表面改性锌镁铝三元类水滑石的摩擦性能及抗磨机理
李 硕,白志民,赵 栋
(中国地质大学材料科学与工程学院,北京100083)
1 Introduction
Friction and wear can lead to materials failure and energy consumption and emission problems.It is difficult for some conventional liquid lubricants to satisfy the modern demands of economic and environmental benefits.Nanoparticles as lubricant additives,such as metal,[1]graphite,[2]metal sulfide,[3]oxide,[4--5]carbonate,[6]rare earth compound,[7]and serpentine powders,[8--9]have attracted considerable attention due to their excellent friction-reducing,anti-wear,load-carrying and self-repairing properties under boundary lubrication condition in the past decades.
Layered double hydroxides(LDHs),also known as anionic clays or hydrotalcite-like compounds(HTlcs),are a family of layered mineral materials,which contain positively charged brucite-like metal hydroxide layers and charge-balancing anions located in the interlayer.Partial divalent metal cations are substituted by trivalent metal cations,leading to surplus positive charges on layers,which are compensated by interlayer anions.[10]The general formula of LDHs can be expressed by[M2+1-xM3+x(OH)2]x+(An-)x/n·mH2O,where xis the molar ratio of trivalent cations M3+/(M2++M3+),An-is the exchangeable anion and mis the amount of water molecule in the interlayer gallery.LDHs have been broadly studied for their applications in the areas of catalysts,absorbents,anion exchange and biosensors[10--12]and will expand their potential uses due to the variability of layer cations and interlayer anions.
In our previous work on the tribological properties of different types of LDHs nanoparticles(Co/Al-LDHs,Mg/Al-LDHs,Zn/Mg/Al-LDHs,etc)as lubricant additives,[13--17]LDHs can reduce friction and wear effectively.Therefore,LDHs are a potential lubricant additive with superior lubricity.In tthis paper,surface-modified Zn/Mg/Al-CO2-3
LDHs(SMZMAC-LDHs)with the average size of 179.4nm were synthesized,and the tribological properties of oil containing LDHs under different loads and rotational speeds were further investigated in a four-ball friction tester.In addition,the anti-wear mechanism was also discussed.
2 Experimental
2.1 Synthesis of LDHs
All raw materials(Zn(NO3)2·6H2O,Mg(NO3)2·6H2O,Al(NO3)3·9H2O,NaOH and Na2CO3,oleic acid)used for the synthesis of SMZMAC-LDHs were analytically pure without further purification.Deionized water was prepared in the laboratory.The Zn/Mg/Al-CO2-3LDHs(ZMAC-LDHs)were synthesized by a coprecipitation method.[15]NaOH and Na2CO3were dissolved in deionized water and then the alkaline solution(n[OH-]=0.8mol/L)was added in dropwise to a salt solution of Zn(NO3)2·6H2O,Mg(NO3)2·6H2O and Al(NO3)3·9H2O with n(Zn)/n(Mg)/n(Al)molar ratio of 1∶1∶1(n[Zn2+]+n[Mg2+]+n[Al3+]=0.4mol/L)until the pH reached 10under stirring at the room temperature for 1h.The mother solution was moved to an autoclave and aged for 14hat 100℃.The ZMAC-LDHs precipitate was centrifuged and washed for several times with deionized water when pH reached 7.Afterwards,the neutral precipitate was dispersed in the stirred deionized water until it turned into a homogeneous slurry and then added indropwise mixed solution of oleic acid to the slurry under ultrasound for 20min.Finally,the SMZMAC-LDHs precipitate was filtered and washed for several times with deionized water and absolute ethanol,and dried at 80℃for 12h.
The crystal structure of LDHs samples was characterized by X-ray diffraction(XRD)with a Rigaku diffractometer(CuKαsource,λ=0.154 06 nm,operated at 40kV and 100mA,scanning rate 8(°)/min from 3°to 70°(2θ)).The images for the morphology were obtained on a model BCPCAS4800 field emission scanning electron microscope(SEM)at an acceleration voltage of 15.0kV.The particle size distribution of LDHs was obtained by Zetasizer(Nano ZS90,Malvern,UK).The chemical composition of LDHs was identified by a wavelength dispersive X-ray fluorescence(WD-XRF,Rigaku ZSX PrimusⅡ).The infrared spectra were recorded in a model NICOLET750 Fourier transform infrared(FTIR)spectrometer at 4 000--450cm--1with KBr sheet.
2.2 Tribological test and worn surface analysis
The friction performance was examined in a model MS-10JR four-ball friction tester,and the balls used are GCr15 steel(AISI 52100 steel,0.95%--1.05% C,1.30%--1.65% Cr,0.15%--0.35%Si,0.25%--0.45%Mn,≤0.027%P,≤0.02%S,≤0.23%Ni,≤0.25%Cu,diameter of 12.70mm and hardness of 64--66 HRC),which were cleaned with petroleum ether under absolute ethanol ultrasound for 10min before the test.The diesel engine oil(CD15W-40)was used as a base oil with the viscosity of 15.02mm2/s at 100℃and 110.60mm2/s at 40 ℃,viscosity index of 228,open flash-point temperature of 228℃and boiling point of 300 ℃.The base oil with 0.5%in mass SMZMAC-LDHs particles were stirred at a high rotational speed of 10 000r/min and under ultrasound to disperse the LDHs particles in the base oil.The base oil with SMZMAC-LDHs was put in a 50mL tube at room temperature to examine its suspension stability.The tribological property of oil containing LDHs was measured at various loads(i.e.,100,200,300and 400N)and rotational speeds(i.e.,600,1 200and 1 800r/min),respectively.The friction coefficient was recorded in a computer.The average friction coefficient for each experimental condition was calculated,and the average wear scar diameter of 3lower balls was determined by an optical microscopy.
The morphologies and elements analysis of the worn surface after friction were characterized by scanning electron microscope equipped with energy dispersive Xray spectrometer.An ESCALAB 250X-ray photoelectron spectrometer was utilized to analyze the chemical state of surface elements,using monochromatic Al Kαradiation as the excitation source and the binding energy of C1s(284.6eV)as a reference,at a passing energy of 30eV.
3 Results and discussion
3.1 X-ray diffraction and morphology of LDHs
Figure 1 shows the XRD pattern of ZMACLDHs.There exist typical diffraction peaks of hydrotalcite phase.[10]The(003),(006)and(110)peaks appear at 11.54°,23.36°and 60.36°,respectively,indicating that synthetic ZMACLDHs crystal grains possess a hydrotalcite-like laminated structure.Table 1shows the lattice parameters.From the XRF results as shown in Table 2,the chemical formula of ZMAC-LDHs can be determined as Zn0.37Mg0.27Al0.36(OH)2(CO3)0.18·0.31H2O.
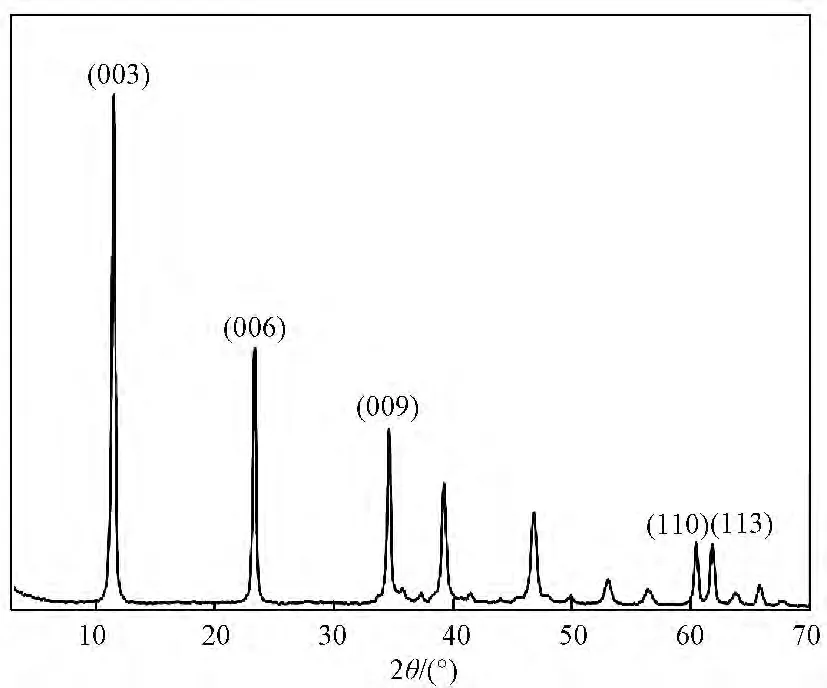
Fig.1 XRD pattern of ZMAC-LDHs

Table 1 Lattice parameters of ZMAC-LDHs
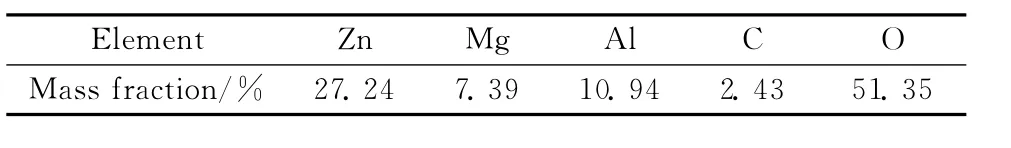
Table 2 Chemical composition of ZMAC-LDHs
Figure 2shows the SEM image and particle size distribution of SMZMAC-LDHs.From the SEM image,it is seen that the particles have a typical hydrotalcite-like hexagonal lamellar structure and most of the partciels disperse uniformly due to surface modification.The particle size range is 100--325nm and the average size is 179.4nm.
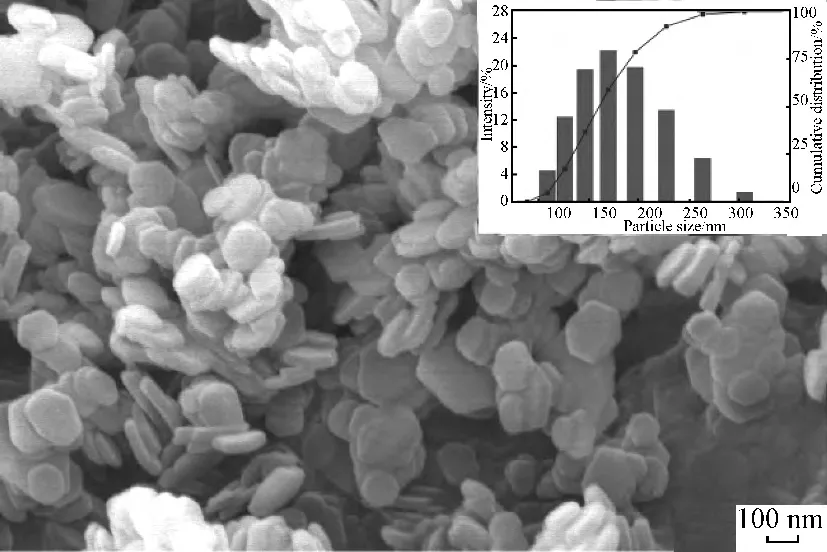
Fig.2 SEM image and particle size distribution of SMZMAC-LDHs
3.2 Fourier transform infrared spectra of SMZMACLDHs
Figure 3shows the FTIR spectra of SMZMACLDHs.The sharp band at 3 451cm--1is attributed to O--H stretching vibration of interlayer water molecular and hydroxyl groups of brucite-like layers.The shoulder observed between 3 150cm--1and 2 980cm--1is assigned to hydrogen bonds between water and,which cause the v3asymmetric stretching vibration of free(v3=1 415cm--1)shifted to the intense band of 1 363cm--1.[13]The v2vibration of interlayercorresponds to 942cm--1.In addition,the bands below 800cm--1can be assigned to the stretching vibration of Zn(Mg,Al)--OH.[14]The asymmetric and symmetric stretching vibrations of C--H of long alkyl chains(2 926cm--1and 2 855cm--1,respectively)demonstrate that oleic acid molecules appear on the surface of LDHs.The band of 1 583cm--1assigning to carboxylate appears,but the carboxyl group-COOH vibration peak of oleic acid spectrum at 1 710cm--1disappears.It indicates that the oleic acid molecules are adsorbed on the surface of ZMAC-LDHs with a monomolecular layer of oleic acid that can improve the suspension stability of LDHs in base oil.[15]The base oil with SMZMAC-LDHs nanoparticles is homogeneous and stable.

Fig.3 FTIR spectra of SMZMAC-LDHs
3.3 Friction and wear performance
3.3.1 Load effect on the tribological properties of LDHs Figure 4shows the friction coefficient of base oil and oil containing LDHs at different loads(i.e.,100,200,300and 400N)for 60min.Compared to base oil,the base oil containing LDHs has little anti-friction effect at 100N.However,the oil containing LDHs has an anti-friction effect and the friction coefficient and fluctuation reduce as the load increases to 200--400N.Figure 5 shows the average friction coefficient of base oil and oil containing LDHs at various loads.It is seen that the friction coefficient decreases gradually in the load range of 100--400N.This phenomena can be explained by the Stribeck curve showing that the friction coefficient(μ)is a function of rotational speed(V),normal force(P)and kinematic viscosity of lubricating oil(η).[18]Under boundary lubrication,the friction coefficient is correlated to the load in a oil used at a certain rotational speed.However,this correlation is not appropriate to the oil containing LDHs since the values ofμat a high load(i.e.,200--400 N)do not increase but decrease instead,compared to that at 100N(see Table 3).Similarly,the wear scar diameters of steel balls lubricated in oil containing LDHs at 200--400Nreduce,compared to the cases in base oil(see Fig.6and Table 4).From the results above,it is indicated that the temperature and pressure between rotating steel balls can promote the nanoparticles to form protective film on the rubbed surfaces to reduce friction and wear at a high load.Besides,there is a competitive relation between the formation and removal of protective film when the load increases.[19--20]Therefore,it can be concluded that LDHs in base oil possess effective anti-friction properties at a high load.

Fig.4 Average friction coefficient of base oil and oil containing LDHs at different loads
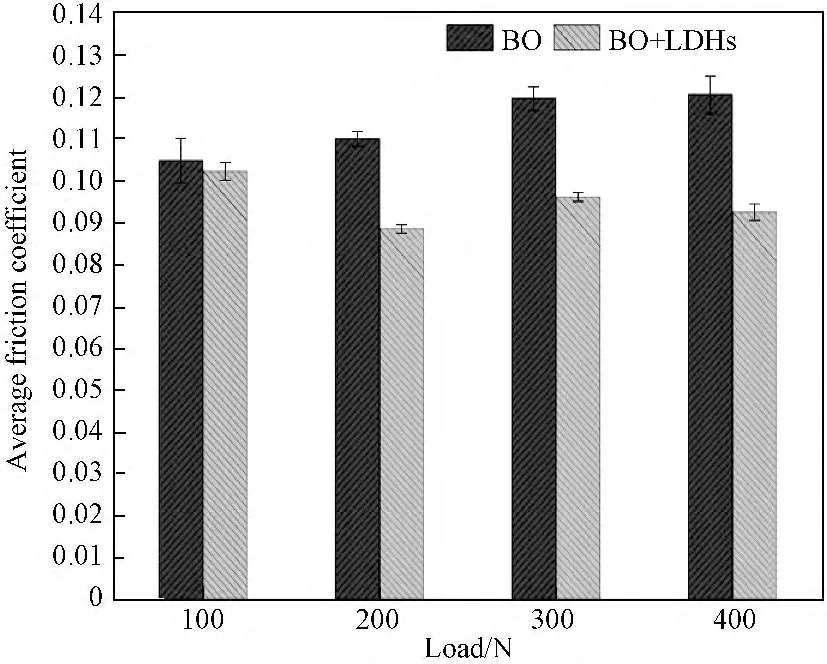
Fig.5 Friction coefficient of base oil and oil containing LDHs at different loads for 60min

Fig.6 Wear scar diameter of steel balls lubricated by base oil and oil containing LDHs at different loads

Table 3 Average friction coefficients of base oil and oil containing LDHs at different loads

Table 4 Wear scar diameters of steel balls lubricated in base oil(A)and oil containing LDHs(B)at different loads
3.3.2 Rotational speed effect on the tribological properties of LDHs Figure 7shows the effect of rotational speed on the friction coefficient at different loads(i.e.,200,300and 400N).It is seen that the value ofμdecreases continually when the speed increases from 600r/min to 1 800r/min.The results can be explained by the Stribeck curve that at definite load and oil,the friction coefficient(μ)has an inverse correlation with the speed(V)under boundary lubrication condition.[18]The wear scar diameters of steel balls lubricated in oil containing LDHs at 200and 300Ndecrease as the rotational speed increases from 600r/min to 1 200r/min(see Fig.8).A high rotational speed enhances the surface temperature and shearing force between the steel balls and LDHs nanoparticles are easy to deposit and form a protective film on the worn surfaces to reduce the wear.When the speed further increases to 1 800r/min,the diameters of wear scars increase.

Fig.7 Average friction coefficient of oil containing LDHs at various rotational speeds for 60min

Fig.8 Wear scar diameter of steel balls lubricated in oil containing LDHs at various rotational speeds
However,the wear scars become greater at a high load of 400Nas the rotational speed increases from 600r/min to 1 800r/min.It is indicated that LDHs nanoparticles can play superior wear-resisting properties only at an appropriate rotational speed in a certain load range.
3.4 Worn surface analysis
Figure 9shows the SEM morphology of worn surfaces lubricated in base oil and oil containing LDHs at various loads of 200,300and 400Nat a rotational speed of 1 200r/min for 60 min.It is seen that the grooves and furrows in sliding direction are wide and deep on the surface lubricated in base oil(see Fig.9(a),(c),(e)and(g)).For the steel balls lubricated in oil containing 0.5%LDHs,the furrows become narrow and shallow with smoother surfaces(see Fig.9(b),(d),(f)and(h)).The metal surface lubricated in base oil with plowing and corrosion is scuffed severely,showing the abrasive and adhesive wear with massive microapertures(see Fig.9(e)and(g)).However,the abrasion of surface lubricated in oil containing LDHs shows mild and smooth wear with tiny scuffing since the nanoparticles form a protective film on the rubbed surfaces(see Fig.9(f)and(h)).Fig.10(b)shows that there exist the elements of Zn,Mg,Fe,C,O,Cr and S on the surface lubricated in oil containing LDHs at 400N.It can be deduced that Zn and Mg stem from the LDHs.

Fig.9 SEM images of worn surface lubricated in(a,c,e and g)base oil and(b,d,f and h)oil containing LDHs at different loads and a rotational speed of 1 200r/min for 60min

Fig.10 EDS spectra of worn surface lubricated in base oil(a)and oil containing LDHs(b)at a load of 400N
Figure 11shows the XPS spectra of worn surface lubricated in oil containing LDHs at 400Nand the speed of 1 200r/min.It is seen that the Zn2pspectrum at a binding energy of 1 021.7eV with the two sub-peaks at 1 021.5and 1 022.1eV illustrates the existence of ZnO and ZnS,which the element of S comes from the steel substrate or base oil.[4]Mg 1s peak at 1 303.5eV indicates Mg on the surface in the state of MgO.[21]Al 2pspectrum with only noise signal represents the absence of Al.The Fe 2p3/2spectrum with the four sub-peaks at binding energies of 709.2,710.5,711.4and 713.6eV declares the presence of FeO,Fe3O4,Fe2O3and Fe-containing organic compounds on the worn surface.[19--20,22--23]C 1s spectrum with three sub-peaks at binding energies of 284.6,285.2and 288.9eV,shows the bonding states of C.A peak at 284.6eV corresponding to CHnor C--C bond reveals long alkyl chains and graphite absorbing on the surface,and peaks at 285.1and 289.0eV demonstrate the presence of C--O and C=O bond of organic compounds.[5,7,19--20,24]The O 1s spectrum with subpeaks at 529.8,531.3and 532.2eV indicates the existence of oxygen that stems from metal oxides(i.e.,zinc oxide,magnesium oxide,iron oxide,etc.)and organic compounds.[6,19--20,24]
From the results above,the following friction reducing and antiwear mechanisms could be given:
1)The surface-modified LDHs nanoparticles deposit and fill the grooves of worn surfaces to compensate the wear loss and polish to improve the surface roughness;[19,25]
2)A hard protective film of oxides,sulfide,graphite and organic compounds with superior antiwear properties can be formed on the rubbed surface.The tribofilm reaction mechanism is similar to that of serpentite.[8--9,19]However,the ceramic phase of alumina in the film formed by serpentite does not exist on the worn surface despite LDHs containing the Al(OH)6octahedron.It could be explained that the temperature between friction pairs is not high enough for the phase transformation from aluminum hydroxides to alumina(i.e.,>1 200℃);[26--27]
3)Due to the removal of interlayer carbonates and water of LDH particles at high surface temperature[10,28]and the reduction of the particle size during friction,the LDHs nanoparticles deposited on the worn surfaces can act as third bodies to reduce the friction and wear.[9]
4 Conclusions

Thick lines represent the XPS spectrum;Thin lines represent the fitted curves.Fig.11 XPS spectra of worn surface lubricated in oil containing LDHs at 400Nand a rotational speed of 1 200r/min
2)The base oil containing LDHs possessed superior anti-friction properties at high loads(i.e.,200--400N)and an appropriate rotational speed.The reduction of average friction coefficient of oil with LDHs at 400N was optimum and the Stribeck curve could not be used to annlyze the relation between friction coefficient and load due to the addition of LDHs nanoparticles.Besides,there was a competition between the formation and removal of protective film at different loads and rotational speeds.
3)The anti-wear mechanism of LDHs could be described as follows:the particles polished the worn surfaces;a hard protective tribo-film containing metal oxides,sulfide,graphite and organic compounds was form;and the nanoparticles acted as third bodies to reduce the friction and wear.
Acknowledgment
Thanks for guidance of XPS analysis from research fellow Bin Cheng of Beijing University of Chemical Technology.
Reference:
[1] TARASOV S,KOLUBAEV A,BELYAEV S,et al.Study of friction reduction by nanocopper additives to motor oil[J].Wear,2002,252(1/2):63-69.
[2] HUHANG H D,TU J P,GAN L P,et al.An investigation on tribological properties of graphite nanosheets as oil additive[J].Wear,2006,261(2):140-144.
[3] TANNOUS J,DASSENOY F,LAHOUIJ I,et al.Understanding the tribochemical mechanisms of IF-MoS2nanoparticles under boundary lubrication[J].Tribol Lett,2011,41(1):55-64.
[4] LIU W M,CHEN S.An investigation of the tribological behaviour of surface-modified ZnS nanoparticles in liquid paraffin[J].Wear,2000,238(2):120-124.
[5] XUE Q J,LIU W M,ZHANG Z J.Friction and wear properties of a surface-modified TiO2nanoparticle as an additive in liquid paraffin[J].Wear,1997,213(1/2):29-32.
[6] ZHANG M,WANG X B,FU X S,et al.Performance and anti-wear mechanism of CaCO3nanoparticles as a green additive in poly-alpha-olefin[J].Tribol Int,2009,42(7):1029-1039.
[7] ZHANG Z F,YU L G,LIU W M,et al.The effect of LaF3nanocluster modified with succinimide on the lubricating performance of liquid paraffin for steel-on-steel system[J].Tribol Int,2001,34(2):83-88.
[8] YU H L,XU Y,SHI P J,et al.Microstructure,mechanical properties and tribological behavior of tribofilm generated from natural serpentine mineral powders as lubricant additive[J].Wear,2013,297(1/2):802-810.
[9] YU H L,XU Y,SHI P J,et al.Tribological behaviors of surface-coated serpentine ultrafine powders as lubricant additive[J].Tribol Int,2010,43(3):667-675.
[10] CAVANI F,TRIFIRÒ F,VACCARI A.Hydrotalcite-type anionic clays:preparation,properties and applications[J].Catal Today,1991,11(2):173-301.
[11] MEYN M,BENEKE K,LAGALY G.Anion-exchange reactions of layered double hydroxides[J].Inorg Chem,1990,29(26):5201-5207.
[12] SHAN D,COSNIER S,MOUSTY C.Layered double hydroxides:an attractive material for electrochemical biosensor design[J].Anal Chem,2003,75(15):3872-3879.
[13] BAI Z M,WANG Z Y,ZHANG T G,et al.Synthesis and characterization of Co-Al-CO3layered double-metal hydroxides and assessment of their friction performance[J].Appl Clay Sci,2012,59/60:36-41.
[14] WANG X B,BAI Z M,ZHAO D,et al.New synthetic route to Mg-Al-CO3layered double hydroxide using magnesite[J].Mater Res Bull,2013,48(3):1228-1232.
[15] LI S,BAI Z M,ZHAO D.Characterization and friction performance of Zn/Mg/Al-CO3layered double hydroxides[J].Appl Surf Sci,2013,284:7-12.
[16] FU F,BAI Z M,YANG N,et al.Preparation and tribological proporties of intercalated Cu-Mg-Al hydrotalcite[J].J Chin Ceram Soc,2012,40(1):165-169.
[17] ZHAO D,BAI Z M.Ni/Al-NO3-LDHs intercalated with dodecanoic acid and its tribological characteristics[J].J Chin Ceram Soc,2012,40(5):769-775.
[18] TALKE F E.A review of‘contact recording’technologies[J].Wear,1997,207(1/2):118-121.
[19] ZHANG B S,XU Y,GAO F,et al.Sliding friction and wear behaviors of surface-coated natural serpentine mineral powders as lubricant additive[J].Appl Surf Sci,2011,257(7):2540-2549.
[20] ZHANG B S,XU B S,XU Y,et al.Cu nanoparticles effect on the tribological properties of hydrosilicate powders as lubricant additive for steel-steel contacts[J].Tribol Int,2011,44(7/8):878-886.
[21] QI X W,LU L,JIA Z N,et al.Comparative tribological properties of magnesium hexasilicate and serpentine powder as lubricating oil additives under high temperature[J].Tribol Int,2012,49:53-57.
[22] MCINTYRE N S,ZETARUK D G.X-ray photoelectron spectroscopic studies of iron oxides[J].Anal Chem,1977,49(11):1521-1529.
[23] YAMASHITA T,HAYES P.Analysis of XPS spectra of Fe2+and Fe3+ions in oxide materials[J].Appl Surf Sci,2008,254(8):2441-2449.
[24] POTTIRAYIL A,KAILAS S V,BISWAS S K.Lubricity of an oil in water emulsion in metal cutting:The effect of hydrophilic/lypophilic balance of emulsifiers[J].Colloids Surf A,2011,384(1/3):323-330.
[25] XU T,ZHAO J Z,XU K.The ball-bearing effect of diamond nanoparticles as an oil additive[J].J Phys D-Appl Phys,1996,29:2932-2937.
[26] LI J G,SUN X D.Synthesis and sintering behavior of a nanocrystallineα-alumina powder[J].Acta Mater,2000,48(12):3103-3112.
[27] HE J,LIU W,ZHU L H,et al.Phase transformation behaviors of aluminum hydroxides to alpha alumina in air and molten salt[J].J Mater Sci,2005,40(12):3259-3261.
[28] RIVES V.Characterisation of layered double hydroxides and their decomposition products[J].Mater Chem Phys,2002,75(1/3):19-25.
