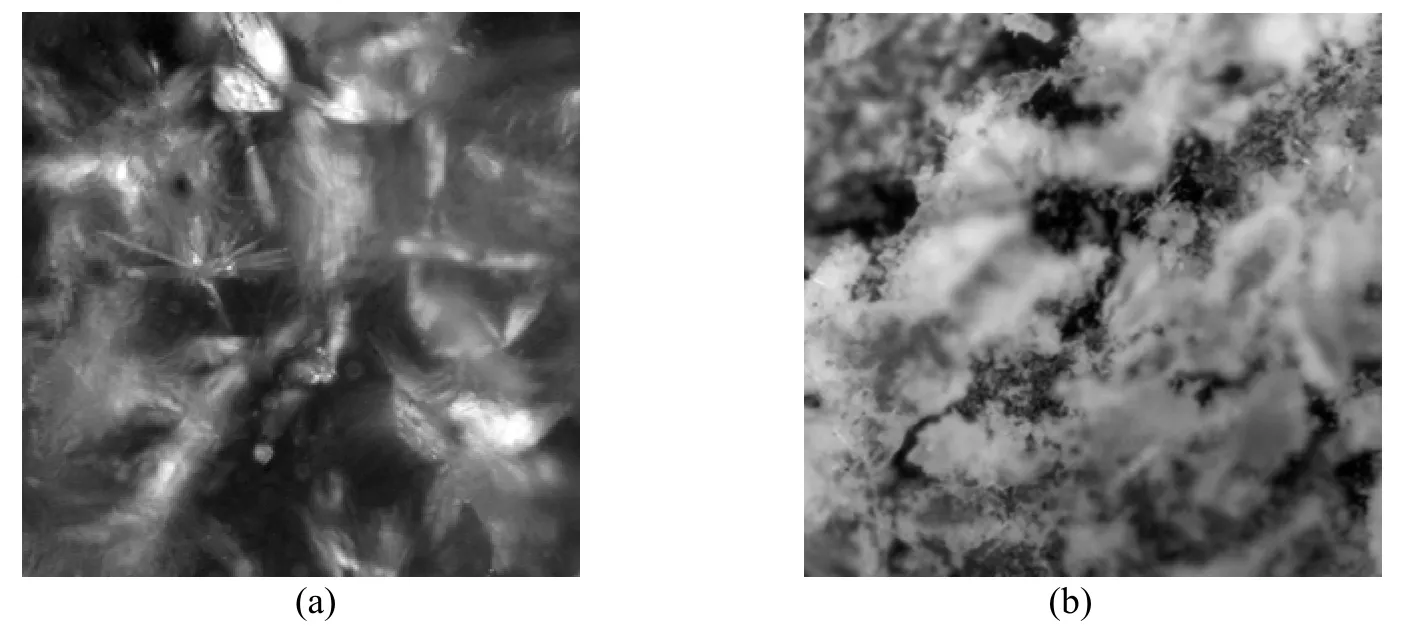
Figure 3 Optical microscopic picture of membrane before (a) and after (b) use as a sensor
The existence of nitrogen and oxygen as donoratoms in the ligand with high lipophilic character seems to form strong complexes with transition metal ions. In order to study the potential response and selectivity of ionophore for different metal ions, BBC was used as neutral carriers to prepare PVC based membrane electrodes for a variety of metal ions including alkali, alkaline earth, transition and heavy metal ions. The potential responses are shown in Fig. 4. Cd2+ion presents the most sensitive response, suitably determined with the membrane electrodes. The emf responses for all other cation-selective electrodes are much lower than that predicted by the Nernst equation. This is probably due to the selective behavior of ionophore against Cd2+in comparison to other metal ions and rapid exchange kinetics of the resulting ionophore with Cd2+complexes.
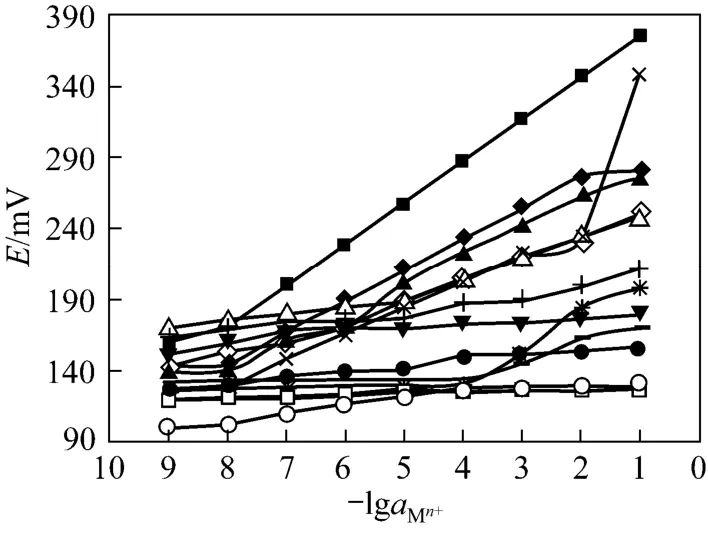
Figure 4 Potential response of ionophore BBC to Mn+ion◆ Hg2+; ■ Cd2+; ▲ Zn2+;Ag+;Pb2+; ● Ni2+;Cu2+;○ Mg2+;Co2+; ◇ Fe3+; □ Na+; △ Cr3+; ▼ Sr2+
The interaction mechanism of Cd2+ion with ionophore was examined on the basis of UV-visible spectrophotometry. UV-visible spectra of carrier without and with cadmium ion were recorded. A UV-visible spectrum of BBC with 0.01 mol·L−1concentration dissolved in DMSO was recorded first. It was then used for the quantitative complexation with Cd2+ion by adding increasing amount of Cd2+ion up to 0.01 mol·L−1concentration. The spectra of the ionophore BBC show the absorption bands at 266 nm [Fig. 5 (a)]. In Fig. 5 (b) the peaks shift and the new peaks are at 329 and 397 nm with increased absorptivity, with Cd2+ion interacted with the carrier, which shows that the size of the complex increases on interaction of Cd2+with the carrier. The absorption intensity changes as a function of molar ratio [Cd2+]/[BBC].
3.4 Optimization of membrane composition
The sensitivity and selectivity for a given membrane depend significantly on membrane ingredients and the nature of plasticizer and additives used. Therefore, 10 membranes of different compositions (Table 1) were prepared and their response characteristics were evaluated according to the International Union of Pure and Applied Chemistry (IUPAC) recommendations [25].
3.4.1 Effect of plasticizer
The improvement for the performance has attempted by the addition of appropriate amount of plasticizer to the membranes. The plasticizers should exhibit high lipophilicity, high molecular mass, low tendency for exudation from the polymer matrix, low vapor pressure and high capacity to dissolve the substrate and other additives present in the membrane and adequate dielectric constant [26, 27]. The addition of four plasticizers, namely o-NPOE, TEHP, DOP and DBP, to the membranes improves the working concentration range and the slope (Table 1). The best performance characteristics are obtained with the membrane having plasticizer TEHP. This sensor exhibits the maximum working concentration range of 1.0×10−1to 1.0×10−8mol·L−1with detection limit 3.2×10−8mol·L−1and a Nernstian slope of 29.7 mV per decade. This indicates that the solvent medium of TEHP probablyprovides the best complexation environment between cadmium ions and their respective carriers.
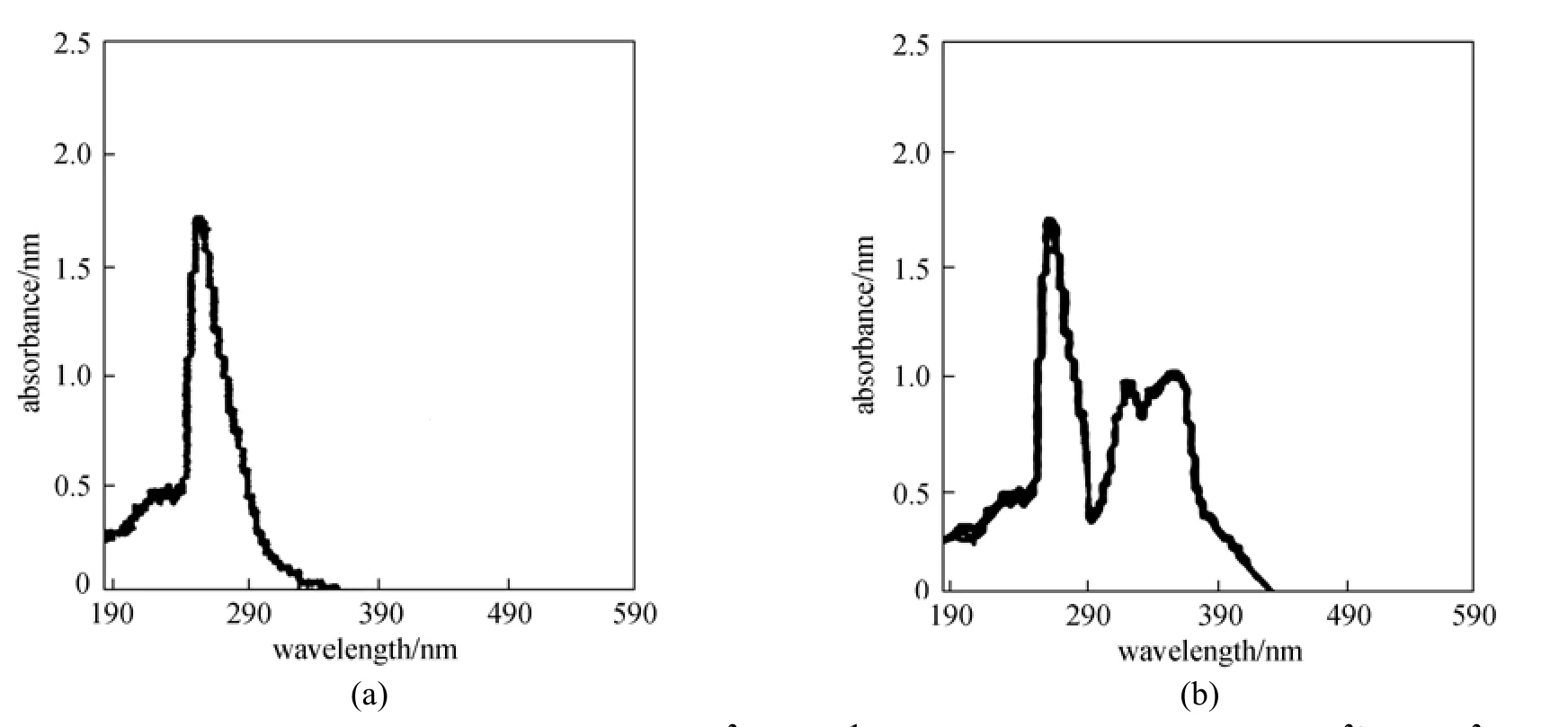
Figure 5 UV-visible interaction mechanism of BBC (1×10−2mol·L−1) in DMSO (a) and BBC with Cd2+(1×10−2mol·L−1) in DMSO (b)
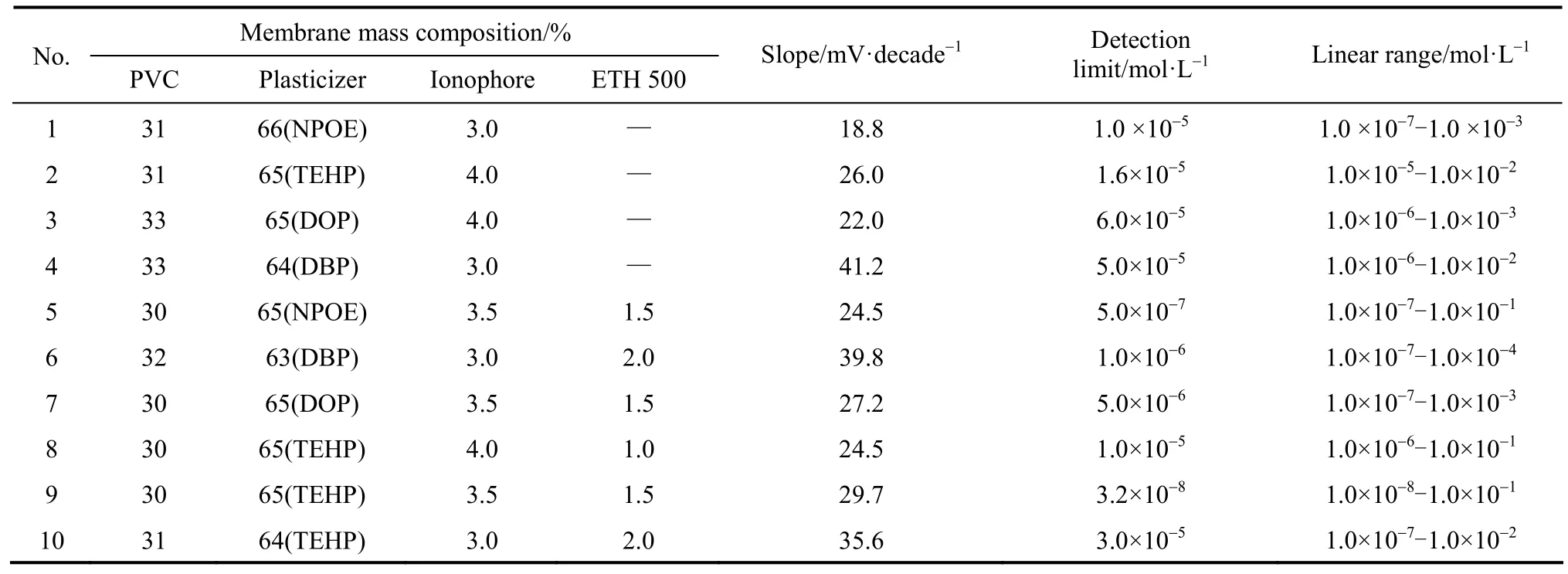
Table 1 Optimization of membrane composition
3.4.2 Effect of ionophore concentration
The response mechanism of neutral carrier-based sensors depends mainly on the extraction equilibrium at the vicinity of the interface between the membrane and aqueous layer [28], as well as concentration of the ionophore complex in the PVC membrane. Therefore, different amounts of ionophore in PVC membranes were tested. An ionophore content of 3.5 mg (based on 100 mg total membrane content) was chosen as an optimum level because the surface conditions of the PVC membranes were deteriorated on decreasing or increasing the amount of ionophore.
3.4.3 Effect of anionic additive concentration
The presence of lipophilic anionic additives in cation selective membrane electrodes is necessary to introduce perm selectivity, and also in cases where the extraction capability is poor, to improve the behavior and sensitivity of membrane electrodes otherwise the electrodes may fail to respond properly [29, 30]. To examine the influence of concentration of lipophilic anionic sites added to the membrane phase on the working parameters of electrode, a series of membranes are studied by using lipophilic additive tetradodecylammoniumtetrakis(4-chlorophenyl) borate (ETH 500). Incorporating ETH 500 in the membrane composition in the proportion of 1.5 mg relative to ionophore shows best performance characteristics, since ETH 500 acts as a charge compensating counter ion in the membrane and facilitates the process of ion charge transduction. The membranes without lipophilic salt show very narrow concentration range and slopes were super-Nernstian, which is likely due to the presence of anionic impurities within the PVC polymer matrix [31].
3.5 Influence of interfering ions
When a Nernstian response is obtained for a sensor, the second challenge is the selectivity. Many methods have been developed to determine the potentiometric selectivity coefficients [32], with the changes of potential in the presence of two ions measured simultaneously, principal and interfering ions. It has been shown theoretically that the potentiometric selectivity of potentiometric membranes based on neutral carriers depends on the complexation specificity of carrier molecules involved and on the composition of membrane. In most instances, the distribution of cation complexes is the predominant factor governing the selectivity of membrane. The distribution process combines complex formation in an aqueous phase and transfer of the complexes formed to the membrane phase. Depending on the ligand structure and cation size, complex formation or extractability is the determining factor for selectivity [33].
In this work, the selectivity coefficient of the sensor towards different cationic species (Mn+) is evaluated by using the fixed interference method (FIM) [34]. In FIM, the selectivity coefficient is evaluated from potential measurement on solutions containing a fixed concentration of interfering ion (1.0×10−2mol·L−1) and varying amount of Cd2+ions. It is given by the expression of
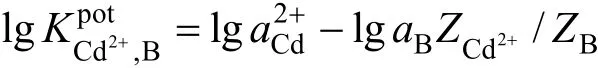
where interfering ion,potK is the selectivity coefficient ofandBZ are the charge on2Cd+ion and cadmium ion and interfering ion respectively.andBa is the activity of2Cd+
The resulting Table 2. For most of the polyvalent cations used, the selectivity coefficients show low values, indicating negligible interference in the performance of the membrane electrode assembly. The selectivity coefficient of an ISE not only depends on ion charge, activity or concentration but also is affected by the type of interaction between ion and ionophore. For example, a tetravalent ion may represent higher selectivity coefficientvalues are summarized inand strong interaction with ionophore in comparison to a univalent ion. Thus, despite their large selectivity coefficients, these ions would not disturb the functioning of the Cd2+sensor significantly.
Table 2 Potentiometric selectivity coefficientfor interfering ions
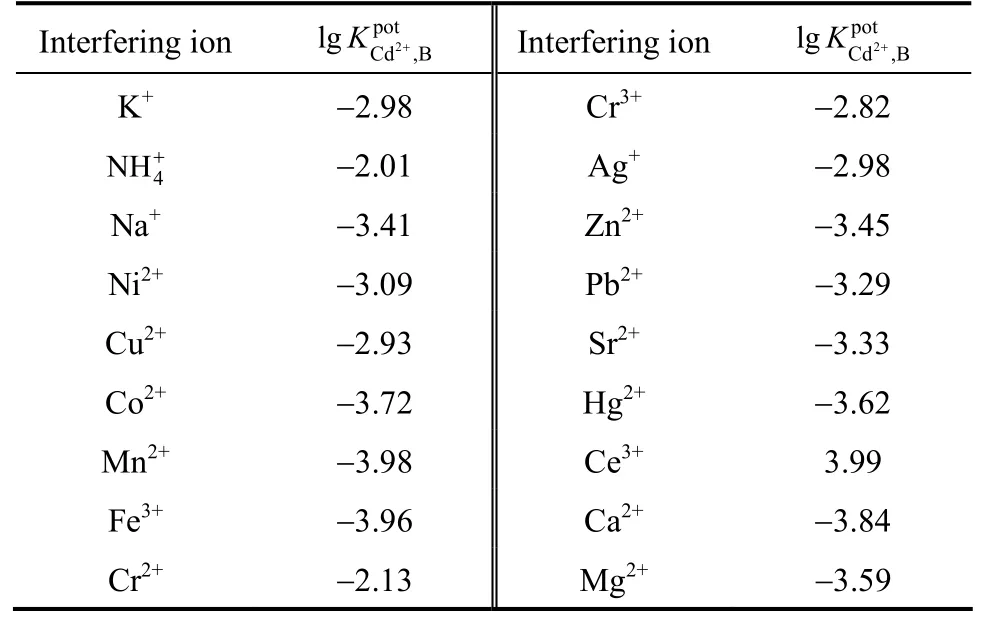
Table 2 Potentiometric selectivity coefficientfor interfering ions
Interfering ion 2 pot Cd,B lgK+Interfering ion 2 pot Cd,B lgK+K+−2.98 Cr3+−2.82 4 NH+−2.01 Ag+−2.98 Na+−3.41 Zn2+−3.45 Ni2+−3.09 Pb2+−3.29 Cu2+−2.93 Sr2+−3.33 Co2+−3.72 Hg2+−3.62 Mn2+−3.98 Ce3+3.99 Fe3+−3.96 Ca2+−3.84 Cr2+−2.13 Mg2+−3.59
3.6 Effect of pH
One of the ions present in aqueous solution is the hydrogen ion. It interferes in many instances in the functioning of the electrode. In view of this, it is necessary to find the optimum pH range in which the electrode functions is not interfered.
The effect of pH of the test solution on the response of the membrane electrode was examined at two Cd2+ion concentrations. As illustrated in Fig. 6, the potentials remain constant from pH 2.0 to 9.0. The electrode responses at pH<2.0 seems ascribable to the competitive binding of proton to the ligand at the surface of membrane electrode, while at pH>9 the potential change may be due to the hydroxylation of Cd2+ions.
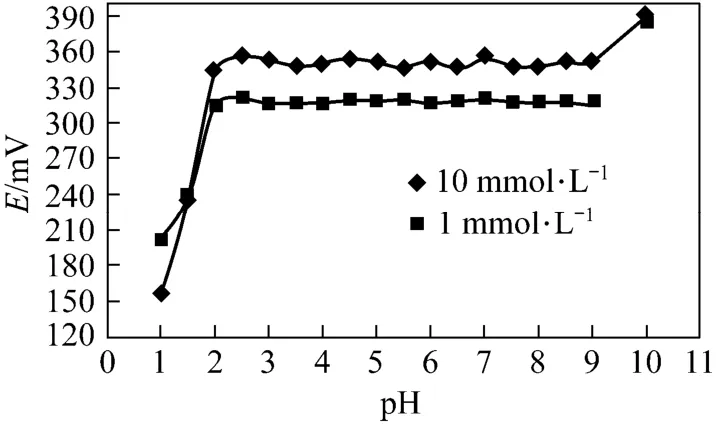
Figure 6 Effect of pH on the response of Cd2+-ISE
3.7 Effect of non aqueous content
Real samples may contain non aqueous content, so the performance of the sensor was also investigated in the media with 20%, 25% and 30% (by volume) non aqueous content in methanol-water, ethanol-water, acetone-water and dioxane-water mixtures and results are shown in Table 3 and Fig. 7. The potentialmeasurements do not show any appreciable change in working concentration range and slope in methanolwater and ethanol-water up to 25% (by volume) non aqueous contents. In acetone-water and dioxane-water mixtures, the membrane could tolerate up to 20% (by volume) non aqueous content. Beyond the value non aqueous content causes a significant interference in the slope. This may be due to the dynamic complexation and decomplexation of Cd2+ion with the ligand.
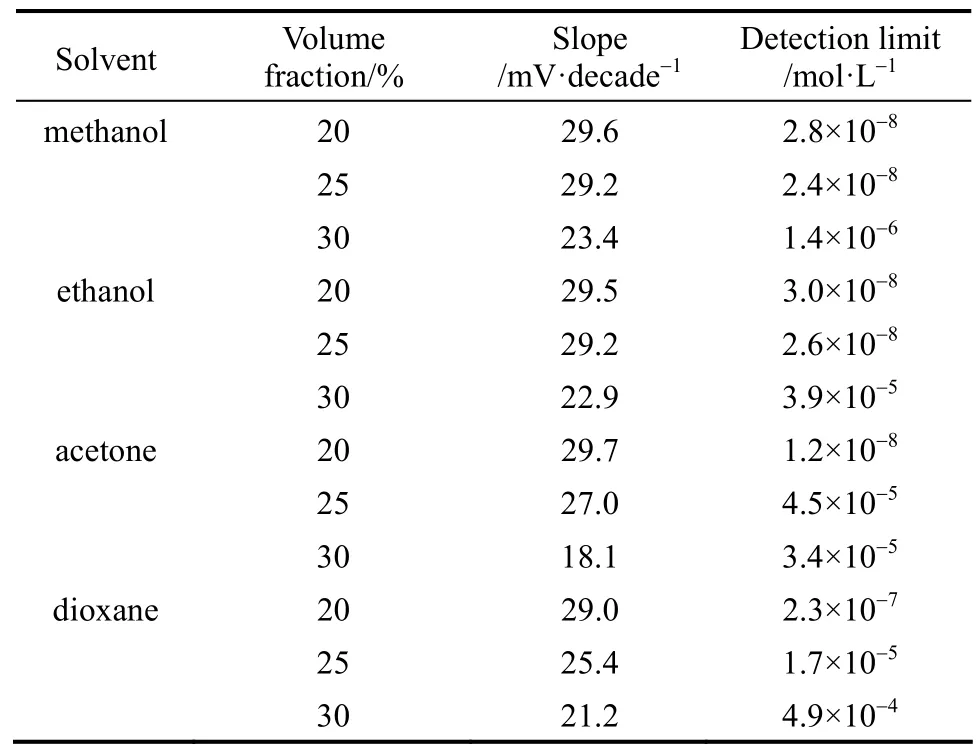
Table 3 Electrode response in mixed solvent media
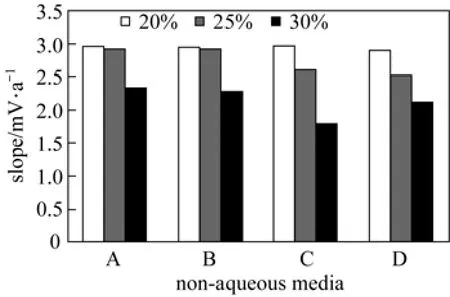
Figure 7 Comparison of slopes of Cd2+-ISE in different non-aqueous mediaA—water-methanol; B—water-ethanol; C—water-acetone; D—water-dioxane
3.8 Dynamic response time and lifetime
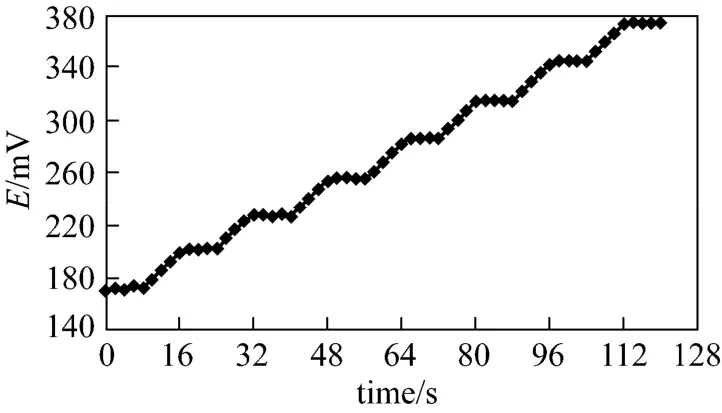
Figure 8 Response time curve for Cd2+-ISE
Dynamic response time is an important factor for any ion-selective electrode. Fig. 8 shows the responsetime in the Cd2+ion concentration from 1.0×10−8to 1.0×10−2mol·L−1. The sensor reaches the equilibrium response in a very short time (~8 s).
To evaluate the reversibility of the electrode, a similar procedure in the opposite direction was adopted. The measurements were performed in the sequence of high-to-low (from 1.0×10−2to 1.0×10−8mol·L−1) concentrations. The results showed that the potentiometric response of the electrodes was reversible, though the time needed to reach equilibrium values (40 s) was longer than that with the sequence of low-to-high concentrations.
The high lipophilicity of ionophore and plasticizer ensure stable potentials and longer lifetime [35] for the membrane. Hence, the lifetime is dependent upon the components of solution and measured specimens. The lifetime of the electrode was determined by performing calibrations periodically with standard solutions and calculating the slopes over the concentration ranges of 1.0×10−2-1.0×10−8mol·L−1Cd2+solutions. The experimental results showed that the lifetime of the present sensor was over 75 days, during which the detection limit and the slope of the electrode remained almost constant. Afterwards, the electrochemical behavior of the electrode gradually deteriorated, which may be due to ageing of the polymer (PVC) and the plasticizers, and leaching of the ionophore. Therefore, the electrode can be used for at least 2 and half months without a considerable change in their response characteristic towards Cd2+.
4 ANALYTICAL APPROACH
The electrode has been applied for direct determination of cadmium in chocolate samples. Chocolate and chocolate-based sweets and candies are common treats, snacks, or gifts for children and adults. The main ingredients in chocolate consist of cocoa, milk and fats, each of which is a potential source of cadmium. The determination of cadmium levels in chocolate is therefore an important issue for chocolate consumers and manufacturers around the globe.
The sample for cadmium determination was prepared by weighing approximately 0.20 g pieces of chocolate (popular global brands of milk and dark variety) accurately and transferred to microwave digestion vessels. 7 ml of nitric acid and 1 ml of hydrogen peroxide were added and left to stand for 5 min, before the vessels were sealed and samples digested in a high pressure microwave digestion system by ramping to 200 °C over 10 min. Samples were maintained at 200 °C for 20 min before cooling. The contents of the vessels were then quantitatively transferred to 100 ml volumetric flasks with deionised water to a final volume of 100 ml.
A comparison of the results obtained by proposed sensor with the results obtained by atomic absorption spectrometry (AAS) shows good agreement as shown in Table 4.
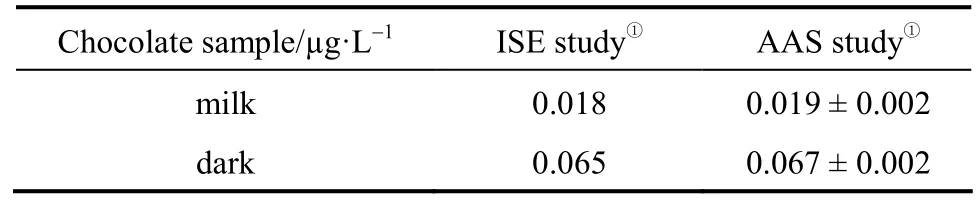
Table 4 Determination of cadmium in chocolate samples using BBC cadmium selective electrode
5 CONCLUSIONS
The potentiometric properties towards sensing of cadmium ion are dependent upon the ionophore, plasticizers and additive used in the preparation of the electrode. The membrane exhibits a linear stable response over a wide concentration range with a near Nernstian response of 29.7 mV per decade. Selectivity coefficients were calculated using FIM. The membrane was found to be stable and recorded steady potential values within 5-8 s with satisfactory reproducibility as compared to previously reported work. The improved pH range for the electrode was 2.0-9.0. The sensor was used for direct determination of cadmium ion in the given chocolate samples with reproducible results.
ACKNOWLEDGEMENT
The author is thankful to the principal, Zakir Husain Delhi College for providing the laboratory facility and Netaji Subhas Institute of Technology for financial assistance.
REFERENCES
1 Andreescu, S., Sadik, O.A., “Trends and challenges in biochemical sensors for clinical and environmental monitoring”, Pure Appl. Chem., 76, 861-878 (2004).
2 Meyerhoff, M.E., Opdyche, M.N., “Ion-selective electrodes”, Adv. Clin. Chem., 25, 1-47 (1986).
3 Ganjali, M.R., Razavi, T., Dinarvand, R., Riahi, S., Norouzi, P.,“New diltiazem potentiometric membrane sensor stands on theoretical calculations as a useful device for diltiazem hydrochloride analysis in pharmaceutical formulation and urine”, Int. J. Electrochem. Sci., 3, 1543-1558 (2008).
4 Ganjali, M.R., Tavakoli, M., Faridbod, F., Riahi, S., Norouzi, P., Salavati-Niassari, M., “Interaction study of a new bis-bidentate schiff’s base with some metal ions and its application in fabrication of Sm(Ⅲ) potentiometric membrane sensor”, Int. J. Electrochem. Sci., 3, 1559-1573 (2008).
5 Ganjali, M.R., Memari, Z., Faridbod, F., Norouzi, P., “Samarium microsensor: An asymetric potentiometric membrane sensor”, Int. J. Electrochem. Sci., 3, 1169-1179 (2008).
6 Gaikwad, D., Shirale, D.J., Savale, P.A., Datta, K., Ghosh, P., Pathan, A.J., Rabbani, G., Shirsat, M.D., “Development of PANI-PVS-GOD electrode by potentiometric method for determination of glucose”, Int. J. Electrochem. Sci., 2, 488-497 (2007).
7 Beheshti, S.S., Amini, M.K., “A simple and selective flow-injection potentiometric method for determination of iodide based on a coated glassy carbon electrode sensor”, Int. J. Electrochem. Sci., 2, 778-787 (2007).
8 Zhang, H., Zhang, Z., Li, J., Cai, S., “Effects of Mg2+on supported bilayer lipid membrane on a glassy carbon electrode during membrane formation”, Int. J. Electrochem. Sci., 2, 788-796 (2007).
9 Codex Alimentarius, Codex General Standard for Contaminants andToxins in Foods and Feeds, Codex Stan 193-1995 (2007).
10 Matza, C.J., Krone, P.H., “Cell death, stress-responsive transgene activation, and deficits in the olfactory system of larval zebrafish following cadmium exposure”, Environ. Sci. Technol., 41, 5143-5148 (2007).
11 Pretto, A., Loro, V.L., Morsch, V.M., Moraes, B.S., Menezes, C., Clasen, B., Hoehne, L., Dressler, V., “Acetylcholinesterase activity, lipid peroxidation, and bioaccumulation in silver catfish (Rhamdia quelen) exposed to cadmium”, Arch. Environ. Contam. Toχicol., 58, 1008-1014 (2010).
12 Verougstraete, V., Lison, D., Hotz, P., “Cadmium, lung and prostate cancer: A systematic review of recent epidemiological data”, J. Toχicol. Environ. Health B: Crit. Rev., 6, 227-255 (2003).
13 Shamsipur, M., Mashhadizadeh, M.H., “Cadmium ion-selective electrode based on tetrathia-12-crown-4”, Talanta, 53, 1065-1071 (2001).
14 Gupta, K.C., d’Arc, M.J., “Cadmium ion-selective electrode based on cyanocopolymer”, Electroanalysis, 12, 1408-1413 (2000).
15 Javanbakht, M., Shabanikia, A., Darvich, M.R., Ganjali, M.R., Shamsipur, M., “Cadmium(II)-selective membrane electrode based on a synthesized tetrol compound”, Anal. Chim. Acta, 408, 75-81 (2000).
16 Gupta, V.K., Chandra, S., Mangla, R., “Dicyclohexano-18-crown-6 as active material in PVC matrix membrane for the fabrication of cadmium selective potentiometric sensor”, Electrochim. Acta, 47, 1579-1586 (2002).
17 Rezaei, B., Meghdadi, S., Bagherpour, S., “Cadmium selective PVC-membranes sensor based on 1, 2-bis (quinoline-2-carboxamido)-4-chlorobenzene as a neutral carrier”, Sensors J. IEEE, 8, 1469-1477 (2008).
18 Wardak, C., Marczewska, B., Lenik, J., “An ion-selective electrode with a polymeric membrane containing an active chelating substance”, Desalination, 163, 69-75 (2004).
19 Abbas, M.N., Zahran, E., “Novel solid-state cadmium ion-selective electrodes based on its tetraiodo- and tetrabromo-ion pairs with cetylpyridinium”, J. Electroanal. Chem., 576, 205-213 (2005).
20 Gupta, V.K., Singh, A.K., Gupta, B., “Schiff bases as cadmium(II) selective ionophores in polymeric membrane electrodes”, Anal. Chim. Acta, 583, 340-348 (2007).
21 Rezaei, B., Meghdadi, S.,Zarandi, R.F., “A fast response cadmiumselective polymeric membrane electrode based on N,N′-(4-methyl-1,2-phenylene)diquinoline-2-carboxamide as a new neutral carrier”, J. Hazard. Mat., 153, 179-186 (2008).
22 Khan, A.A., Khan, A., “Ion-exchange studies on poly-o-anisidine Sn(IV) phosphate nano composite and its application as Cd(II) ion-selective membrane electrode”, Cent. Eur. J. Chem., 8, 396-408 (2010).
23 Craggs, A., Moody, G.J., Thomas, J.D.R., “PVC matrix membrane ion-selective electrodes”, J. Chem. Educ., 51, 541-544 (1974).
24 Katsu, T., Ido, K., Takaishi, K., Yokosu, H., “Ethanol sensors by using RuO2-modified Ni electrode”, Sens. Actuators B, 87, 331-335 (2002).
25 Guilbault, G.G., Durst, R.A., Frant, M.S., Freiser, H., Hansen, E.H., Light, T.S., Pungor, E., Rechnitz, G., Rice, M.N., Rohm, T.J., Simon, W., Thomas, J.D.R., “Recommendations for nomenclature of ion-selective electrodes”, Pure Appl. Chem., 48, 127-132 (1976).
26 Delosa, M., Perez, A., Martin, L.P., Quintana, J.C., Yazdani-Pedram, M., “Influence of different plasticizers on the response of chemical sensors based on polymeric membranes for nitrate ion determination”, Sens. Actuators B, 89, 262-268 (2003).
27 Khayatian, G., Shariati, S., Salimi, A., “Thallium(Ⅰ)-selective membrane potentiometric sensor based on dibenzyldiaza-18-crown-6”, Bull. Kor. Chem. Soc., 24, 421-426 (2003).
28 Schaller, U., Bakker, E., Spichiger, H.E., Pretsch, E., “Ionic additives for ion-selective electrodes based on electrically charged carriers”, Anal. Chem., 66, 391-398 (1994).
29 Hodinar, A., Tyo, A., “Contribution of membrane components to the overall response of anion carrier based solvent polymeric membrane ion-selective electrodes”, Anal. Chem., 61, 1169-1171 (1989).
30 Buhlmann, .P, Pretsch, E., Bakker, E., “Carrier-based ion-selective electrodes and bulk optodes. 2. Ionophores for potentiometric and optical sensors”, Chem. Rev., 98, 1593-1687 (1998).
31 Yua, S., Li, F., Qin, W., “An all-solid-state Cd2+-selective electrode with a low detection limit”, Sens. Actuators B, 155, 919-922 (2011).
32 Umezawa, Y., Buhlmann, P., Umezawa, K., Tohda, K., Amemiya, S.,“Potentiometric selectivity coefficients of ion-selective electrodes Part I. Inorganic cations”, Pure Appl. Chem., 72, 1851-2082 (2000).
33 Chandra, S., Singh, D.R., “Polymeric membrane neodymium(Ⅲ)-selective electrode based on 11,13-diaza-4,7,12-trioxo-2(3),8(9)-dibenzoyl-cyclotetridecane-1,11-diene”, Mat. Sci. Eng. A, 502, 107-110 (2009).
34 Gupta, V.K., Jain, A.K., Ludwig, R., Maheshwaria, G., “Electroanalytical studies on cadmium(II) selective potentiometric sensors based on t-butyl thiacalix[4]arene and thiacalix[4]arene in poly(vinyl chloride)”, Electrochim. Acta, 53, 2362-2368 (2008).
35 Rawat, A., Chandra, S., Sarkar, A., “Highly selective potentiometric oxalate ion sensors based on Ni(II) bis-(m-aminoacetophenone) ethylenediamine”, Chin. J. Chem., 28, 1140-1146 (2010).
2012-08-12, accepted 2013-01-08.
* To whom correspondence should be addressed. E-mail: deepshikha.research@gmail.com; schandra_00@yahoo.com
 Chinese Journal of Chemical Engineering2014年4期
Chinese Journal of Chemical Engineering2014年4期
- Chinese Journal of Chemical Engineering的其它文章
- Determination and Correlation of Solubilities of Four Novel Benzothiazolium Ionic Liquids with6PF−in Six Alcohols*
- An Approach to Formulation of FNLP with Complex Piecewise Linear Membership Functions
- One Step Preparation of Sulfonated Solid Catalyst and Its Effect in Esterification Reaction*
- Impacts of Power Density on Heavy Metal Release During Ultrasonic Sludge Treatment Process*
- Determination and Correlation of Solubility for D-Xylose in Volatile Fatty Acid Solvents*
- Effects of Assistant Solvents and Mixing Intensity on the Bromination Process of Butyl Rubber*
