A Modified Peng-Robinson Equation State for Prediction of Gas Adsorption Isotherm
Ali Akbar Amooey*Department of Chemical Engineering, University of Mazandaran, Babolsar 47416-95447, Iran
A Modified Peng-Robinson Equation State for Prediction of Gas Adsorption Isotherm
Ali Akbar Amooey*
Department of Chemical Engineering, University of Mazandaran, Babolsar 47416-95447, Iran
This research proposes a modified two-dimensional Peng-Robinson equation model to predict adsorption isotherm in adsorbate-adsorbent systems. The parameters of the proposed model are calculated by using the optimization of experimental data for the different single gas adsorption systems at various temperatures. The experimental adsorption equilibrium data of adsorbate-adsorbent systems was compared with the calculated results in our proposed model and the two-dimensional Hill-deBoer equation model. The proposed model as indicated in the results shows a better prediction of the experimental results compared with two others.
gas adsorption isotherm, Peng-Robinson equation state
1 INTRODUCTION
Man was aware of adsorption phenomena for a very long time and used it in an increasing way to perform desired bulk separation or purification purposes. A porous solid medium is usually core to the adsorption process. In describing single component of the adsorption equilibrium it is undoubtedly necessary to use a suitable isotherm equation so as to represent adsorption kinetics [1].
Such fluids as gas and liquid adsorbed on the solid surface are actually distributed on the solid surface un-uniformly and are inhomogeneous, i.e., their density is a function of the distance from the solid surface. In this case, the modern density functional theory [2, 3] is a powerful tool to describe this adsorption equilibrium. However, density functional theory needs the integral equations to be solved by the numerical iterations so as to obtain density profiles of the fluids in the first place. Therefore some simplified models appear to be simple enough for real gas adsorption. This requires that the surface layer be treated as a third homogeneous phase, as done in the Flowler-Guggenheim and Langmuir models.
There are several simplified models for adsorption isotherm. Langmuir model [4] is the primary theory in adsorption; Langmuir suggested a model for adsorption of gases onto a flat surface based on a kinetic viewpoint. It is clear that Langmuir’s approach is kinetic by nature, but adsorption equilibria can also be explained by the thermodynamic approach. In Gibbs approach the classical thermodynamic, in the bulk phase of the volume concept and gas pressure is replaced by the area and spreading pressure, respectively. Using Gibbs equation, a number of two dimensional isotherm equations were derived such as the Volmer isotherm [5] and the Hill-deBoor isotherm [6, 7]. Volmer used imperfect gas low-effect of covolume term in ideal equation state and Hill-deBoor obtained his model on the basis of the van der Wall’s equation state. In other approach it was assumed that the surface of an adsorbent can be treated as a solvent and the adsorption process as the formation of the solution between the adsorbate and adsorbent; this model is called the vacancy solution model (VSM). Suwanayuen and Danner [8] used Wilson activity equation while Cochran et al. [9] used the Flory-Huggins equation that leads to one lower parameter in comparison with the original vacancy solution model. Based on the vacancy solution theory (VST) and using the non-random two liquid model (NRTL) activity coefficient model, recently Haghtalab and et al. [10] developed a new adsorption isotherm. Basic knowledge of classic adsorption isotherm equations as mentioned above is discussed in article of Terzyk et al [11].
Generally speaking, in the thermodynamic representation of adsorption equilibrium, there are two methods to describe the adsorbate-adsorbent system, either a two-dimensional gas or as a solution which is composed of adsorbate and vacancies. In this research, we followed the two-dimensional approach to develop a new isotherm using the Peng-Robinson and our proposed modified Peng-Robinson equation state. Comparison between the pressure deviation in the modified Peng-Robinson equation state and the results from this and Hill-deBoer equation model shows that the former works much better than the others.
2 THE MODIFIED TWO-DIMENSIONAL PENGROBINSON EQUATION MODEL
The study of gas-liquid equilibria plays an important role in solution thermodynamics. It resembles very much the study of gas-solid adsorption equilibria. Van Ness [12] considered the actual interfacial region as transformable into an imaginary mathematical surface in which the properties of the gas phase imagined to be the same until it reaches this surface which is treated as a two-dimensional phase with its own thermodynamic properties.
According to Do [5] when the adsorbed phase is treated as a two-dimensional surface, the fundamental equations in classical thermodynamics can still be applied. Thus, the Gibbs-Duhem equation at constant temperature for a planar surface is as follows [5]

where A is the surface area of an adsorbent, niis the mole amount of species i, π is the spreading pressure, playing the same role as the pressure in the bulk phase, and μiis the surface chemical potential of species i. In a two-dimensional approach we have only one component (i.e., an adsorbate). If this component is shown by the subscript “1,” Eq. (1) can take the form below:
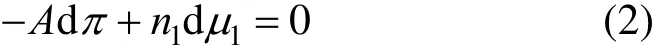
At equilibrium, the chemical potential of the adsorbed phase is equal to that of the gas phase, which is supposed to be ideal; i.e., [5]
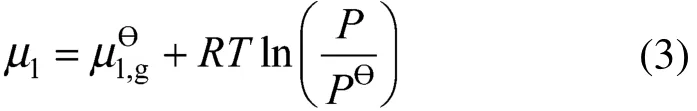
where P is the pressure of “1” in the gas phase and is the standard pressure.
Inserting Eq. (3) into Eq. (2), the fundamental Gibbs formula is derived as follows [1],

where σ is equal to

Ω1and θ represent the surface area occupied by one mole of the adsorbate molecules, and the surface coverage respectively which are as below:

Two-dimensional Peng-Robinson equation state is as follows:
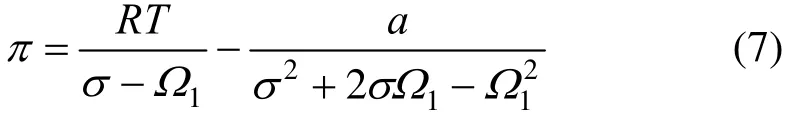
the a is a characteristic constant, and T is the absolute temperature.
Inserting Eq. (7) into Eq. (4) the following purecomponent adsorption isotherm would be resulted:

The Henry’s law constant k in the above equation is defined as:

The modified Two-dimensional Peng-Robinson equation state used in this work is as a result of introducing a parameter m into Eq. (7),
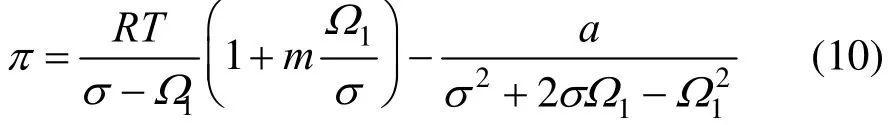
Inserting Eq. (10) into Eq. (4) the following purecomponent adsorption isotherm would be resulted:
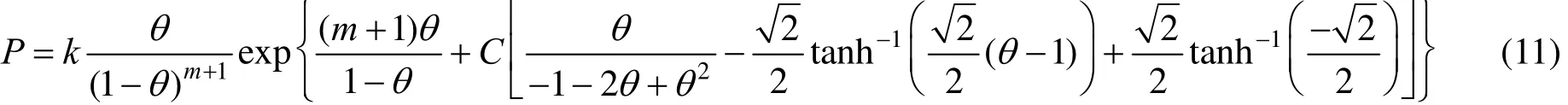
As a result, we derived Eq. (8) and then we modified Peng-Robinson equation state and Eq. (11) is derived for the prediction of adsorption isotherm in adsorbate-adsorbent systems.
3 RESULT AND DISCUSSION
The new isotherm model, Eq. (8) and Eq. (11), is tested with pure gas adsorption data for different gases such as CH4, C2H6, C3H8, H2S, CO2and water vapor on the diverse adsorbents at three different temperature. Adsorption data for 10 different systems of adsorbate-adsorbent from various sources are collected at different temperature as shown in Table 1.
Table 2 reports the parameters of each Eq. (8), Eq. (11) and Hill-deBoer equation model resulted from the least-square optimization procedure. The new isotherm parameters are as a result of the optimization of the following objective function:

In the objective function in use, N is the number of experimental data.
The parameters of Hill-deBoer equation model are also optimized here, using both the new models and Hill-deBoer equation model the deviation for the same systems are presented in Table 3. As is observed the results from modified Peng-Robinson equation model is better than those of Peng-Robinson and Hill-deBoer equation models. One of the advantages of the present isotherm is much better correlation between the pressure
of such polar systems as water vapor and H2S compared with the isotherm using Peng-Robinson and Hill-deBoer equation models.

Table 1 Experimental data on diverse gas adsorption isotherms used in this research
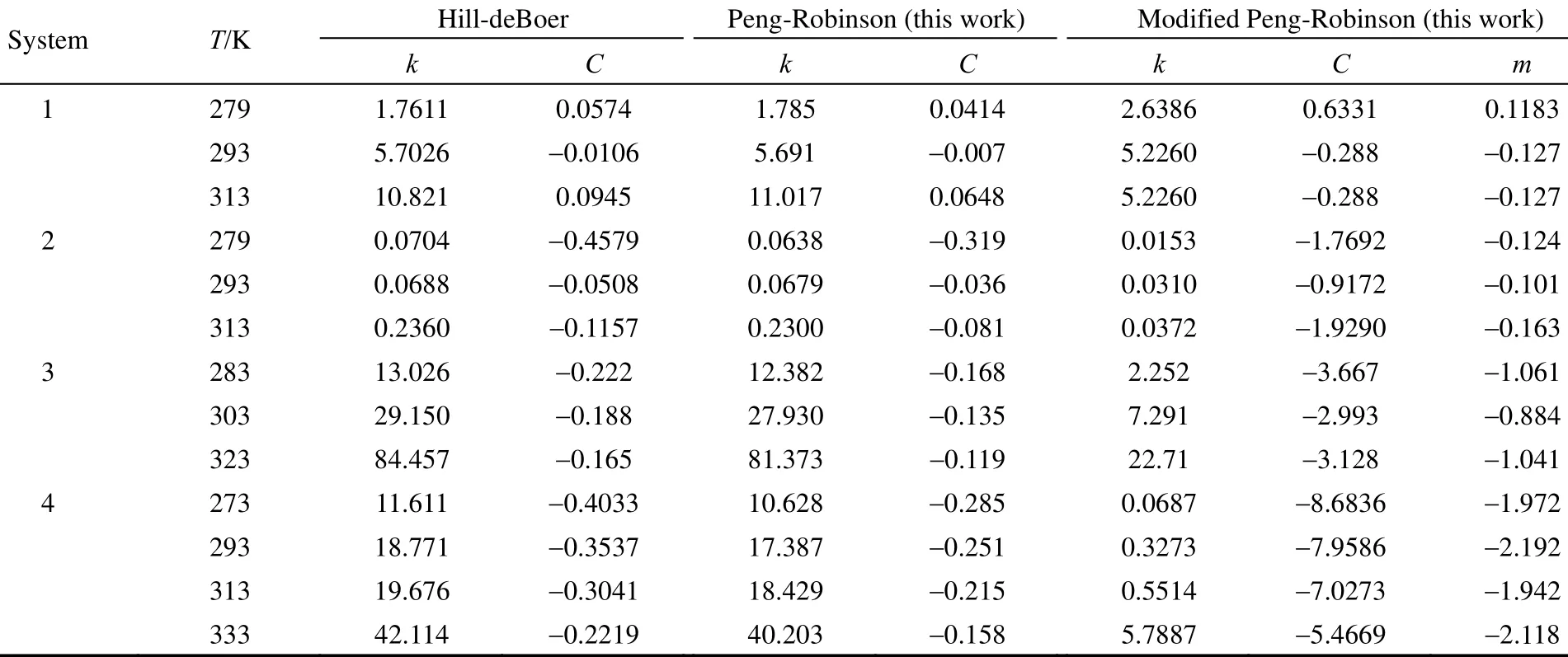
Table 2 The parameter of the new adsorption isotherm for the systems listed in Table 1
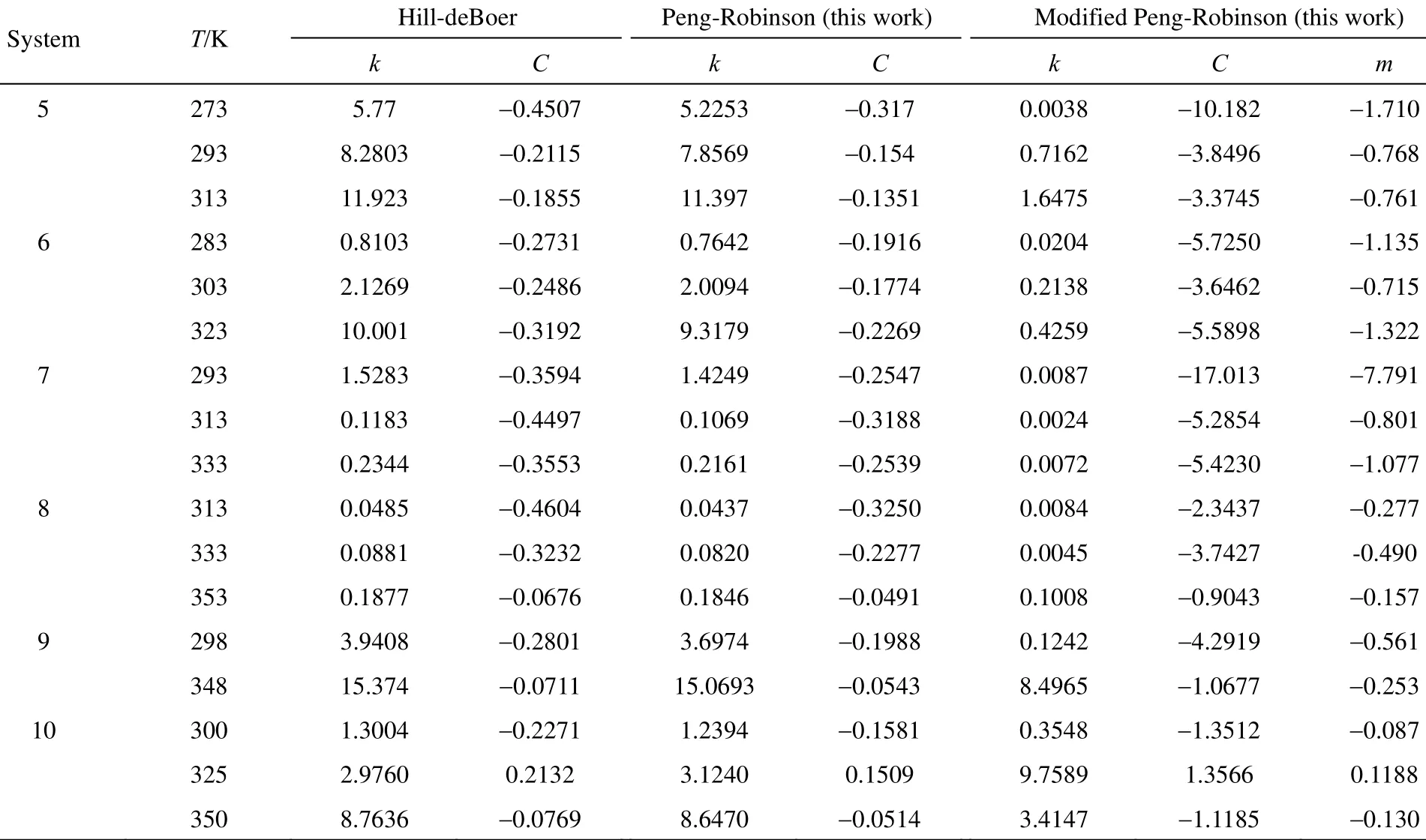
Table 2 (Continued)
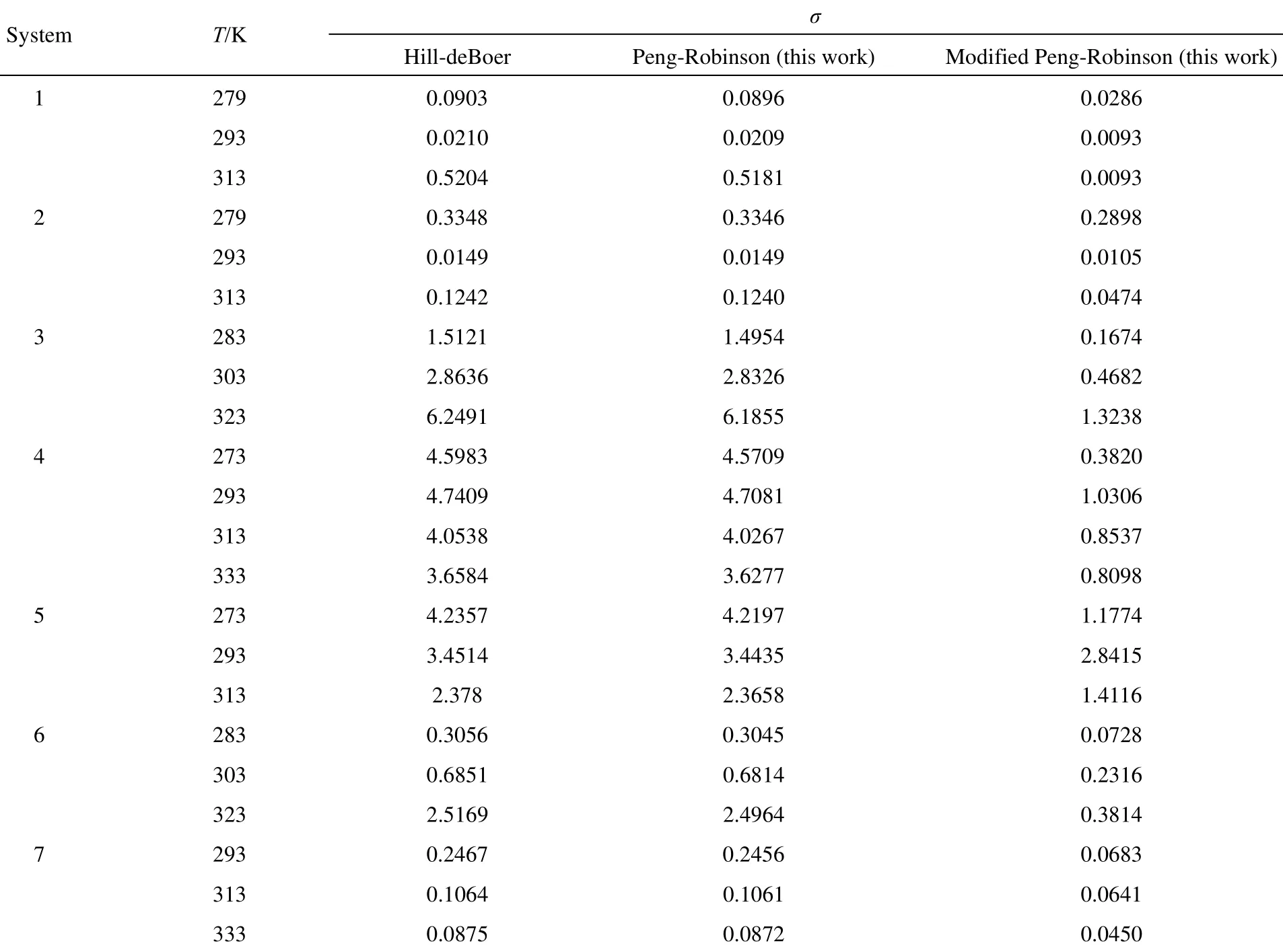
Table 3 Comparison of the new model and other models
Figure 1 demonstrates the calculated and experimental adsorption pressure versus number of moles of the adsorbed gas at various temperatures for the CO2on zeocarbon. The figure shows that the results of the modified Peng-Robinson equation model are in a very good agreement with the experimental. This system as understood represents a polar system the way that the calculated result is in a very good agreement with experimental data. Similarity, Fig. 2 presents the adsorption H2S on mordenite and it is clear that the results of modified Peng-Robinson equation model are in a good agreement with the experimental. Fig. 3 presents the adsorption of water vapor on zeolite and it is clear that the results of modified Peng-Robinson equation model are in a good agreement with the experimental.
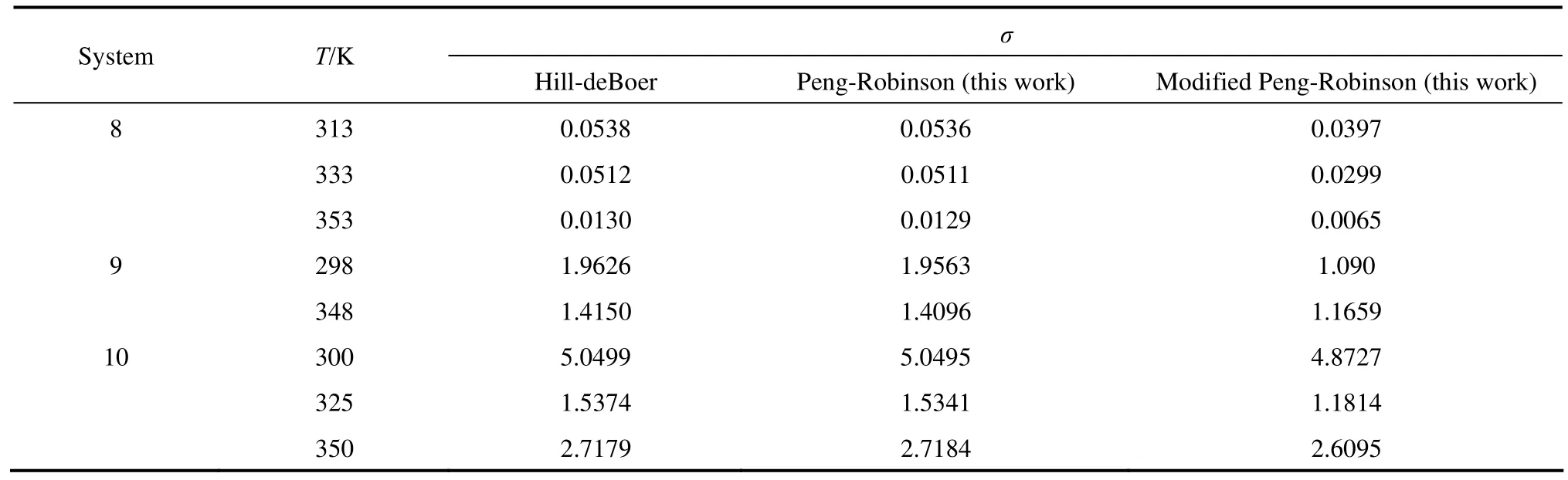
Table 3 (Continued)
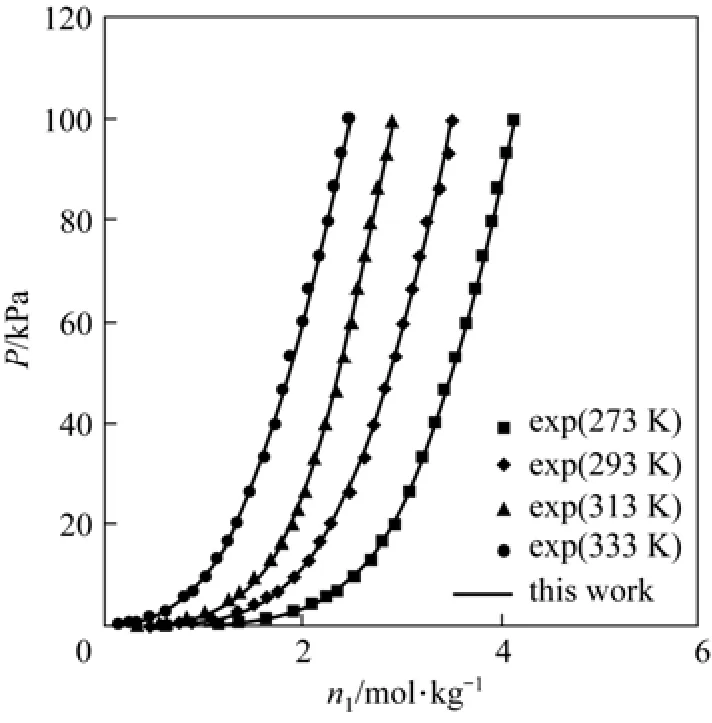
Figure 1 Adsorption isotherms of CO2on zeocarbon at different temperature
As shown in Table 2, values of the parameters resulted from such equation models as Hill-deBoer, Peng-Robinson and modified Peng-Robinson have not any correlation with adsorbents for the same adsorbate gas, and each system has the unique parameters.

Figure 2 Adsorption isotherms of H2S on mordenite at different temperature
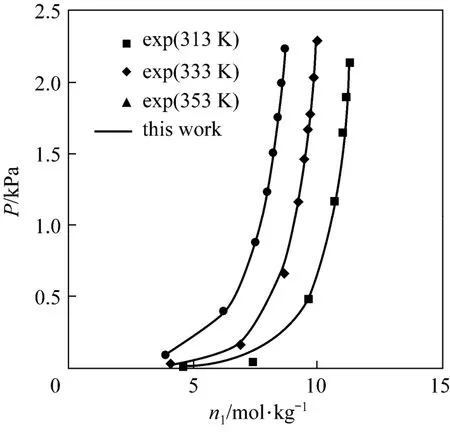
Figure 3 Adsorption isotherms of water vapor on zeolite at different temperature
4 CONCLUSIONS
We proposed new adsorption isotherm based on modified Peng-Robinson equation model for adsorption of pure gases on the solid adsorbent. The optimized experimental data determined parameters of the new model. The latter used for the 10 single gas adsorbateadsorbent systems at different temperature and the results compared with Peng-Robinson and Hill-deBoerequation models. The modified Peng-Robinson equation model as indicated in the results showed a better prediction of the experimental results compared with two others.
NOMENCLATURE

REFERENCES
1 Ding, L.P., Bhatia, S.K., Liu, F., “Kinetics of adsorption on activated carbon: Application of heterogeneous vacancy solution theory”, Chem. Eng. Sci., 57 (18), 3909-3928 (2002).
2 Yu, Y.X., “A novel weighted density functional theory for adsorption, fluid-solid interfacial tension, and disjoining properties of simple liquid films on planar solid surfaces”, J. Chem. Phys., 131 (2), 024704-1-024704-11 (2009).
3 Peng, B., Yu, Y.X., “A density functional theory for Lannard-Jones fluids in cylindrical pores and its applications to adsorption of nitrogen on MCM-41 materials”, Langmuir, 24 (21), 12431-12439 (2008).
4 Langmuir, I., “The adsorption of gases on plane surfaces of glass, mica and platinum”, J. Am. Chem. Soc, 40 (9), 1361-143 (1918).
5 Do, D.D., Adsorption Analysis: Equilibria and Kinetics, Imperial College Press, London (1998).
6 Hill, T.L., “Localized and mobile adsorption and adsorption”, J. Chem. Phys., 14 (7), 441-552 (1946)
7 de Boer, J.H., The Dynamical Character of Adsorption, Oxford University Press, Oxford (1953).
8 Suwanayuen, S., Danner., R.A., “A gas adsorption isotherm equation based on vacancy solution theory”, AIChE J., 26 (1), 68-76 (1980).
9 Cochran, T.W., Kabel, R.L., Danner, R.P., “Vacancy solution theory of adsorption using Flory-Huggins activity coefficient equations”, AIChE J., 31 (2), 268-277 (1985).
10 Haghtalab, A., Farzad, S., “A new gas adsorption isotherm using the vacancy solution theory and NRTL activity coefficient model”, Fluid Phase Equilibria, 292 (1-2), 36-41 (2010).
11 Koter, S., Terzyk, A.P., “Two-dimensional gas and vacancy solution approaches in the thermodynamic description of adsorption equilibrium”, J. Colloid and Inter Sci, 282 (2), 335-339 (2005).
12 Van Ness, H.C., “Adsorption of Gases on Solids. Review of Role of Thermodynamics”, Ind. Eng. Chem. Fundam., 8 (3), 464-473 (1969).
13 Costa, E., Guillermo, C., Jimenez, A., Pau, J., “Adsorption equilibrium of ethylene, propane, propylene, carbon dioxide, and their mixtures on 13X zeolite”, J. Chem. Eng. Data, 36 (2), 218-224 (1991).
14 Talu, O., Zwiebel, I., “Multicomponent adsorption equilibria of nonideal mixtures”, AIChE J., 32 (8), 1263-1276 (1986).
15 Seok, J., Kim, J.H., “Adsorption equilibria of CO2on Zeolite 13X and zeolite X/Activated carbon composite”, J. Chem. Eng. Data., 47 (5), 1237-1242 (2002).
16 Hwa, K.J., Chang, H.L., Woo, S.K., Jung, M.L., “Adsorption equilibria of water vapor on alumina, zeolite 13X, and a zeolite X/activated carbon composite”, J. Chem. Eng. Data, 48 (1), 137-141 (2003).
17 Moon, D.J., Chung, M.J., Cho, S.Y. Ahn, B.S., Park, K.Y., “Adsorption equilibria of chloropentafluoroethane and pentafluoroethane on activated carbon pellet”, J. Chem. Eng. Data, 43 (5), 861-864 (1998).
18 Loughlin, K.F., Hasanain, M.A., Abdul-Rehman, H.B., “Quaternary, ternary, binary, and pure component sorption on zeolites. 2. Light alkanes on Linde 5A and 13X zeolites at moderate to high pressures”, Ind. Eng. Chem. Res., 29 (7), 1535-1546 (1990).
2013-02-20, accepted 2013-05-22.
* To whom correspondence should be addressed. E-mail: aamooey@umz.ac.ir
 Chinese Journal of Chemical Engineering2014年6期
Chinese Journal of Chemical Engineering2014年6期
- Chinese Journal of Chemical Engineering的其它文章
- Roles of Biomolecules in the Biosynthesis of Silver Nanoparticles: Case of Gardenia jasminoides Extract*
- Modeling and Optimization for Short-term Scheduling of Multipurpose Batch Plants*
- Symbiosis Analysis on Industrial Ecological System*
- Phase Behavior of Sodium Dodecyl Sulfate-n-Butanol-Kerosene-Water Microemulsion System*
- Photochemical Process Modeling and Analysis of Ozone Generation
- Unified Model of Purification Units in Hydrogen Networks*
