Exogenous Hyaluronic Acid Reduces R5-HIV Infection of CD4+T Cells
LI Peilin
(Department of Medicine,University of California San Francisco(UCSF),San Francisco CA 94121,USA)
0 Introduction
Prevention of HIV transmission is still the most direct way to stem the HIV/AIDS epidemic[1].However,to date,large-scale clinical trials of vaccines to produce an HIV-specific antibody or a T-cell response to prevent HIV infection have been disappointing[2-3].Since 80%of HIV infection occurs through sexual contact[4],there is intense interest in the prevention of HIV mucosal transmission,and CCR5-tropic viruses(R5 viruses)are preferentially transmitted.To design a better strategy to prevent mucosal transmission of HIV,it needs more fully understand the mechanism of HIV mucosal transmission[5].
Mucosal tissues are the front-line defense against pathogen invasion and greatly impede HIV transmission.Studies using the simian immunodeficiency virus(SIV)rhesus macaque model demonstrate that the genital tract mucosal barrier limits exposure of CD4+T cells,dendritic cells(DCs),and macrophages to the majority of the viral inoculum,and only a small number of infectious virions pass through the mucosal barrier to establish the infected founder population[6-7].These findings are confirmed by clinical studies showing that a small number of infectious virions breach the mucosal barrier to infect resting CD4+T cells,generating a clonal or oligoclonal founder population[5,8-9].
Mucosal integrity plays an important role in HIV transmission,and mucosal inflammation can increase HIV transmission[10-12].The mucosal tissues are composed of epithelial cells,extracellular matrix,interstitial cells and surface mucus.In addition to providing a full complement of host immune cells that variably facilitate or impede HIV infection,the mucosal surface also serves as a physical barrier to mucosal HIV invasion.Mucosal mucus can trap HIV virions[13]and reduce virion movement[14].An acidic vaginal mucosal environment can decrease the rate of HIV sexual transmission[15].How these effects on mucosal HIV transmission are mediated remains largely unknown[5,9].
The surface of the mucosal layer is a scaffold with extracellular matrix,a major component of the extracellular matrix is hyaluronic acid(HA,or hyaluronan).HA is a large glycosaminoglycan(GAG)that can be remodeled and degraded by hyaluronidase.On the surface of the cells,HA polymers extend up to 25μm in length,forming pericellular coats.HA interaction with its receptors can induce cellular signaling and is involved in mucosal tissue homeostasis and maintenance of tissue integrity[16-18].HA is also a regulator of immunity.HA interaction with its main receptor,CD44,regulates recruitment and extravasation of T-cells into sites of inflammation[19-20]and participates in the inflammatory process[16,21].HA interaction with CD44 can reduce cytokine production from macrophages in the setting of inflammation[22]and lowers PKCa activity to decrease histamine release from leukemic cell lines[23].
In our lab,recently we observed that exogenous HA reduced HIV infectivity when both virions and CD4+T cells expressed CD44.Effects were seen on both early infection events like viral binding and probably later events through reduction of PKCa activation,while treatment with hyaluronidase reduced endogenous HA thickness and enhanced susceptibility of CD4+T cells to infection,but in that study the HIV was generated from NL4-3 plasmid,which is a T-cell-tropic(CXCR4-dependent,X4)[24].Clinical studies have found that CCR5-tropic viruses(R5 viruses)are preferentially transmitted[5].The main aim of this study was to assess the role of HA in CCR5-tropic HIV infection on un-stimulated CD4+T cells.We observed that exogenous HA reduced both X4,and R5-HIV infectivity on un-stimulated CD4+T cells.Effects were seen on viral binding,while treatment with hyaluronidase reduced endogenous HA thickness and enhanced susceptibility of CD4+T cells to infection.
1 Material and Methods
1.1 Material
Hyaluronic acid(HA)(Sigma,St.Louis,MO),hyaluronidase(Hase)(Sigma,St.Louis,MO),Phytomeagglu-tinin-M(PHA-M)(Roche,Indianapolis IN),human-Interleukin 2(hIL-2)(Roche,Indianapolis,IN),MDEM and PRMI(Life Technologies,Carlsbad,CA),anti-HIV-1-p24 antibody(Santa Cruz Biotechnology,Dallas,Texas),Alexa Fluor647(Life Technologies,Carlsbad,CA),cell membrane staining(Life Technologies,Carlsbad,CA),stained nuclei with DAPI(Life Technologies,Carlsbad,CA),anit-CD4-PE(R&D System),EasySep Human CD4+T cell Enrichment kit(STEMCell,Vancouver,BC),HIV-1p24 ELISA kit(PerkinElmer,Waltham,MA).Beta-Glo®assay system(Promega,Madison WI).
1.2 Methods
1.2.1 Isolation of CD4+T cells from healthy donors CD4+T cells were isolated from healthy donors PBMCs using the EasySep Human CD4+T cell Enrichment kit(STEMCell,Vancouver,BC)according to the manufacturer′s instructions.
1.2.2 Generation of X4(NL4-3)and R5(YK-JRCSF)HIV virus stock from PBMC Isolated PBMCs were stimulated with 3μg/mL of phytomeagglutinin-M(PHA-M)(Roche,Indianapolis IN);and 20 unit/mL of human-Interleukin 2(hIL-2)(Roche,Indianapolis,IN)for 3 days,X4 and R5 HIV viral supernatant from transfection of 293T cells was used to infect pooled mixed stimulated PBMCs prepared from 2 healthy donors.The supernatant from HIV infected PBMCs was harvested at day 7 and day 11.Viral titers were determined by HIV-1p24 ELISA(PerkinElmer,Waltham,MA).
1.2.3 Reduction of HA from cell surface 1x106/mL of unstimulated CD4+T cells were washed 3 times in RPMI without FCS and incubated in RPMI with 12 unit/mL of hyaluronidase(Hase)(Sigma,St.Louis,MO)for 60 min,followed by removal of supernatant and three washes with RPMI without FCS.
1.2.4 Staining HIV-p24 on unstimulated CD4+T cells HIV staining:Unstimulated CD4+T cells were incubated with R5-HIV for 5 hours,fixed with 2%formaldehyde,and incubated with mouse anti-HIV-1 p24 antibody at 4℃overnight,then stained with donkey anti-mouse IgG antibody conjugated with Alexa Fluor647(Life Technologies,Carlsbad,CA)for 2 hours.HIV-p24 staining was measured by 2 methods:laser scanning microscopy(LSM),and flow cytometry(FACS).For LSM analysis:the cells were further stained with anit-CD4-PE(R&D System),cell membranes with cell membrane staining(Life Technologies,Carlsbad,CA),and stained nuclei with DAPI(Life Technologies,Carlsbad,CA),and LSM510 META laser scanning microscope(Carl Zeiss Microimaging Inc.Thornwood,NY,USA)was used to scan the cells with Z-stack projections.For FACS analysis:50000 HIV-p24 stained cells were analyzed by EasyCyte6HT-2L(Millipore,MA).
1.2.5 Assays for HIV Infection (1)TZM-bl cells(NIH AIDS Research and Reference Reagent Program):3×104cells plated into 96 well plate for overnight,then removed supernatant and cells were washed with complete DMEM without FCS,then treated with study reagents,inoculated with 100pg HIV-p24 of HIV/well for 5 hours,washed 3 times with complete DMEM,and cultured in complete DMEM.HIV infectivity was measured by using Beta-Glo®assay system(Promega,Madison WI)and measured with a plate reader(VICTOR3)(PerkinElmer)at 3 days post-infection[25].The luciferase activity is presented as relative light units(RLU).(2)Unstimulated CD4+T cells:1×106healthy donor unstimulated cells were washed with RPMI without FCS,treated with different reagents,inoculated with 1000 pg HIV-p24 of HIV for 5 hours,washed 3 times with complete RPMI,resuspended in complete RPMI with 5μg of PHA-M and 20 unit of hIL-2 for 24 hours,washed 3 times with completed RPMI,and finally resuspended in completed RPMI with 20 unit of hIL-2.HIV infectivity was measured by HIV-1 p24 production in supernatant at day 7 post-infection by using HIV-1p24 ELISA kit(PerkinElmer,Waltham,MA).
1.3 Statistical analysis
TZM cell assay was run in triplicate and repeated at least 3 times.Experiments with primary CD4+T cells were run in duplicate and repeated with at least 3 different donors.Data averages and standard deviations(SD)were calculated in Microsoft Excel 2008 and presented as means±standard deviation(x±s).Significance was assessed by a t-test,with P<0.05 considered significant.
2 Results
2.1 Exogenous HA reduces both X4 and R5-HIV infectivity on TZM-bl cells line,and unstimulated CD4+T cells
Earlier studies reported that exogenous HA could interrupt T cell rolling and impede extravasation of T cells into an inflamed site through interactions with CD44[19].To study whether exogenous HA can impact HIV infectivity,TZM-bl cells[25]were treated with exogenous HA for 1 hour,then inoculated with X4(NL4-3)or R5(YK-JRCSF)produced from PBMCs cells and normalized by p24.HIV infectivity was measured at day 3 post infection by luciferase activity[25]as relative light units(RLU).Exogenous HA reduced infectivity of both X4(NL4-3)and R5(YK-JRCSF)HIV significantly(Fig.1A).
In vivo studies of acute HIV and SIV infection demonstrate that virus initially targets resting CD4+T cells and establishes an infected founder population during mucosal transmission[5,8-9].To study whether exogenous HA treatment can reduce infectivity of both R5(YK-JRCSF)and X4(NL4-3)HIV on CD4+T cells,primary unstimulated CD4+T cells from healthy donors were treated with exogenous HA,and then inoculated with viral stocks of both X4(NL4-3)or R5(YK-JRCSF)HIV normalized by p24,then stimulated with PHA and IL-2 for 24 hours.HIV infectivity was assessed by measurement of supernatant HIV-1 p247 days post-infection.Exogenous HA treatment significantly reduced HIV-1 p24 production during both X4(NL4-3)and R5(YK-JRCSF)infection(Fig.1B).
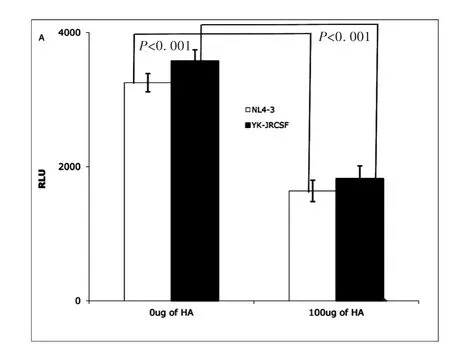
Fig.1A TZM-bl cells assay:Exogenous HA(Sigma,St.Louis,MO)reduced infectivity of both NL4-3(X4)(open bar)and YK-JRCSF(R5)HIV(filled bar).(Data are mean±SEM of triplicate samples and represent 3 independent experiments)
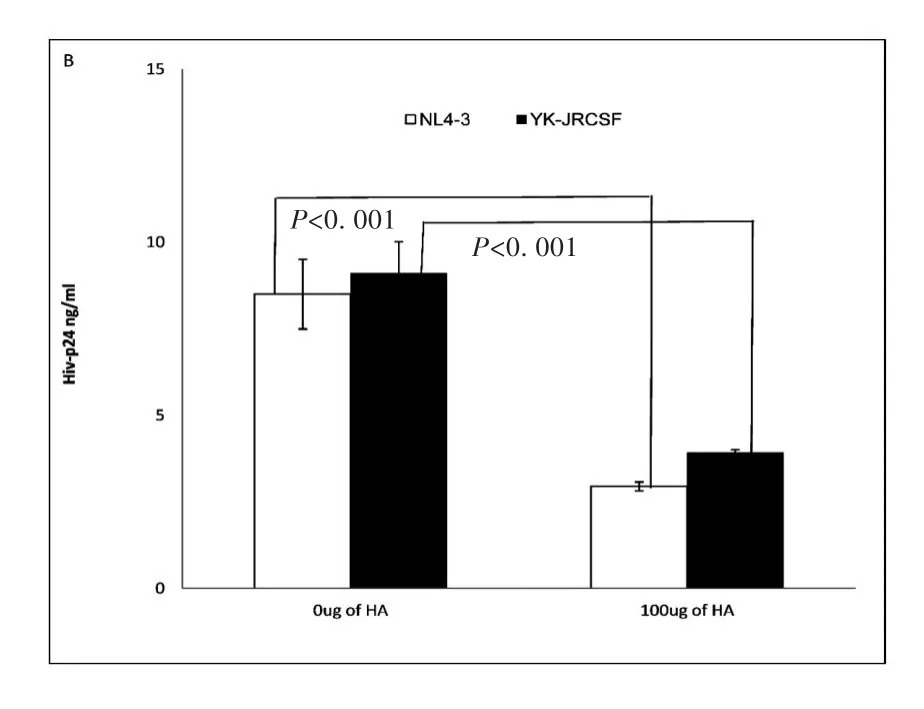
Fig.1B Un-stimulated CD4+T cells assay:Exogenous HA decreased infectivity of both NL4-3(X4)(open bar)and YK-JRCSF(R5)on primary un-stimulated CD4+T cells.(Data are mean±SEM of duplicate samples and representative of 3 donors)
2.2 Hyaluronidase treatment can enhance both X4 and R5-HIV infectivity on unstimulated CD4+T cells
Endogenous HA has been observed to form a cushion on the cell surface,to play an anti-adhesive role[18]and to impede ligand access to cell-surface receptors[26].Hyaluronidase treatment can reduce endogenous HA from the cell surface,thus promoting cell-cell contact[17].Hyaluronidase is highly activated during sexual intercourse as well as inflammation[16-18].Our initial experiments showed that exogenous HA reduced both X4(NL4-3)and R5(YK-JRCSF)HIV infection of unstimulated CD4+T cells.Thus,we studied the impact of hyaluronidase treatment on HIV infection of primary unstimulated CD4+T cells.Healthy donor,unstimulated primary CD4+T cells were treated with hyaluronidase prior to inoculation with an equivalent input(by p24)of either X4(NL4-3)or R5(YK-JRCSF),then stimulated with PHA and IL-2 for 24 hours.At day 7 post-infection,infectivity was assessed by measurement of HIV-1 p24 in culture supernatants.Consistent with the ability of endogenous HA to inhibit HIV infection,hyaluronidase treatment significantly increased both X4(NL4-3)and R5(YK-JRCSF)infection of unstimulated CD4+T cells,but this enhancement could be completely reversed by addition of exogenous HA(Fig.2).
These results indicate that hyaluronidase treatment renders unstimulated CD4+T cells more susceptible to HIVCD44infection,possibly through reduction of cell surface HA.
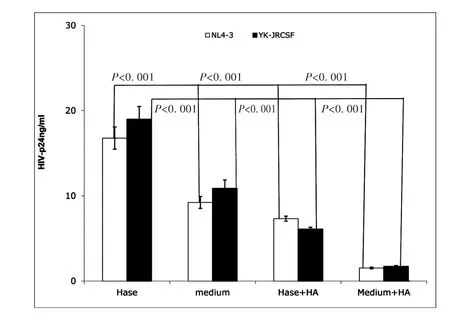
Fig.2 Hyaluronidase treatment enhanced HIV infection of unstimulated CD4+T cells.Hase(Hyaluronidase treatment)(Sigma,St.Louis,MO);medium(medium treatment);Hase+HA:(Hyaluronidase treatment with 100μg of exogenous HA(Sigma,St.Louis,MO));Medium+HA:(medium treatment with100μg of exogenous HA).Treatment of healthy donor unstimulated CD4+T cells by hyaluronidase(Hase)boosted both X4(open bar)and R5(filled bar)HIV infection,and exogenous HA reduced this enhancement(Hase+HA).(All data are mean±SEM from duplicate samples and representative of at least 3 donors)
2.3 Hyaluronidase treatment enhances R5-HIV binding on unstimulated CD4+T cells
Next,we investigated how hyaluronidase treatment impacts HIV binding HA on the surface of unstimulated CD4+T cells.Our early results shown that hyaluronidase treatment can reduce endogenous HA thickness on surface of the un-stimulated CD4+T cells[24].Because the thickness of endogenous HA has been postulated to prevent ligand binding to cognate receptors on the surface of T-cells[27]and hyaluronidase treatment enhanced infectivity of both X4(NL4-3)and R5(YK-JRCSF),we hypothesized that hyaluronidase-mediated reduction of HA on the cell surface may enhance HIV binding.To test this hypothesis,unstimulated,primary CD4+T cells were treated by hyaluronidase and then incubated with R5(YK-JRCSF)in the presence 10μg/mL of the peptide fusion inhibitor T-20[25]for 5 hours.Cells were washed 5 times to remove unbound HIV,stained with anti-HIV-1 p24 antibody,and studied by laser scanning microscopy.Hyaluronidase treatment significantly increases HIV binding on surface of the unstimulated,primary CD4+T cells(Fig.3A,B,C,D,E).
3 Discussion

Fig.3(A,B,C) LSM510 META laser scanning microscope(Carl Zeiss Microimaging Inc.Thornwood,NY,USA)was used to scan the cells.Images are 40x;CD4+T cells were stained with mouse anti-HIV-1p24(Santa Cruz Biotech)and donkey anti-mouse IgG antibody with AlexaFluor@680(Life Technologies,Carlsbad,CA).CD4-PE(R&D Sytem),cell membrane staining(Life Technologies,Carlsbad,CA);and nuclear staining-DAPI(Life Technologies,Carlsbad,CA).(A)CD4+T cells treated with 100μg of exogenous HA;(B)CD4+T cells treated with medium;(C)CD4+T cells treated with hyaluronidase
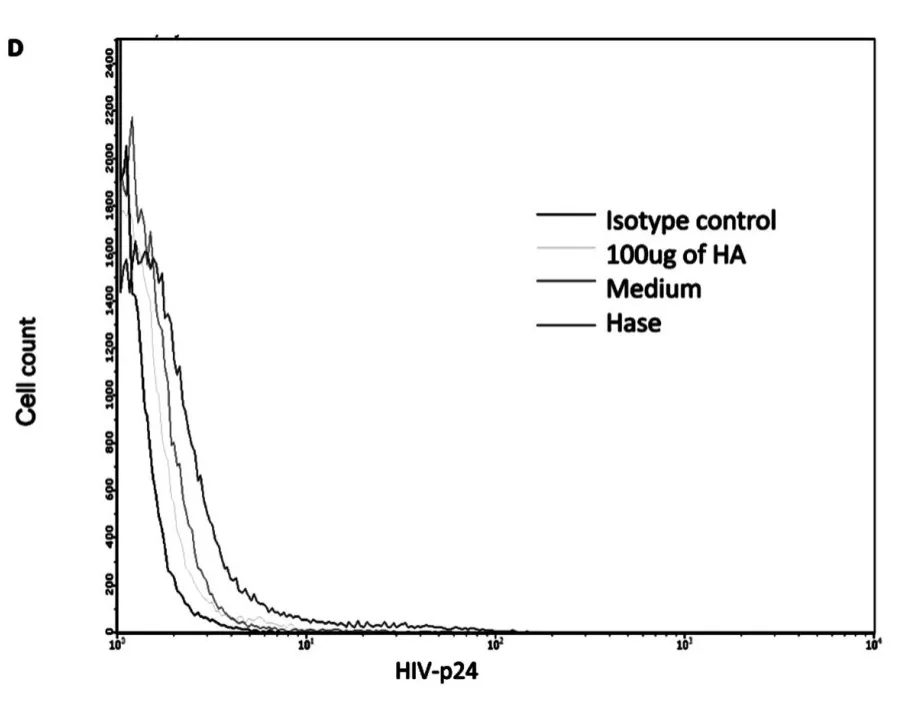
Fig.3 D FACS results:cells with isotype control,cells treated with 100μg of HA,cells treated with medium and cells treated with hyaluronidase
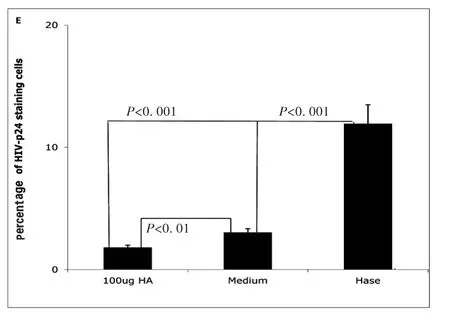
Fig.3E Percentage of HIV-1-p24 positive cells treated with hyaluronidase(Hase)or with medium(Medium)or with 100μg of HA(100μg HA).(All data are mean ±SEM from duplicate experiments representative of at least 3 donors)
Devising more effective interventions to prevent mucosal HIV transmission will require a deeper understanding of the interplay between virus and target cells in the mucosal environment.Studies of both human and rhesus macaque models indicate that mucosal tissues provide multiple mechanisms to prevent HIV and SIV infection,including physical barriers and innate and adaptive immune responses[5,9].However,a small amount of HIV-1 and SIV can penetrate the mucosal barrier,gaining access to the sub-mucosa and the initial recipient cell targets,which appear to be predominantly resting CD4+T cells[8].Details of the interaction of HIV and SIV with resting CD4+T cells in the sub-mucosa are not fully understood,but CCR5-tropic viruses(R5-HIV)are preferentially transmitted[5,9].In this study,we show that endogenous HA on unstimulated CD4+T cells is an obstacle for HIV infection and that hyaluronidase treatment can increase binding of HIV particles,and enhance HIV infectivity.Furthermore,addition of exogenous HA at 100μg/mL,the concentration of HA in human epidermis[28],reduces HIV infectivity on unstimulated CD4+T cells.These data collectively argue that HA interferes with HIV infection during early stage,perhaps by directly impacting the interactions between CD44 and other molecules,or altering the distribution and/or activation status of local CD4+T cells,thus reducing targets which are needed for efficient transmission.
HA is the main component of the extra cellular matrix(ECM)in the mucosal tissue.The main receptor for HA,CD44,is highly expressed in all cells,including CD4+T cells,in mucosal tissues,especially those along the reproductive tract[18,29].HA interactions with CD44 are involved in mucosal immunity and participate in mucosal inflammation[16,21].It has been reported that exogenous HA can interfere with interaction of endogenous HA with CD44,limiting recruitment of T cells by reducing T cell adhesion[30],preventing T cell rolling,and inhibiting T cell extravasation into an inflamed site[19-20].Other studies identify the recruitment of T cells into submucosa as important and necessary for mucosal HIV transmission[5,9].
Our study demonstrates that exogenous HA reduces both X4 and R5 HIV infection of unstimulated CD4+T cells.Others have reported that the endogenous HA coat on the surface of cells can prevent extraneous or indiscriminant ligand binding[18]and can prevent infection with newcastle disease virus(NDV),vesicular stomatitis virus(VSV),and rubella virus(RV).Reducing the thickness of HA on the cell surface by hyaluronidase treatment can make target cells more susceptible to NDV,VSV,and RV infection[31].We also observed that treatment of unstimulated CD4+T cells with hyaluronidase reduced the thickness of endogenous HA on the cell surface[24],allowing more HIV particles to bind(Fig.3),an effect that could be reversed by the addition of exogenous HA(Fig.2).While these experimental results suggest that the inhibitory effects of HA on HIV infection occur at least in part at an early stage of the viral life cycle,including effects localizable to viral binding,further work is needed.
It is notable that HA,and hyaluronidase are all highly expressed and play important roles in maintaining the integrity and function of the mucosal tissue along the reproductive tract.Hyaluronidase is also a major constituent of semen[29].Both HA and hyaluronidase are highly up-regulated during sexual intercourse as well as inflammation[18].The processes of reproductive fertilization and HIV transmission intersect at many levels anatomically and functionally.Both involve the reproductive tract and permissiveness of mucosal tissue and surface barriers.Clinical studies have demonstrated that inflammation in the mucosal tissue can facilitate HIV transmission[32],and that transmission rates are higher for HIV in semen compared to blood[33].The identification of topical agents that can be applied to mucosal surfaces to prevent mucosal HIV transmission has been identified as a priority area for research and development in the effort to control the HIV/AIDS epidemic[34].
In summary,HA present on the surface of unstimulated CD4+T cells can influence both X4 and R5-HIV infection.Both HA and hyaluronidase have well described roles in reproductive and inflammatory processes.Our data now show that the status of the HA coat may play an important role in the initial interaction of X4 and R5-HIV virions with resting CD4+T cells,a feature that may be particularly relevant for mucosal HIV transmission.Intriguingly,exogenous HA appears to block HIV engagement and infection of target cells.HA is a non-immunogenic natural biopolymer that has been used in a variety of clinical applications.These findings should be further explored in vivo and,if confirmed,could lead to the development of novel interventions to reduce HIV mucosal transmission.
Conflict of Interest
The authors declare that they have no conflict of interest.
This work was supported by the National Institutes of Health[grants 1R21AI104445-01A1(PL)].
(
)
[1]PADIAN N S,MCCOY S I,KARIM S S,et al.HIV prevention transformed:the new prevention research agenda[J].Lancet,2011,378:269-278.
[2]PITISUTTITHUM P,GILBERT P,GURWITH M,et al.Randomized,double-blind,placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok,Thailand[J].J Infect Dis,2006,194:1661-1671.
[3]WATKINS D I,BURTON D R,KALLAS E G,et al.Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans[J].Nat Med,2008,14:617-621.
[4]UNAIDS.(Global report 2013.Geneva)[EB/OL].[2013]http://www.unaids.org/globalreport/global_report.htm.
[5]SHAW G M,HUNTER E.HIV transmission[J].Cold Spring Harb Perspect Med,2012,2:1-23.
[6]MILLER C J,LI Q,ABEL K,et al.Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus[J].J Virol,2005,79:9217-9227.
[7]LI Q,ESTES J D,SCHLIEVERT P M,et al.Glycerol monolaurate prevents mucosal SIV transmission[J].Nature,2009,458:1034-1038.
[8]ZHANG Z,SCHULER T,ZUPANCIC M,et al.Sexual transmission and propagation of SIV and HIV in resting and activated CD4+T cells[J].Science,1999,286:1353-1357.
[9]HAASE A T.Early events in sexual transmission of HIV and SIV and opportunities for interventions[J].Annu Rev Med,2011,62:127-139.
[10]GIAVEDONI L D,CHEN H L,Hodara V L,et al.Impact of mucosal inflammation on oral simian immunodeficiency virus transmission[J].J Virol,2013,87:1750-1758.
[11]LAJOIE J,JUNO J,BURGENER A,et al.A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers[J].Mucosal Immunol,2012,5:277-287.
[12]HAALAND R E,HAWKINS P A,SALAZAR-GONZALEZ J,et al.Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1[J].PLoS Pathog,2009,5:e1000274.
[13]MAHER D,WU X,SCHACKER T,et al.HIV binding,penetration,and primary infection in human cervicovaginal tissue[J].Proc Natl Acad Sci U S A,2005,102:11504-11509.
[14]SHUKAIR S A,ALLEN S A,CIANCI G C,et al.Human cervicovaginal mucus contains an activity that hinders HIV-1movement[J].Mucosal Immunol,2013,6:427-434.
[15]LAI S K,HIDA K,SHUKAIR S,et al.Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus[J].J Virol,2009,83:11196-11200.
[16]TOOLE B P.Hyaluronan:from extracellular glue to pericellular cue[J].Nat Rev Cancer,2004,4:528-539.
[17]GIRISH K S,KEMPARAJU K.The magic glue hyaluronan and its eraser hyaluronidase:a biological overview[J].Life Sci,2007,80:1921-1943.
[18]EVANKO S P,TAMMi M I,TAMMI R H,et al.Hyaluronan-dependent pericellular matrix[J].Adv Drug Deliv Rev,2007,59:1351-1365.
[19]DE GRENDELE H C,ESTESS P,SIEGELMAN M H.Requirement for CD44 in activated T cell extravasation into an inflammatory site[J].Science ,1997,278:672-675.
[20]SIEGELMAN M H,DE GRENDELE H C,ESTESS P.Activation and interaction of CD44 and hyaluronan in immunological systems[J].J Leukoc Biol,1999,66:315-321.
[21]JIANG D,LIANG J,NOBLE P W.Hyaluronan as an immune regulator in human diseases[J].Physiol Rev,2011,91:221-264.
[22]MUTO J,YAMASAKI K,TAYLOR K R,et al.Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS[J].Mol Immunol,2009,47:449-456.
[23]KIM Y,LEE Y S,HAHN J H,et al.Hyaluronic acid targets CD44 and inhibits FcepsilonRI signaling involving PKCdelta,Rac1,ROS,and MAPK to exert anti-allergic effect[J].Mol Immunol,2008,45:2537-2547.
[24]LI P,FUJIMOTO K,BOURGUINGNON L,et al.Exogenous and endogenous hyaluronic acid reduces HIV infection of CD4+T cells[J].Immunol Cell Biol,2014,Jun 24,doi:10.1038.
[25]DERDEYN C A,DECKER J M,SFAKIANOS J N,et al.Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120[J].J Virol,2000,74:8358-8367.
[26]ZHANG L S,MA H W,GREYNER H J,et al.Inhibition of cell proliferation by CD44:Akt is inactivated and EGR-1 is down-regulated[J].Cell Prolif,2010,43:385-395.
[27]MCBRIDE W H,BARD J B.Hyaluronidase-sensitive halos around adherent cells.Their role in blocking lymphocyte-mediated cytolysis[J].J Exp Med ,1979,149:507-515.
[28]VOLPI N 1,SCHILLER J,STERN R,et al.Role,metabolism,chemical modifications and applications of hyaluronan[J].Curr Med Chem,2009,16:1718-1745.
[29]RODRIGUEZ HURTADO I,STEWART A J,WOLFE D F,et al.Immunolocalization of the hyaluronan receptor CD44 in the reproductive tract of the mare[J].Theriogenology,2010,75:276-286.
[30]MOHAMADZADEH M,DEGRENDELE H,ARIZPE H,et al.Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion[J].J Clin Invest,1998,101:97-108.
[31]PATTERSON R L,PETERSON D A,DEINHARDT F,et al.Rubella and rheumatoid arthritis:hyaluronic acid and susceptibility of cultured rheumatoid synovial cells to viruses.(resistant to virus)[J].Proc Soc Exp Biol Med,1975,149:594-598.
[32]GALVIN S R,COHEN M S.The role of sexually transmitted diseases in HIV transmission[J].Nat Rev Microbiol,2004,2:33-42.
[33]PILCHER C D,TIEN H C,ERON J J Jr,et al.Duke-UNC-Emory Acute HIV Consortium.Brief but efficient:acute HIV infection and the sexual transmission of HIV[J].J Infect Dis,2004,189:1785-1792.
[34]HLADIK F,DONCEL G F.Preventing mucosal HIV transmission with topical microbicides:challenges and opportunities[J].Antiviral Res,2010,88S:S3-9.
- 江汉大学学报(自然科学版)的其它文章
- 江汉大学学报(自然科学版)2014年第42卷总目次

