Evolution of limestone fracture permeability under coupled thermal, hydrological, mechanical, and chemical conditions*
LI Feng-bin (李凤滨), SHENG Jin-chang (盛金昌), ZHAN Mei-li (詹美礼), XU Li-meng (徐力猛), WU Qiang (吴强), JIA Chun-lan (贾春兰)
College of Conservancy and Hydropower Engineering, Hohai University, Nanjing 210098, China, E-mail: lanxue151@163.com
Evolution of limestone fracture permeability under coupled thermal, hydrological, mechanical, and chemical conditions*
LI Feng-bin (李凤滨), SHENG Jin-chang (盛金昌), ZHAN Mei-li (詹美礼), XU Li-meng (徐力猛), WU Qiang (吴强), JIA Chun-lan (贾春兰)
College of Conservancy and Hydropower Engineering, Hohai University, Nanjing 210098, China, E-mail: lanxue151@163.com
(Received March 12, 2013, Revised July 3, 2013)
The effect of temperature on the rock fracture permeability is a very important factor in the prediction of the permeability of enhanced geothermal systems and in reservoir engineering. In this study, the flow-through experiments were conducted on a single limestone fracture at different temperatures of 25oC, 40oC and 60oC, and with differential pressures of 0.3 MPa and 0.4 MPa. The experimental results suggest a complex temporal evolution of the fracture aperture. The aperture increases considerably with increasing temperature and reduces gradually to a steady value at a stable temperature. The results of three short-term experiments (QT-1, QT-2, QT-3) indicate an exponential relationship between the permeability and the temperature change ratio (ΔT/ T), which provides a further evidence that the rising temperature increases the aperture. It is shown that the changing temperature has its influence on two possible accounts: the chemical dissolution and the pressure dissolution. These two processes have opposite impacts on the fracture permeability. The chemical dissolution increases the permeability with a rising temperature while the pressure dissolution reduces the permeability with a stable temperature. These make a very complex picture of the permeability evolution. Our results show that the fracture permeability reduces 39.2% when the temperature increases by 15oC (during the 25oC-40 C interval) and 42.6% when the temperature increases by 20oC (during the 40oC-60oC interval). It can be concluded that the permeability decreases to a greater extent for larger increases in temperature.
permeability, temperature, fracture aperture, rock fracture
Introduction
In underground engineering, particularly, in the petroleum reservoirs, the geological sequestration of CO2, and the reservoirs used for the sequestration of energy by-products such as radioactive waste, the permeability evolution law in fractured rocks coupled by thermal-hydrologic-mechanical-chemical processes is very important. The temperature, in particular, has considerable effects on the flow and transport behavior in fractured rocks coupled by thermal-hydrologicmechanical-chemical processes[1,2]. At moderate temperatures and wet environment, resulting of chemical reaction and stress, the subcritical crack growth takes place in the rocks[3], and the removal of the mineral mass as a result of the occurrence of bridging asperities within the fracture[4], leading to a decrease of the fracture aperture. At high temperatures, the rock fracture heals and seals microcracks and reduces the permeability, and especially at long time of elevated stress and temperature[5], caused by violent chemical reactions impacted on the process of the dissolution and the precipitation on the fracture surface[6]. The flowthrough tests were conducted at moderate temperatures of 20oC, 80oC, 90oC, 120oC and 150oC[4,7]to study the evolution of the fracture permeability, but not at 40oC and 60oC.
Both at moderate temperatures, and at high temperatures[6], the fracture permeability in the quartz-domain rock, all decreases with increasing time, and monotonically at stepped-temperatures[7]. However, for different components, the effects of temperature, stre-ss, and chemical have different responses in the limestone, leading to more complicated flow and transport behaviors in the fracture. The dissolution on the fractured surface in the limestone is sensitive to aqueous conditions[8,9]. The changing temperature has a great effect on the chemical reaction, the fracture aperture is controlled by the competition of stress and reactivity, leading to greater complexity.
In the present study, a flow-through experiment was conducted on a single rock fracture in the limestone at temperatures of 25oC, 40oC and 60oC under differential pressures of 0.3 MPa and 0.4 MPa. The fracture aperture was evaluated from the measured flow rate. The results of the experiments are complicated, thus, two different methods are employed to analyze the experimental results. First, three other temperature-dependent experiments were conducted to allow a detailed study of the effects of rising temperature on the permeability characteristics. Then, a more detailed analysis of the influence of temperature on the fluid flow is made to help elucidate the influence of the temperature on the fracture permeability at higher and more stable temperatures.
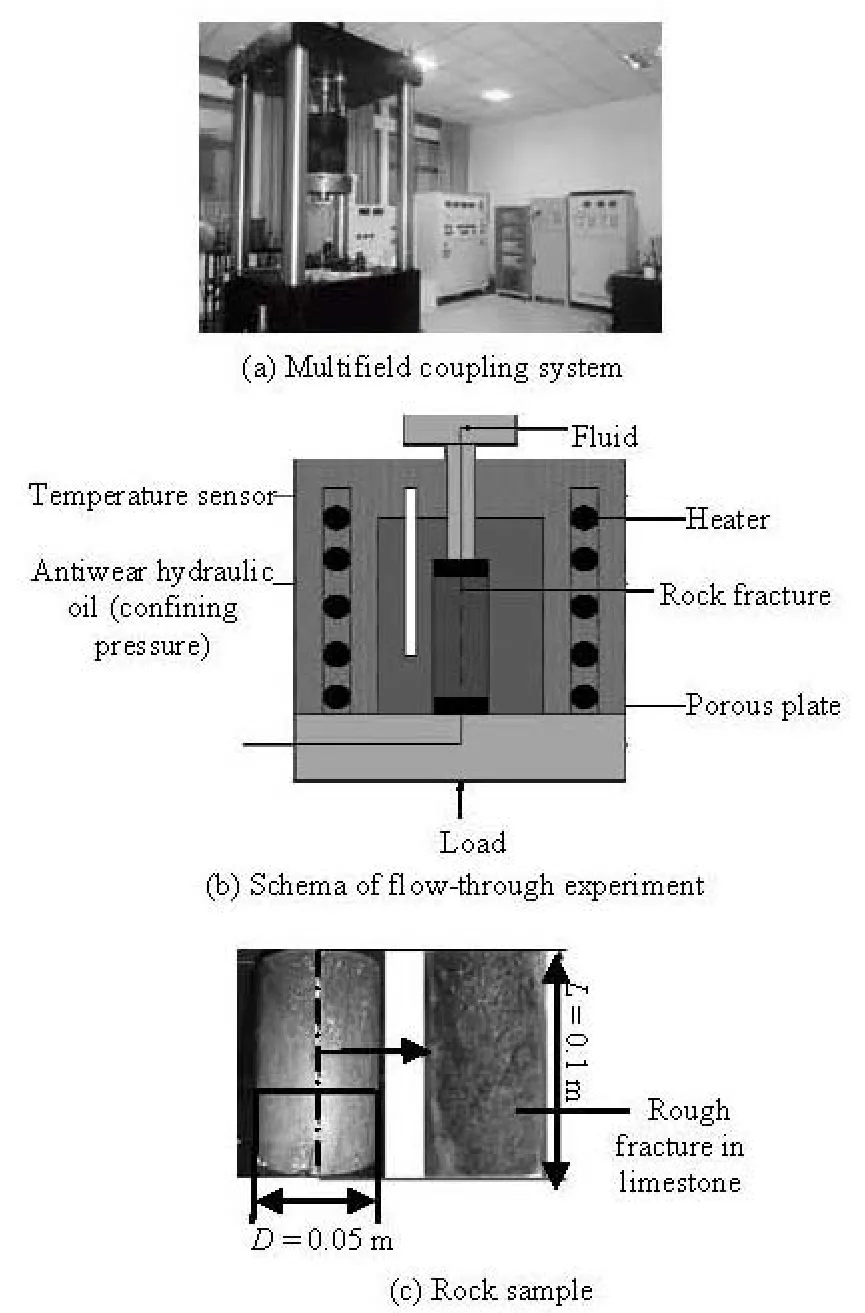
Fig.1 Experimental equipment and rock sample
1. Experimental method
In order to study the behavior of the rock fracture permeability characteristics during heating and at constant moderate temperatures (25°C-60°C), a water flow-through experiment was conducted based on a multifield coupling system for rock fractures. The tap water was used as the permeant fluid to replicate the characteristics of the natural fluid in deep rock fractures under complex environmental conditions. The rock used is a cylindrical sample of limestone (0.05 m diameter, 0.1 m length) containing a single diametral fracture along the cylindrical axis (Fig.1). This sample was collected from a mountain in Jiaxiang Country, Jining City, Shandong Province, China. The fracture was induced using the Brazilian test by tensile stress within the sample. The same fractured rock sample was used throughout the test, and the measured flow rates yield a continuous record of the average fracture transmissivity, which can be converted to obtain the average hydraulic aperture. The ion concentration in the effluent flow is also examined after the experiment, but will not be discussed in this paper.
1.1 Experimental system
The fluid flow system is illustrated in Fig.1. The rock sample is saturated according to the operational standard. After gently washing the fracture surfaces with distilled water, the two half-rock samples are fitted together carefully to avoid introducing small debris into the fracture. Then, the fitted core is coated with a thin silicone gel layer of 704. The fitted core is again wrapped with a double heat-shrink tube. Such a careful treatment is applied to avoid the flow of the fluid through the existing void in the outside wall of the rock, and to prevent the confining-pressure leakage occurring as a result of punching or rupturing of the sleeve under high temperature and pressure conditions. After the silicone consolidation, the core is placed inside a pressure cell to be adequately jacketed. The confining pressure, the axial compression, and the osmotic pressure are controlled by computer, with dynamic accuracies within ±0.15 MPa, ±7.5 kN and ±0.002 MPa , respectively. A casting body composed of the founded aluminum stainless steel heating pipe is used to heat the cell and complete the heating process, from room temperature to 150oC, within 3 h. The flow rates are evaluated by weighing the effluent water flowing out of the pressure cell directly, the rates are recorded every minute throughout the flowthrough experiments.
1.2 Experimental procedure
The flow-through experiment was conducted in four stages (1-4), under the confining pressure of 2.5 MPa, the axial compression of 15 MPa, the differential water pressure of 0.3 MPa or 0.4 MPa, and the temperatures of 25°C, 40°C and 60°C (Table 1). Two of the experimental stages (1 and 2) were conducted under the confining pressure of 2.5 MPa and at 25°Cin order to compare the hydraulic aperture under different differential water pressures.
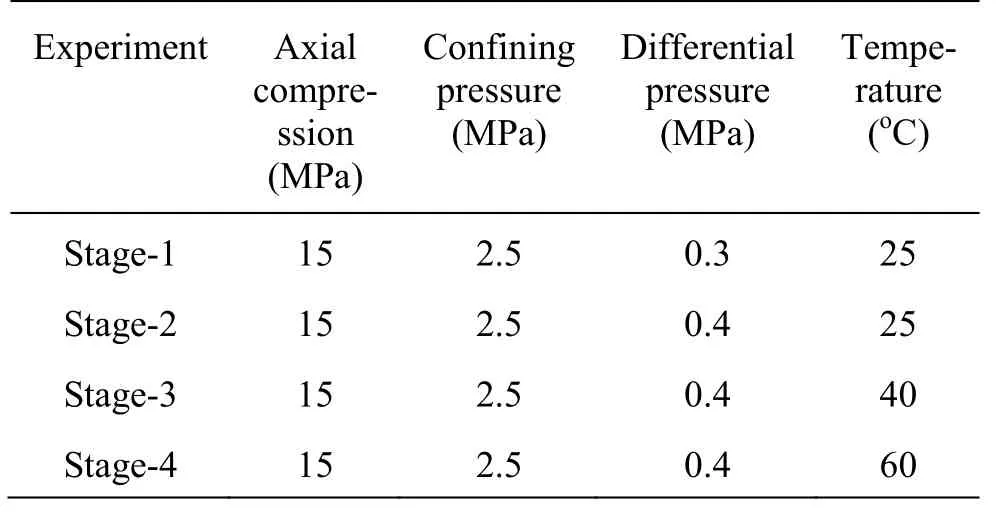
Table 1 Experimental conditions
After being jacketed, the rock sample was constrained within the pressure cell and pressurized to 3 MPa (or more) by axial compression to ensure an adequate connection between the porous plate and the force transmission column, and to prevent the water pathway from becoming immersed by the confining pressure oil. Then, the antiwear hydraulic oil was injected into the cell, the confining fluid was pressurized to a specified confining pressure, and the axial compression was modulated accordingly. The flow-through experiment was initiated at a controlled water pressure differential and at room temperature; the flow rate intervals were measured and the water samples were kept for the ion concentration measurement. In each experiment, a stable flow rate was reached and maintained for at least one day before the subsequent experiment.
Experiments last nearly one month, to measure the effluent flow rate at the outlet of the fracture rock. The fluid flow could be considered as the laminar flow for the small flux in the rock fracture. The cubic law for seepage through rock fractures, proposed by Boussinesq (1868) for the description of the movement of liquid in the gap between parallel plates, is suitable for describing the laminar flow movement. The measured flow rate, q, is formulated as follows:

where A is the cross-sectional area of the rock fracture, V is the flow velocity across the rock fracture, K is the hydraulic conductivity, J is the hydraulic gradient, D is the diameter of the rock, b is the hydraulic aperture, g is the gravity acceleration, L is the length of the rock sample, Δp is the differential water pressure, k is the fracture permeability, and ν and ρ are the kinetic viscosity and the density of the fluid, respectively (Table 2).
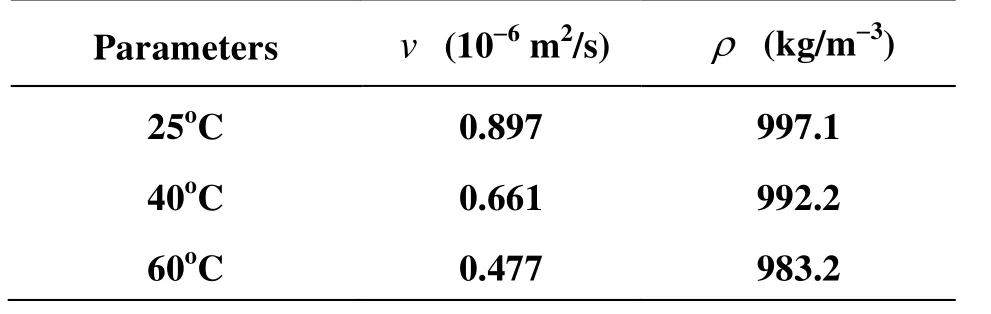
Table 2 Parameters in Eqs.(1)-(3)
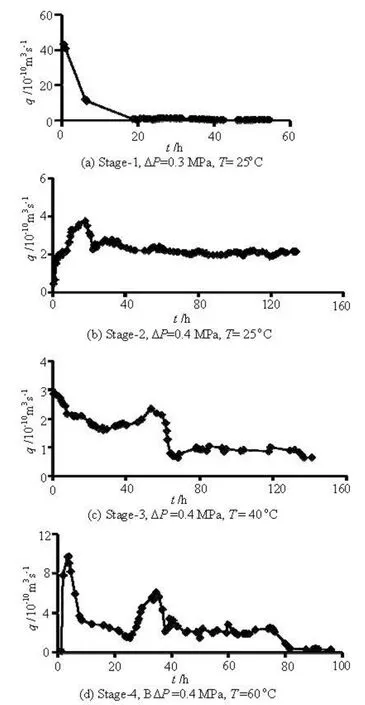
Fig.2 Evolution of flow rate measured at different experimental stages
2. Experimental results
The results of the flow-through experiment are presented in Figs.2 and 3 where Δp is differentialpressure, T is temperature. Stage-1 starts 10 h after the loading of the differential pressure. The flow rate is stabilized during this stage and the differential pressure is increased to 0.4 MPa in about 50 h after the start of the stage, thereafter, stage-2 is initiated. In stage-2, the experiment is stabilized, the temperature is increased to 40oC and then stage-3 is initiated. Some short auxiliary experiments are conducted at the end of stage-3, resulting in a discontinuity in the experimental timeline prior to the start of stage-4. The rock cell is heated from room temperature at the beginning of stage-4. Due to some improper operation, a temperature higher than 60oC appears at initial several hours. The rock cell is cooled, and then heated to 60oC. Accordingly, the peaks of the flow rate and the fracture aperture appear at the beginning of stage-4 (Figs.2 and 3).

Fig.3 Evolution of fracture aperture measured at 25oC (stage-1, stage-2), 40°C (stage-3), and 60oC (stage-4), and differential pressure of 0.3 MPa (stage-1) and 0.4 MPa (stage-2, stage-3, stage-4). The fracture aperture is evaluated from the recorded flow rates and the cubic law and is displayed against experimental time
The fracture aperture exhibits a complex behavior during the four experimental stages. It decreases rapidly with time in stage-1 and then approaches a stable value, as in good agreement with the results of Yasuhara et al.[10]. The fracture aperture is decreased by 80.2% during this stage. After the differential pressure is increased to 0.4 MPa, the fracture aperture measured in stage-2 is increased considerably before it is once again decreased and approaches a more stable value. This indicates that the permeability is switched gradually from an increasing trend to a decreasing trend in response to the change in the differential pressure. During this process, the fracture aperture is increased by 17.9% in conjunction with a differential pressure increase of 33.3%. The peaks of the fracture aperture occur in response to the heating in stage-3 and stage-4, suggesting that the fracture aperture is enhanced by these increases of temperature. The promoted transmission may be helped by the process of mineral dissolution[11]. In the experiment, a constant temperature is maintained after these peaks of the fracture aperture. The fracture aperture is decreased to 1.37 μm after 140.95 h in stage-3 and to 0.94 μm after 91.6 h in stage-4. The decrease of the fracture aperture with time may be resulted from the irreversible inelastic crushing (i.e., mechanical failure) of the propping asperities, the dissolution at the contacting asperities, and the clogging of the fracture void by mineral precipitation[12]. The fracture aperture is decreased by 39.6% in stage-3 accompanied with an increase of 15oC of the temperature and by 63.7% in stage-4 accompanied with an increase of 20oC of the temperature, that is, the fracture aperture is decreased to a greater extent for a larger increase of the temperature.
In the experiments conducted by Polak[7], the measurement of fluid and dissolved mass fluxes, the concurrent X-ray CT and imaging, and the post-test sectioning and SEM were used to constrain the progress of the mineral dissolution and its effect on the transport properties. The results of previous studies indicate a general correspondence between the mineral mass removed from the contacting asperities of a conductive fracture, the measured change in the hydraulic aperture, and the nondestructive observed removal of mass from the fracture contact area. The evolution of the fracture aperture (i.e., the hydraulic aperture) is used to describe the mean removal of mass from the fracture contact area in this study.
The results in Fig.3 indicate that the temperature has a considerable effect on the fracture aperture, in particular, the aperture changes dramatically in response to the temperature increases at the beginning of stage-4. To some extent, the evolution of the fracture aperture can be considered to express the fracture permeability characteristics of the rock (e.g., Eq.(3)).
3. Rising temperature and rock fracture permeability
To study the effects of the temperature on the fracture permeability, three short-term temperature-dependent experiments (QT-1, QT-2, QT-3) were conducted under the axial compression of 15 MPa, the confining pressure of 2.5 MPa, and the differential pressure of 0.4 MPa (Fig.4). The results of these experiments suggest that the flow rate increases with the increase of the temperature. The temperature and the flow values were collected simultaneously in the experiments, the temperature was measured in the confining pressure oil by a temperature sensor and is not entirely representative of the current temperature in the fracture. However, the computer simulation indicates that, from the temperature in the confining pressure oil, the surface temperature of the rock fracture delays only seven minutes to reach that value. This lag time is considered negligible when compared with the overall experiment time.
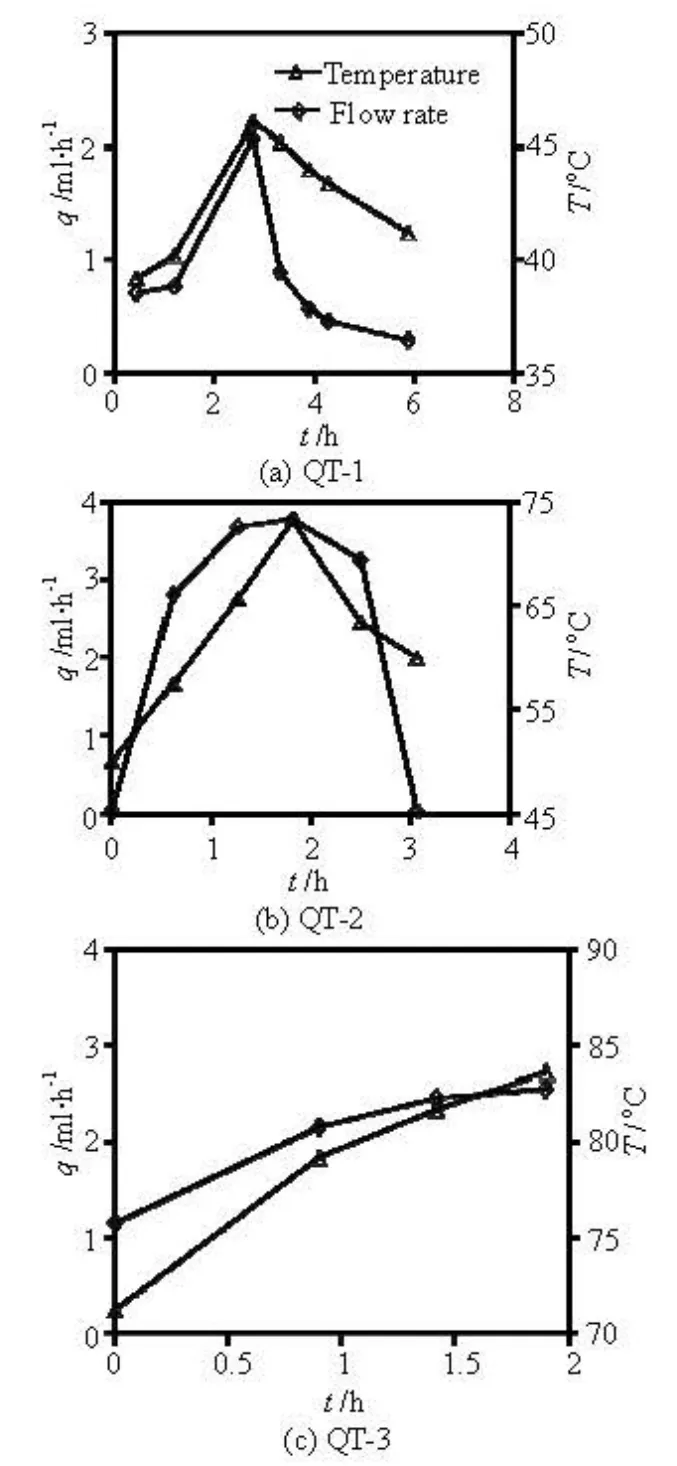
Fig.4 Comparison of the flow rate and temperature for QT-1, QT-2, and QT-3 under axial compression of 15 MPa, confining pressure of 2.5 MPa, and differential pressure of 0.4 MPa
Table 3 shows the experimental results of the rising temperature sections of the experiments QT-1, QT-2, QT-3, leaving out the cooling sections. Each test case results are shown in the same row in Table 3. Temperature increment, ΔT, is the difference between the current temperature and the previous-row temperature (listed at the previous column of the same row). The data for 39.2oC is omitted
Theoretical and experimental data are combined to illustrate the phenomena apparent in the experiments. Yasuhara[12]produced an equation describing the fracturing rate for a tensile opening mode
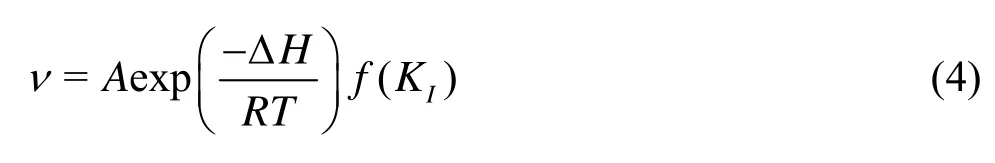
where A is a pre-exponential factor, R is universal gas constant, f( KI) is a stress intensity factor, which becomes a constant under the stable confining pressure. In view of the relationship between the flow rate and the fracture aperture (i.e., Eqs.(1)-(3)), the relationship between the flow variation rate, dq/dT, and the temperature might be similar to that between the fracturing rate and the temperature, as

where A is a pre-exponential factor related to the stress intensity factor. Table 3 illustrates the reduction of the change rate of the flow rate with increasing temperature and the change behaviors relating to the initial temperature. Accordingly, Eq.(5) is modified as follows

The experimental data from QT-1, QT-2 and QT-3 are used to fit curves for the parameters A and B (Table 4).
The relationship between the flow rate and the permeability is provided by Eq.(1). When the differential pressure and the cross-sectional area of the fracture are constant, the changes of the permeability resulting from increasing temperature can be described as follows
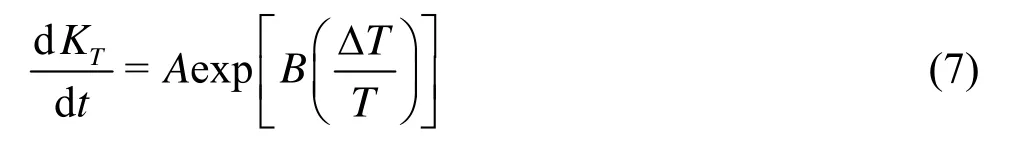
where KTis a coefficient of the permeability related to the increasing temperature. However, it should be noted that this section discusses only the condition where all variables except the temperature are kept constant.
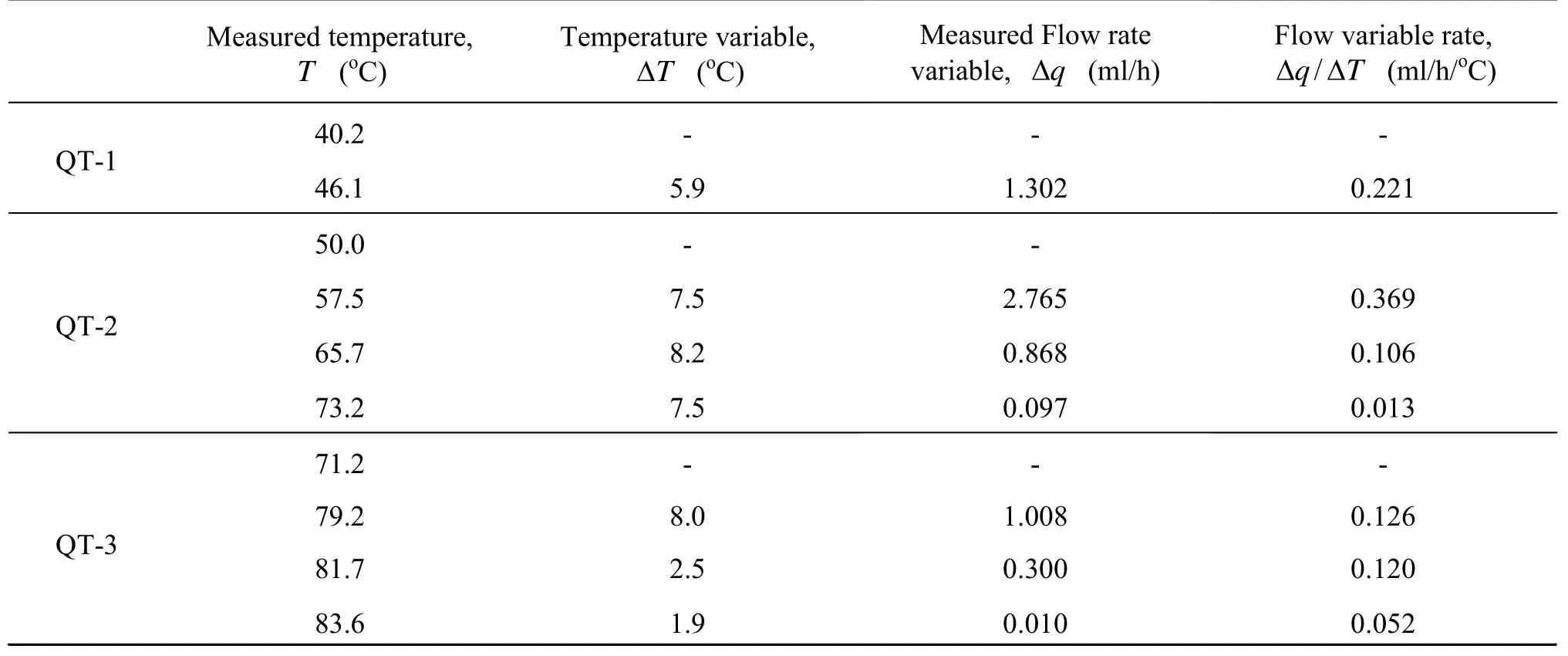
Table 3 The relationship between temperature and flow rate in QT-1, QT-2 and QT-3

Table 4 Fitting of Eq.(6) based on experimental data from QT-1, QT-2 and QT-3
The parameters A and B are found to be greater than zero (Table 4), indicating that increasing temperature causes an increase in the coefficient of the permeability of the rock fracture, which corresponds to peaks of the flow rate and the fracture aperture during stage-3-1 and stage-4 in macroscopic observations (i.e., during the first 10 h and for 26 h-37 h, respectively). Parameter B is related to the activation enthalpy (e.g., Eq.(4)). Parameter A may be mediated by the material characteristics, the stress intensity factor, and various temperature-dependent factors, accordingly. The value of A determined from QT-3 is four orders of magnitude smaller than those derived from the other two experiments. Only two groups of experimental data from QT-1 produce less accurate results for the fitting curve, and these results are used for reference. Moreover, the flow rate through the fracture is affected in a complex way by the cooling and the effective stress during temperature decreasing (i.e., the decreasing trends illustrated in Figs.4(a) and 4(b), to show the appearance of a slow declining.
4. Effects of temperature on the physical and chemical properties of rock fracture
The changes of the temperature influence the fracture apertures through changes of material properties, chemical reaction rates, and flow-through characteristics, all of which are temperature-dependent processes that affect the rate of mineral dissolution.
In particular, the critical stress of the propping particles in the fracture apertures is decreased with increasing temperature. The stress-mediated process of subcritical cracking was investigated in detail[5,13-15], and their findings were applied to describe the compaction behavior in granular aggregates[16,17]. The pressure solutions may result in a time-dependent decrease of the fracture permeability through compaction driven by the fracturing (or crushing) of the propping asperities and by the dissolution at contacting asperities. The critical stress, cσ, can be determined through the energy balance under the applied stress and temperature conditions[18], which defines the stress state where the compaction of the grain aggregate will effectively halt[12]. The variation of the critical stress on the propping particles in the fracture, Δcσ, is defined as

According to Eq.(8),cσ decreases with increasing temperature. Before heating, the fracture closure rate is decreased, indicating a balance between σcand the asperity-contact stress,aσ, on the contact area.Conversely, after heating,aσ is greater thancσ, which drives the process of the pressure dissolution. Thus, a high stable temperature is dominated by the effects of the pressure solution mechanisms, and the fracture permeability is decreased during this process.
It is well known that the increase of temperature can promote the chemical dissolution, on the propping particle and the free surface in the rock fracture. The transition state theory (TST) holds that the chemical reaction processes are initiated only by the collision of reactant molecules with sufficient energy (i.e., activation energy), and with appropriate collision angles. The number of molecules with activation energy is increased with increasing temperature. Moreover, according to the Arrhenius equation, the reaction rate constant is higher for a higher temperature. In such cases, the chemical dissolution of the contacting particle and the free surface are accelerated.
The fluid viscosity is known to decrease with increasing temperature. The relationship between the dynamic viscosity of water, μ, and the temperature, t, can be expressed as follows

where μ is calculated. ν=μρ-1, where ν is the kinetic viscosity of water. Thus, ν decreases with increasing temperature. According to Eqs.(1) and (3), the fracture permeability and the flow rate are increased with all other conditions kept unchanged.
5. Effects of temperature changes on permeability
Increasing temperature will increase the rate of the mineral dissolution on the free surface, and enhance the fracture permeability. The transmission in dominant fractures can be enhanced by processes such as the shear dilation, the mineral dissolution[9,11], and the strain energy driven free-face dissolution. In particular, the mineral dissolution, driven by the chemical reactions in the fracture void during compaction, is accelerated as a result of increasing temperature, this changes the morphology of the fracture surfaces and increases the hydraulic aperture. Moreover, the changes in the fracture surface result in permeability changes, such that the fracture permeability increases over time during the periods of increasing temperature in stage-3-1 and stage-4-1. Furthermore, the increasing temperature promotes the expansion of the rock fracture, opening the fracture. Under such combined action, the increasing temperature increases the fracture permeability.
During a higher stable temperature, the fracture permeability decreases. In the stable temperature intervals in stage-3-2 and stage-4-2, the decreasing critical stress drives the pressure solution. The propping particles in the fracture are dissolved in the pressure solution process and the fracture closes slowly at compaction. However, the dissolution rate slows over time with the increase of the contact area, and the closure of the fracture decreases the permeability.
Throughout the intervals of increasing and stable temperature, the fracture permeability decreases significantly at the end of each experimental stage. The permeability increases by 13.3% in stage-3-1, decreases by 34.7% in stage-3-2, and decreases by 39.2% in stage-3 in the lump. Conversely, the permeability increases by 59.5% in stage-4-1, decreases by 73.24% in stage-4-2, and decreases by 42.6% in stage-4 in the lump. The permeability characteristics are important in the prediction of the permeability of the earthquake-disturbed belt, the radioactive waste repositories, and the basic facilities for underground reservoir engineering, when the temperature changes.
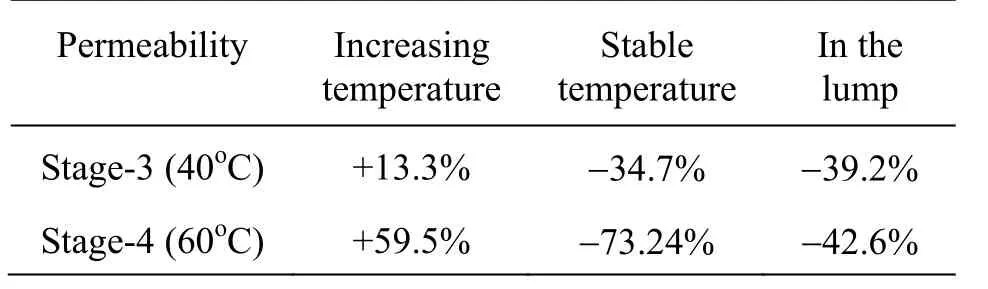
Table 5 Percentage changes in the permeability at different temperatures
The values with a percent sign in Table 5 show the changing of the permeability. “+”sign means increasing, and “-” means decreasing. The change is the compared value at the end time with the value at the beginning time at the same phase. So the general trends in stage-3 and stage-4 are decreasing in the permeability.
6. Conclusions
Flow-through experiments were conducted to measure the evolution of the aperture in a fracture rock at different temperatures.
(1) The experimental results indicate that the evolution of the fracture aperture with time is complex: the aperture increases significantly during the intervals of rising temperature, but reduces gradually to a steady state under stable temperature conditions.
(2) Rising temperature can improve the rock fracture permeability. The fitting data for these three short-term experiments (QT-1, QT-2 and QT-3) confirm an exponential relationship between the permeability and the temperature change ratio, ΔT/T, which helps explain the increases of the fracture aperture during stage-3-1 and stage-4-1.
(3)Temperature affects the transport behavior in the rock fracture in three respects. First, the increased temperature diminishes the critical stress-driven pressure solution, reducing the permeability of the fra-cture by compaction. Second, the increased temperature promotes the chemical dissolution, leading to an accelerated dissolution of the propping particles and the free surface in the fracture. Third, the increased temperature reduces the fluid viscosity, enhancing the flow rate.
(4) The increasing temperature accelerates the chemical dissolution and opens the fracture, leading to an increased permeability; at higher stable temperatures, the enhanced pressure dissolution mechanisms reduce the fracture permeability.
(5) Under a combined action, the permeability decreases by 39.2% for a temperature increase of 15oC (i.e., during the experimental stage of 25oC-40oC) and by 42.6% for a temperature increase of 20oC (i.e., during the experimental stage of 40oC-60oC). This result indicates that reductions of the permeability are greater for larger temperature increases.
[1] YASUHARA H., ELSWORTH D. and POLAK A. Evolution of permeability in a natural fracture: Significant role of pressure solution[J].Journal of Geophysical Research:Solid Earth,2004,109(B3): B03204.
[2] YASUHARA H., ELSWORTH D. and POLAK A. et al. Spontaneous switching between permeability enhancement and degradation in fractures in carbonate: Lumped parameter representation of mechanicallyand chemically-mediated dissolution[J].Transport inPorous Media,2006, 65(3): 385-409.
[3] NARA Y., KANEKO K. Study of subcritical crack growth in andesite using the double torsion test[J].International Journal of Rock Mechanics andMining Sciences,2005, 42(4): 521-530.
[4] YASUHARA H., KINOSHITA N. and OHFUJI H. et al. Temporal alteration of fracture permeability in granite under hydrothermal conditions and its interpretation by coupled chemo-mechanical model[J].AppliedGeochemistry,2011, 26(12): 2074-2088.
[5] RUTQVIST J., FREIFELD B. and MIN K. B. et al. Analysis of thermally induced changes in fractured rock permeability during 8 years of heating and cooling at the Yucca Mountain Drift Scale Test[J].International Journal of Rock Mechanics and MiningSciences,2008, 45(8): 1373-1389.
[6] MORROW C. A., MOORE D. E. and LOCKNER D. A. Permeability reduction in granite under hydrothermal conditions[J].Journal of Geophysical Research: Solid Earth,2001, 106(B12): 30551-30560.
[7] POLAK A., YASUHARA H. and ELSWORTH D. et al. The evolution of permeability in natural fracturesthe competing roles of pressure solution and free-face dissolution[J].Elsevier Geo-Engineering Book Se-ries,2004, 2: 721-726.
[8] POLAK A., ELSWORTH D. and LIU J. et al. Spontaneous switching of permeability changes in a limestone fracture with net dissolution[J].Water Resou-rces Research,2004, 40(3): W03502.
[9] LIU J., SHENG J. and POLAK A. et al. A fully-coupled hydrological-mechanical-chemical model for fracture sealing and preferential opening[J].International Journal of Rock Mechanics and Mining Scie-nces,2006, 43(1): 23-36.
[10] YASUHARA H., POLAK A. and MITANI Y. et al. Evolution of fracture permeability through fluid-rock reaction under hydrothermal conditions[J].Earth Pla-net Science Letters,2006, 244(1-2): 186-200.
[11] TARON J., ELSWORTH D. Thermal-hydrologic-mechanical-chemical processes in the evolution of engineered geothermal reservoirs[J].International Journal of Rock Mechanics and Mining Sciences,2009, 46(5): 855-864.
[12] YASUHARA H., ELSWORTH D. Compaction of a rock fracture moderated by competing roles of stress corrosion and pressure solution[J].Pure AppliedGeophysics,2008, 165(7): 1289-1306.
[13] DOVE P. M. Geochemical controls on the kinetics of quartz fracture at subcritical tensile stresses[J].Journal of Geophysical Research:Solid Earth,1995, 100(B11): 22349-22359.
[14] FENG X., CHEN S. and LI S. Effects of water chemistry on microcracking and compressive strength of granite[J].International Journal of Rock Mechanicsand Mining Sciences,2001, 38(4): 557-568.
[15] WILTSCHKO D. V., MORSE J. W. Crystallization pressure versus ‘‘crack seal’’ as the mechanism for banded veins[J].Geology,2001, 29(1): 79-82.
[16] KARNER S. L., CHESTER F. M. and KRONENBERG A. K. et al. Subcritical compaction and yielding of granular quartz sand[J].Tectonophysics,2003, 377(3-4): 357-381.
[17] CHESTER J. S., LENZ S. C. and CHESTER F. M. et al. Mechanisms of compaction of quartz sand at diagenetic conditions[J].Earth Planet Science Letters,2004, 220(3-4): 435-451.
[18] REVIL A. Pervasive pressure-solution transfer: A poro-visco-plastic model[J].Geophysical Research Letters,1999, 26(2): 255-258.
10.1016/S1001-6058(14)60026-3
* Project supported by the National Natural Science Foundation of China (Grant Nos. 50779012, 51009053 and 51079039).
Biography: LI Feng-bin (1986-), Female, Ph. D. Candidate
SHENG Jin-chang, E-mail: jinchang@hhu.edu.cn
- 水动力学研究与进展 B辑的其它文章
- Numerical prediction of 3-D periodic flow unsteadiness in a centrifugal pump under part-load condition*
- Experimental investigations of transient pressure variations in a high head model Francis turbine during start-up and shutdown*
- Improved conservative level set method for free surface flow simulation*
- Capillary effect on the sloshing of a fluid in a rectangular tank submitted to sinusoidal vertical dynamical excitation*
- Effect of compressive stress on the dispersion relation of the flexural–gravity waves in a two-layer fluid with a uniform current*
- Comprehensive analysis on the sediment siltation in the upper reach of the deepwater navigation channel in the Yangtze Estuary*

