Hepatic ischemic preconditioning increases portal vein flow in experimental liver ischemia reperfusion injury
Estela RR Figueira, Joel A Rocha-Filho, Mauro Nakatani, Marcelo FS Buto, Eduardo R Tatebe, Vitor O Andre, Ivan Cecconello and Luiz AC D'Albuquerque
São Paulo, Brazil
Hepatic ischemic preconditioning increases portal vein flow in experimental liver ischemia reperfusion injury
Estela RR Figueira, Joel A Rocha-Filho, Mauro Nakatani, Marcelo FS Buto, Eduardo R Tatebe, Vitor O Andre, Ivan Cecconello and Luiz AC D'Albuquerque
São Paulo, Brazil
BACKGROUND:Ischemic preconditioning (IPC) has been shown to decrease liver injury and to increase hepatic microvascular perfusion after liver ischemia reperfusion. This study aimed to evaluate the effects of IPC on hemodynamics of the portal venous system.
METHODS:Thirty-two rats were randomized into two groups: IPC group and control group. The rats of the IPC group underwent IPC by 10 minutes of liver ischemia followed by 10 minutes of reperfusion before liver ischemia, and the rats of the control group were subjected to 60 minutes of partial liver ischemia. Non-ischemic lobes were resected immediately after reperfusion. The animals were studied at 4 hours and 12 hours after reperfusion. Mean arterial pressure, heart rate, portal vein flow and pressure were analyzed. Blood was collected for the determination of the levels of aspartate aminotransferase, alanine aminotransferase, calcium, lactate, pH, bicarbonate, and base excess.
RESULTS:IPC increased the mean portal vein flow at 4 hours and 12 hours after reperfusion. IPC recovered 78% of the mean portal vein flow at 12 hours after reperfusion. IPC decreased the levels of aspartate aminotransferase, alanine aminotransferase and lactate, and increased the levels of ionized calcium, bicarbonate and base excess at 12 hours after reperfusion.
CONCLUSIONS:This study demonstrated that IPC increases portal vein flow and enhances hepatoprotective effects in liver ischemia reperfusion. The better recovery of portal vein flow after IPC may be correlated with the lower levels of transaminases and with the better metabolic profile. (Hepatobiliary Pancreat Dis Int 2014;13:40-47)
ischemic preconditioning; portal vein flow;
liver ischemia
Introduction
Hepatic ischemia reperfusion (IR) injury can result in liver dysfunction and even in liver failure that complicates liver surgery and transplantation as an important cause of immediate and late postoperative adverse outcomes.[1, 2]Despite all the advances incorporated in recent years, liver IR injury remains the main limiting factor that has restricted the surgical treatment of liver diseases.
Disturbance of hepatic microcirculation that occurs after liver reperfusion is a key factor to the development of liver IR injury and subsequent liver dysfunction.[3]There is a progressive narrowing of sinusoids with a decrease of microcirculatory blood flow and appearance of non-perfused areas.[3, 4]Several studies[3, 5-7]reported that the extension of microcirculatory derangement is correlated with the severity of liver IR injury. A recent study[8]demonstrated that severe liver IR injury is associated with reduced portal vein flow, hepatic artery fl ow, and total liver blood fl ow.
Moreover, several therapeutic strategies have beendesigned to reduce liver IR injury. Ischemic preconditioning (IPC) is a surgical strategy increasing tissue tolerance to prolonged ischemia by previous exposure to a brief period of ischemia followed by a brief period of reperfusion.[9, 10]The protective effects of IPC were initially demonstrated in the heart,[9]and since then these effects have been found in the liver and other organs.[11-14]
IPC has been reported to increase microvascular blood flow and to reduce microcirculatory dysfunction that is followed by liver IR.[15-18]However, the influence of IPC on hepatic circulation has not been confirmed. Accordingly, the present study was undertaken to investigate the effects of IPC on hemodynamics of the portal vein after liver IR.
Methods
Animals and ethical approval
The present study was approved by the Ethics Committee of University of São Paulo School of Medicine. Fixtysix animals were cared for in accordance with the recommendations of the National Research Council Guide (1996) for the Care and Use of Laboratory Animals. Adult male Wistar rats weighing 185 to 300 g were housed in a temperature-controlled environment under 12-hour light/12-hour dark cycle, with access to water and standard laboratory rat chow ad libitum.
Anesthesia and surgical procedures
The rats were anesthetized with intraperitoneal injection of 5% ketamine (Ketalar, Cristalia, SP, Brazil), 30 mg/kg, and 2% xylazine (Rompum, Bayer, SP, Brazil), 30 mg/kg. During the surgical procedure, the animal was placed on a heating mat to maintain body temperature between 35 ℃ and 37 ℃. A midline laparotomy of 4 cm in length from the xiphoid process was performed. The common pedicles of the median and left lateral hepatic lobes were occluded with an atraumatic microclamp, inducing ischemia of approximately 70% of the total liver volume.[10, 19, 20]During the period of ischemia, the abdominal wall was closed to avoid dehydration. After 60 minutes of ischemia, the microclamp was removed allowing hepatic reperfusion, the non-ischemic right and caudate lobes were immediately removed, and the abdomen was closed.[10]
At 4 hours and 12 hours after reperfusion rats were re-anesthetized and subjected to orotracheal intubation and mechanical ventilation with a tidal volume of 0.08 mL/g body weight, 60 breaths per minute, and 21% FiO2(683 Small Animal Ventilator, Harvard Apparatus, Holliston, MA, USA). A polyethylene catheter PE-50 was inserted into the right carotid artery for hemodynamic monitoring and blood sampling. At the end of the experiment, blood was drawn via cardiac puncture for the measurement of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Finally the rats were euthanized by exsanguination.
Study groups and experimental design
Thirty-two rats were randomized into two groups: control group (n=16) and IPC group (n=16). The rats of the control group were subjected to 60 minutes of liver ischemia, and those of the IPC group underwent IPC by 10 minutes of liver ischemia followed by 10 minutes of reperfusion performed at the common pedicle of the median and left lateral hepatic lobes before 60 minutes of liver ischemia. Eight rats of each group were studied at 4 hours after reperfusion and the other 8 rats, at 12 hours. In the sham-operated group (n=8), animals were subjected to the resection of the right and caudate lobes without liver ischemia. The study design is shown in Fig. 1.
Biochemical assays
AST and ALT levels were determined to assess the degree of hepatocellular injury 4 hours and 12 hours after reperfusion (Cobas-Mira, Roche, Rotkreuz, Switzerland). The levels of pH, bicarbonate, base excess, lactate, and ionized calcium in arterial blood samples were analyzed 4 hours and 12 hours after reperfusion (ABL800 Flex Radiometer, Copenhagen, Denmark).
Hemodynamic monitoring
Hemodynamic parameters were recorded 4 hours and 12 hours after reperfusion. Heart rate and mean arterial pressure (MAP) were recorded by the MP150 Starter System (Biopac Systems Inc., CA, USA). The mean portal vein flow (MPVF) was assessed with the perivascular probe (Transonic Systems Inc., NY, USA) connected to a flowmeter (Animal Research Flowmeter TS420, Transonic Systems Inc., NY, USA). The mean portal vein pressure (MPVP) was assessed with a microprobe inserted into the ileal vein (SAMBA BiopacSystems Inc., CA, USA).
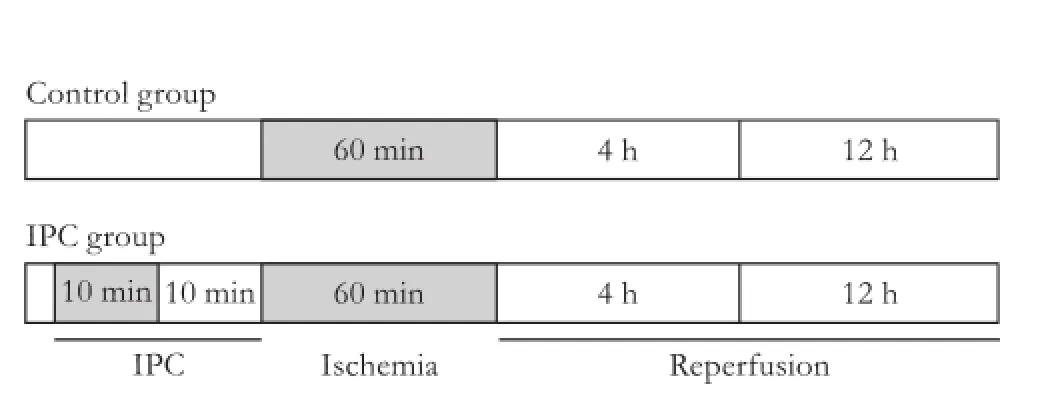
Fig. 1.Study design. Control group: animals subjected to 60 minutes of liver ischemia. IPC group: animals subjected to 10 minutes of ischemia followed by 10 minutes of reperfusion before 60 minutes of liver ischemia.
Histological evaluation of the liver
Liver specimens of the rats were fi xed in 10% formalin and stained with hematoxylin and eosin, and a pathologist who knew nothing about the study performed a microscopic study. The liver reperfusion injury score was assessed 12 hours after reperfusion according to Quireze et al's method.[21]Hepatocellular tumefaction, apoptosis, coagulation necrosis, hepatocellular steatosis, portal inflammation, lobular inflammation and sinusoidal cellularity were scored as absence (0), mild (1), moderate (2) or severe (3) degrees. The degree of coagulation necrosis was multiplied by three.
Survival rate
Eight additional rats of the control and IPC groups were observed for 7 days to assess the survival rate after surgical procedures. The rats alive at the seventh day were considered survivors.
Statistical analysis
Values were expressed as mean±standard deviation or a median of range. Student's t test or the Mann-Whitney U test was performed to evaluate significant differences between the groups. One-way analysis of variance was used when both normality and homogeneity assumptions of variances were satisfied. If only homogeneity was satisfied, the Kruskal-Wallis test was applied. Multiple comparisons were performed using the parametric Tukey test or the non-parametric Tukey test.[22]In cases that both normality and homogeneity were not satisfied, the non-parametric Tukey test was used for two-by-two comparisons to identify differences between the groups. The survival rate of the rats was analyzed by the Kaplan-Meier method, and survival curves were compared using the log-rank test. Differences were considered significant when P<0.05. Statistical analysis was made with the R Statistical Software for Windows, version 2.12.
Results
Hemodynamic evaluation
The levels of MPVF and MPVP were decreased in the control and IPC groups at 4 hours and 12 hours after reperfusion, compared to the sham-operated group, indicating that liver ischemia decreases portal vein flow and pressure (Fig. 2). At 4 hours and 12 hours after reperfusion, the level of MPVF was increased in the IPC group compared to the control group. In the IPC group, the level of MPVF increased from 4 hours up to 12 hours after reperfusion. There was no difference in MPVP between the control and IPC groups during the study (Table, Fig. 2).

Table.Hemodynamic variables at 4 hours and 12 hours after reperfusion
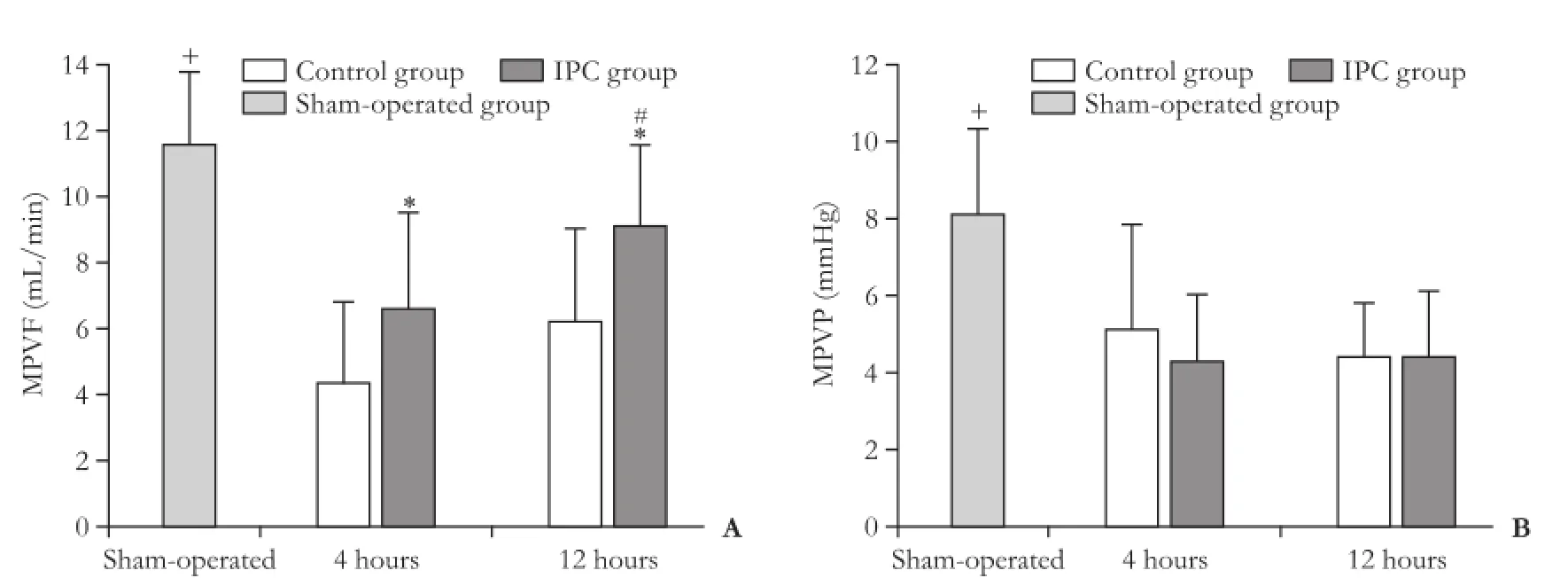
Fig. 2.MPVF and MPVP at 4 hours and 12 hours after reperfusion. Bars represent mean±standard deviation from 8 rats in each group. *: P<0.05, control group vs IPC group; #: P<0.05, 4 hours vs 12 hours; +: P<0.05, sham-operated group vs any other group. MPVF: mean portal vein flow; MPVP: mean portal vein pressure.
There were no differences in MAP after reperfusion at 4 hours and 12 hours between the control and IPC groups. The heart rate increased from 4 hours up to 12 hours after reperfusion in both groups (Table).
AST and ALT levels after hepatocellular injury
At 4 hours after reperfusion, the levels of AST and ALT were increased in the control and IPC groups compared to the sham-operated group, there were no differences between the control and IPC groups. At 12 hours after reperfusion, the levels of AST and ALT were decreased in the IPC group compared with the control group. In the IPC group, the levels of AST and ALT decreased from 4 hours up to 12 hours after reperfusion (Fig. 3).
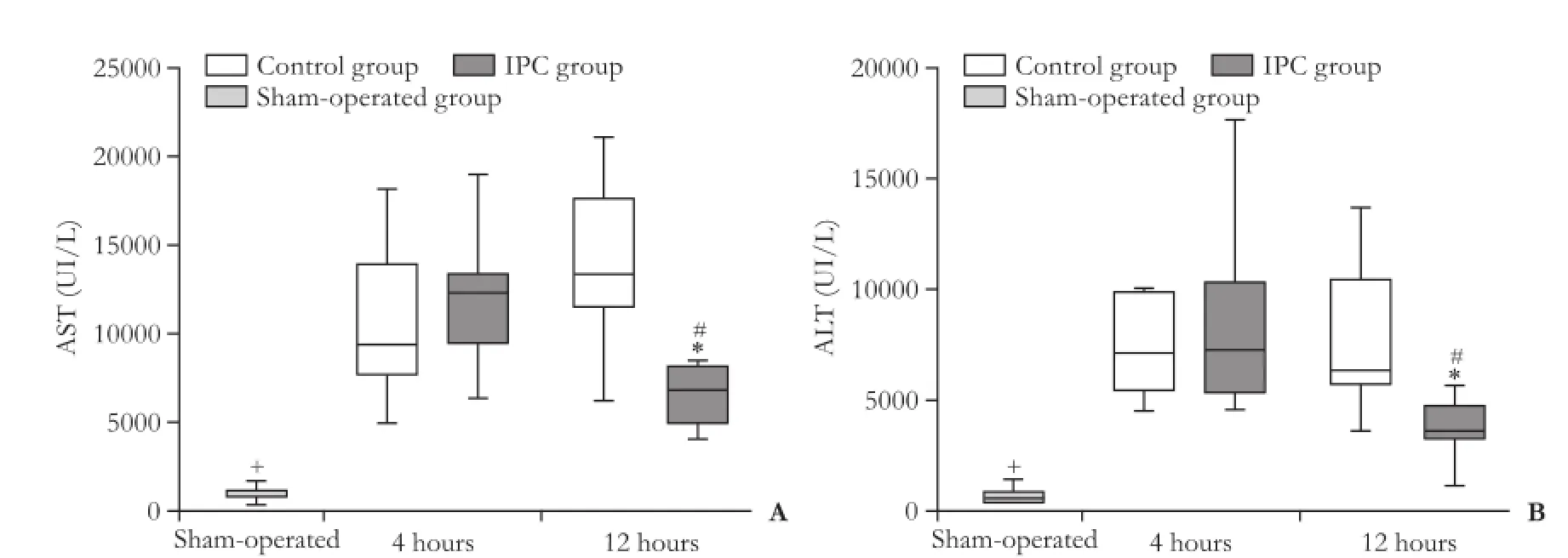
Fig. 3.AST and ALT levels 4 hours and 12 hours after reperfusion. Data are presented as box-whisker plots from 8 rats in each group. *: P<0.05, control group vs IPC group; #: P<0.05, 4 hours vs 12 hours; +: P<0.05, sham-operated group vs any other group.
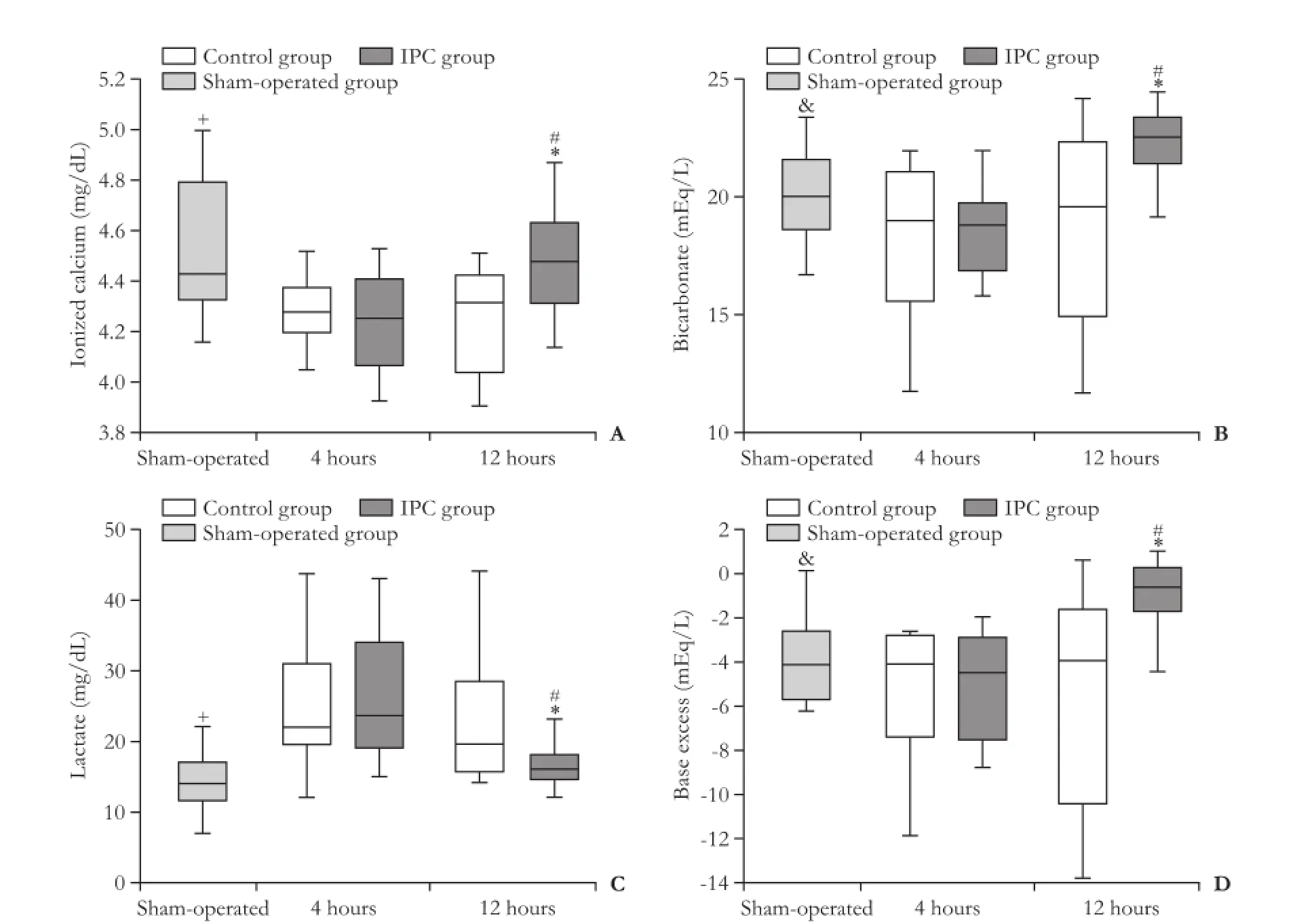
Fig. 4.Ionized calcium (A), bicarbonate (B), lactate (C), and base excess (D) arterial levels 4 hours and 12 hours after reperfusion. Data are presented as box-whisker plots from 8 rats in each group. +: P<0.05, sham-operated group vs control group-4 hours, control group-12 hours, IPC group-4 hours; &: P<0.05, sham-operated-group vs IPC group; *: P<0.05, control group vs IPC group; #: P<0.05, 4 hours vs 12 hours.
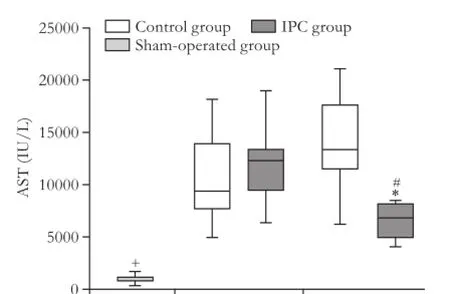
Fig. 5.Seven days survival analysis. Survival rate was estimated by Kaplan-Meier log-rank analysis. Control group (n=8), animals submitted to 60 minutes of liver ischemia, resulted in a survival rate of 50% compared to 12.5% for the IPC group (n=8), animals submitted to 10 minutes of liver ischemic preconditioning before 60 min of liver ischemia. Difference between the control and IPC groups is not significant.
Metabolic and acid-base profile
At 4 hours of reperfusion, there were no differences in levels of bicarbonate, base excess, lactate and ionized calcium between the control and IPC groups. At 12 hours of reperfusion, the levels of ionized calcium, bicarbonate and base excess were increased but the level of lactate was decreased in the IPC group compared with the control group. In the IPC group the levels of ionized calcium, bicarbonate, and base excess were increased, but the level of lactate was decreased from 4 hours up to 12 hours after reperfusion (Fig. 4). There was no difference in pH levels between the control and IPC groups at 4 hours of reperfusion, but at 12 hours, the pH was decreased in the control group compared with the IPC group (7.37±0.09 vs 7.44±0.11, P<0.05).
Liver histology
After 12 hours of reperfusion, there were no differences in the liver reperfusion injury score, 9.5 (7 to 10) and 8 (5 to 11), and in the degree of necrosis, 2.5 (2 to 3) and 2 (0 to 3), respectively between the control and IPC groups.
Analysis of survival rates
After 7 days of reperfusion, there were no differences in the survival rates between the control (12.5%) and IPC (50.0%) groups (Fig. 5).
Discussion
The present study aimed to determine the effects of hepatic ischemic preconditioning on hemodynamics of the portal venous system after 60 minutes of liver ischemia. The results of the study demonstrated that this experimental model is appropriate to determine the effects of IPC on the hemodynamics of the portal vein during liver IR and that IPC can promote the recovery of the portal vein flow and metabolic profile while lowering the level of liver transaminases.
This model of partial liver ischemia with subsequent resection of non-ischemic lobes seems appropriate to evaluate global liver ischemia. Preservation of portal fl ow through the caudate and right lobes during liver ischemia prevents splanchnic congestion, and resection of the non-ischemic lobes at the beginning of reperfusion reduces its influence on hepatic hemodynamics. Preservation of the non-ischemic lobes during reperfusion may lead to a significant amount of hepatic blood flow shunting to the non-ischemic lobe until normalization of the increased vascular resistance in the postischemic lobes.[23]We found previously that 1 hour of partial liver ischemia (70% of the liver mass) with preservation of non-ischemic lobes led to a 3 times increase in the level of liver transaminases.[20]The model with resection of the non-ischemic lobes used in the present study seems appropriate to represent total liver ischemia which occurs during liver transplantation or in liver resection with Pringle maneuver,[24]and to study hepatic hemodynamics during liver IR.
The impairment of hepatic microcirculation plays a central role in the development of reperfusion injury. The imbalance between endothelin, a potent vasoconstrictor, and nitric oxide (NO) is a major contributor to progressive reduction of sinusoidal perfusion. Neutrophil adhesion and activation and platelet aggregation with additional release of cytokines and reactive oxygen species (ROS) could amplify cellular injury and microvascular dysfunction during reperfusion.[3, 25]Studies[3, 5]found that the severity of microcirculatory derangement is closely correlated with the severity of IR injury.
IPC is a surgical strategy to increase tissue tolerance to prolonged ischemia by previous exposure to a brief period of ischemia followed by a brief period of reperfusion.[9, 10]Several studies[26-33]showed the effects of IPC on liver IR, i.e. the precise protective mechanism of IPC against liver IR is not fully understood, but it involves improvement of hepatic microcirculation, preservation of hepatic energy metabolism, decrease of neutrophil infiltration, ROS production, oxidative stress, reduction of necrosis, and apoptotic cell death. The administration of N-nitro-L-arginine methyl ester (L-NAME), a suppressor of NO synthase, could abolish the positive effect of IPC, suggesting that IPC improves microcirculation through an increase in NO synthesis that is mediated by activation of adenosineA2b receptor.[17, 28, 34, 35]Hepatic microcirculation was monitored with laser Doppler flowmetry during liver IR, showing that IPC promotes a faster and more effective return of hepatic tissue blood flow after reperfusion.[15, 28]Recent studies[36, 37]have also demonstrated the protective effects of remote IPC on hepatic microcirculation by using intravital fluorescence microscopy.
A study[18]found that severe liver IR injury, determined by increased levels of AST, is associated with reduced portal vein flow, hepatic artery flow, and hepatic blood flow. During liver IR, decreased portal blood flow may be related to increased presinusoidal and sinusoidal resistance that occurs in the dysfunctional microcirculation.[38]Our data showed that portal vein flow was significantly reduced by 62% after 4 hours of reperfusion and by 46% after 12 hours. The decreased portal flow was associated with increased levels of AST and ALT. However IPC induced a mjnor decrease of MPVF, and after 4 hours of reperfusion the MPVF was decreased by 43%-22% after 12 hours. At 12 hours after reperfusion, IPC caused a recovery of 78% of MPVF. Despite studies on the effects of IPC on liver microcirculation, the effects of IPC on liver macrocirculation have not been confirmed. However, a recent study[39]showed that in patients subjected to liver resection with Pringle maneuver, IPC prevented the decrease of postischemic portal vein flow but increased arterial perfusion, suggesting that the improvement of hepatic microcirculation is related to the protective mechanism of IPC after hepatectomy.
In the present study, IPC increased portal vein flow after reperfusion, but did not increase the portal vein pressure 12 hours after reperfusion. MAP and heart rate were similar in the control and IPC groups, suggesting that the increase of portal vein flow in the IPC group was not related to hemodynamics. IPC decreased liver transaminases at 12 hours after reperfusion, suggesting IPC protection against liver IR. Several studies[8, 40, 41]showed inverse correlation between hepatic perfusion and the level of liver transaminases after reperfusion.
Despite the markedly decreased levels of AST and ALT 12 hours after reperfusion in the IPC group, no histological advantages of IPC were observed in liver injury score and necrosis. A study[42]reported that 24 hours after reperfusion IPC promoted less hepatocellular necrosis with decreased apoptosis in a model with 60 minutes of liver ischemia. Quireze et al[21]also reported the similar finding 4 hours after reperfusion with 40 minutes of ischemia. In contrast to our experiment, non-ischemic lobes were not removed in all the studies. Possibly, our model with 60 minutes of ischemia was associated with resection of non-ischemic lobes which caused more severe liver injury. We believe that the protective effects of IPC in liver histology might be detected later.
The metabolic and acid-base profile showed a decreased level of lactate and increased levels of bicarbonate and base excess 12 hours after reperfusion in the IPC group. IPC also promoted a recovery of the levels of lactate, bicarbonate and base excess, which reached normal values from 4 hours to 12 hours of reperfusion. The level of ionized calcium decreased after 4 hours of reperfusion in both the control and IPC groups. Interestingly, IPC increased the level of ionized calcium after 12 hours of reperfusion.
Increased intracellular calcium is considered an early event of cell injury or cell death in liver IR.[43-45]Reperfusion after ischemia can increase the level of total hepatocyte calcium.[46, 47]This increase is thought to activate numerous enzymes, apoptotic and necrotic pathways modulating hepatocyte death in liver IR.[44, 45]Although the increased level of ionized calcium in the cytoplasmic space on reperfusion is due to an increased calcium inflow across the plasma membrane secondary to ischemia, the mechanism of calcium inflow remains to be elucidated.[48, 49]Moreover, IPC can increase the release of adenosine.[34, 35]The mechanisms of adenosine hepatoprotection are not fully elucidated. Adenosine was found to decrease Ca2+influx via activation of ATP-dependent K+channels.[18]In our study, IPC increased ionized calcium in arteries. But it is difficult to correlate increased ionized calcium in the IPC group with decreased calcium influx through the plasma membrane. We suggest that IPC could reduce the calcium inflow across the plasma membrane.
Notwithstanding the general recovery presented in the IPC group, this study did not show a clear benefit in the 7 days survival rate of animals. We believe that a large sample size is required to detect if IPC can reduce the mortality of rats with severe liver IR injury.
In conclusion, this experimental model is appropriate to evaluate the effects of IPC on hemodynamics of the portal vein after liver IR. IPC increases the blood flow of the portal vein and promotes hepatoprotective effects 12 hours after reperfusion. Further studies are needed to clarify the protective mechanisms of IPC and if the IPC hepatoprotection is related to inhibition of liver IR-induced increase of cytoplasmic calcium.
Acknowledgements:We thank Cinthia Lanchotte (Laboratory of Medical Investigations LIM37 FMUSP) for assistance in data processing, Dr Rosely Antunes Patzina (Department of Pathology HCFMUSP) for histological evaluation, Marcio Augusto Diniz (Institute of Mathematics and Statistics USP) for statistical analysis and Professor Eleazar Chaib (Director of the Laboratory of MedicalInvestigations LIM37 FMUSP) for research consultation. Nakatani M FAPESP Scientific Research Initiation 2011/09434-8, Andre VO FAPESP Scientific Research Initiation 2011/08759-0.
Contributors:FERR and RFJA conceived and designed the study, contributed to the acquisition of data and supervised the experimental work, analysed the data and wrote the manuscript. NM, BMFS, TER and AVO helped to design the study and participated in animal preparation and performance of experimental work. CI and DLAC participated in experimental design and helped to draft the manuscript. All authors read and approved the final manuscript. FERR is the guarantor.
Funding:This study was supported by a grant from São Paulo Foundation Research FAPESP 2011/05214-3.
Ethical approval:The study was approved by the Ethics Committee of University of São Paulo School of Medicine.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Pine JK, Aldouri A, Young AL, Davies MH, Attia M, Toogood GJ, et al. Liver transplantation following donation after cardiac death: an analysis using matched pairs. Liver Transpl 2009;15:1072-1082.
2 Beck-Schimmer B, Breitenstein S, Urech S, De Conno E, Wittlinger M, Puhan M, et al. A randomized controlled trial on pharmacological preconditioning in liver surgery using a volatile anesthetic. Ann Surg 2008;248:909-918.
3 Vollmar B, Glasz J, Leiderer R, Post S, Menger MD. Hepatic microcirculatory perfusion failure is a determinant of liver dysfunction in warm ischemia-reperfusion. Am J Pathol 1994;145:1421-1431.
4 Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, Piña E, Geller DA. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res 2008;147:153-159.
5 Clemens MG, McDonagh PF, Chaudry IH, Baue AE. Hepatic microcirculatory failure after ischemia and reperfusion: improvement with ATP-MgCl2 treatment. Am J Physiol 1985; 248:H804-811.
6 Horiuchi T, Muraoka R, Tabo T, Uchinami M, Kimura N, Tanigawa N. Optimal cycles of hepatic ischemia and reperfusion for intermittent pedicle clamping during liver surgery. Arch Surg 1995;130:754-758.
7 Menger MD, Richter S, Yamauchi J, Vollmar B. Role of microcirculation in hepatic ischemia/reperfusion injury. Hepatogastroenterology 1999;46:1452-1457.
8 Kelly DM, Shiba H, Nakagawa S, Irefin S, Eghtesad B, Quintini C, et al. Hepatic blood flow plays an important role in ischemia-reperfusion injury. Liver Transpl 2011;17: 1448-1456.
9 Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74:1124-1136.
10 Yoshizumi T, Yanaga K, Soejima Y, Maeda T, Uchiyama H, Sugimachi K. Amelioration of liver injury by ischaemic preconditioning. Br J Surg 1998;85:1636-1640.
11 Peralta C, Hotter G, Closa D, Gelpí E, Bulbena O, Roselló-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology 1997;25:934-937.
12 Lloris-Carsí JM, Cejalvo D, Toledo-Pereyra LH, Calvo MA, Suzuki S. Preconditioning: effect upon lesion modulation in warm liver ischemia. Transplant Proc 1993;25:3303-3304.
13 Glazier SS, O'Rourke DM, Graham DI, Welsh FA. Induction of ischemic tolerance following brief focal ischemia in rat brain. J Cereb Blood Flow Metab 1994;14:545-553.
14 Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000;232:155-162.
15 Szijártó A, Hahn O, Lotz G, Schaff Z, Madarász E, Kupcsulik PK. Effect of ischemic preconditioning on rat liver microcirculation monitored with laser Doppler flowmetry. J Surg Res 2006;131:150-157.
16 Glanemann M, Vollmar B, Nussler AK, Schaefer T, Neuhaus P, Menger MD. Ischemic preconditioning protects from hepatic ischemia/reperfusion-injury by preservation of microcirculation and mitochondrial redox-state. J Hepatol 2003;38:59-66.
17 Koti RS, Yang W, Dashwood MR, Davidson BR, Seifalian AM. Effect of ischemic preconditioning on hepatic microcirculation and function in a rat model of ischemia reperfusion injury. Liver Transpl 2002;8:1182-1191.
18 Cutrn JC, Perrelli MG, Cavalieri B, Peralta C, Rosell Catafau J, Poli G. Microvascular dysfunction induced by reperfusion injury and protective effect of ischemic preconditioning. Free Radic Biol Med 2002;33:1200-1208.
19 Madrahimov N, Dirsch O, Broelsch C, Dahmen U. Marginal hepatectomy in the rat: from anatomy to surgery. Ann Surg 2006;244:89-98.
20 Figueira ER, Bacchella T, Coelho AM, Sampietre SN, Molan NA, Leitão RM, et al. Timing-dependent protection of hypertonic saline solution administration in experimental liver ischemia/reperfusion injury. Surgery 2010;147:415-423.
21 Quireze C, Montero EF, Leitão RM, Juliano Y, Fagundes DJ, Poli-de-Figueiredo LF. Ischemic preconditioning prevents apoptotic cell death and necrosis in early and intermediate phases of liver ischemia-reperfusion injury in rats. J Invest Surg 2006;19:229-236.
22 Brunner E, Puri ML. Nonparametric methods in factorial designs. Stat Pap 2001;42:1-52.
23 Hayashi H, Chaudry IH, Clemens MG, Baue AE. Hepatic ischemia models for determining the effects of ATP-MgCl2 treatment. J Surg Res 1986;40:167-175.
24 Pringle JH. V. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg 1908;48:541-549.
25 Ramalho FS, Fernandez-Monteiro I, Rosello-Catafau J, Peralta C. Hepatic microcirculatory failure. Acta Cir Bras 2006;21:48-53.
26 Yadav SS, Sindram D, Perry DK, Clavien PA. Ischemic preconditioning protects the mouse liver by inhibition of apoptosis through a caspase-dependent pathway. Hepatology 1999;30:1223-1231.
27 Sindram D, Rüdiger HA, Upadhya AG, Strasberg SM, Clavien PA. Ischemic preconditioning protects against cold ischemic injury through an oxidative stress dependent mechanism. J Hepatol 2002;36:78-84.
28 Caban A, Oczkowicz G, Abdel-Samad O, Cierpka L. Influence of ischemic preconditioning and nitric oxide on microcirculation and the degree of rat liver injury in the model of ischemia andreperfusion. Transplant Proc 2006;38:196-198.
29 Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol 2003;39:55-61.
30 Ofluoglu E, Kerem M, Pasaoglu H, Turkozkan N, Seven I, Bedirli A, et al. Delayed energy protection of ischemic preconditioning on hepatic ischemia/reperfusion injury in rats. Eur Surg Res 2006;38:114-121.
31 Yuan GJ, Ma JC, Gong ZJ, Sun XM, Zheng SH, Li X. Modulation of liver oxidant-antioxidant system by ischemic preconditioning during ischemia/reperfusion injury in rats. World J Gastroenterol 2005;11:1825-1828.
32 Navarro-Sabaté A, Peralta C, Calvo MN, Manzano A, Massip-Salcedo M, Roselló-Catafau J, et al. Mediators of rat ischemic hepatic preconditioning after cold preservation identified by microarray analysis. Liver Transpl 2006;12:1615-1625.
33 Serafín A, Fernández-Zabalegui L, Prats N, Wu ZY, Roselló-Catafau J, Peralta C. Ischemic preconditioning: tolerance to hepatic ischemia-reperfusion injury. Histol Histopathol 2004;19:281-289.
34 Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpí E, et al. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology 1999;29: 126-132.
35 Choukèr A, Ohta A, Martignoni A, Lukashev D, Zacharia LC, Jackson EK, et al. In vivo hypoxic preconditioning protects from warm liver ischemia-reperfusion injury through the adenosine A2B receptor. Transplantation 2012;94:894-902.
36 Tapuria N, Kumar Y, Habib MM, Abu Amara M, Seifalian AM, Davidson BR. Remote ischemic preconditioning: a novel protective method from ischemia reperfusion injury--a review. J Surg Res 2008;150:304-330.
37 Tapuria N, Junnarkar S, Abu-Amara M, Fuller B, Seifalian AM, Davidson BR. Modulation of microcirculatory changes in the late phase of hepatic ischaemia-reperfusion injury by remote ischaemic preconditioning. HPB (Oxford) 2012;14: 87-97.
38 Hanboon BK, Ekataksin W, Alsfasser G, Schemmer P, Urbaschek B, McCuskey RS, et al. Microvascular dysfunction in hepatic ischemia-reperfusion injury in pigs. Microvasc Res 2010;80:123-132.
39 Heizmann O, Meimarakis G, Volk A, Matz D, Oertli D, Schauer RJ. Ischemic preconditioning-induced hyperperfusion correlates with hepatoprotection after liver resection. World J Gastroenterol 2010;16:1871-1878.
40 Klar E, Bredt M, Kraus T, Angelescu M, Mehrabi A, Senninger N, et al. Early assessment of reperfusion injury by intraoperative quantification of hepatic microcirculation in patients. Transplant Proc 1997;29:362-363.
41 Puhl G, Schaser KD, Pust D, Köhler K, Vollmar B, Menger MD, et al. Initial hepatic microcirculation correlates with early graft function in human orthotopic liver transplantation. Liver Transpl 2005;11:555-563.
42 Knudsen AR, Kannerup AS, Grønbæk H, Dutoit SH, Nyengaard JR, Funch-Jensen P, et al. Quantitative histological assessment of hepatic ischemia-reperfusion injuries following ischemic pre- and post-conditioning in the rat liver. J Surg Res 2013;180:e11-20.
43 Nieuwenhuijs VB, De Bruijn MT, Padbury RT, Barritt GJ. Hepatic ischemia-reperfusion injury: roles of Ca2+ and other intracellular mediators of impaired bile flow and hepatocyte damage. Dig Dis Sci 2006;51:1087-1102.
44 Mattson MP, Chan SL. Calcium orchestrates apoptosis. Nat Cell Biol 2003;5:1041-1043.
45 Sakon M, Ariyoshi H, Umeshita K, Monden M. Ischemiareperfusion injury of the liver with special reference to calcium-dependent mechanisms. Surg Today 2002;32:1-12.
46 Isozaki H, Fujii K, Nomura E, Hara H. Calcium concentration in hepatocytes during liver ischaemia-reperfusion injury and the effects of diltiazem and citrate on perfused rat liver. Eur J Gastroenterol Hepatol 2000;12:291-297.
47 Osawa M, Takemoto K, Kikuyama M, Uchiyama H, Hiramoto Y, Kuroda H. Sperm and its soluble extract cause transient increases in intracellular calcium concentration and in membrane potential of sea urchin zygotes. Dev Biol 1994;166:268-276.
48 Crenesse D, Hugues M, Ferre C, Poiree JC, Benoliel J, Dolisi C, et al. Inhibition of calcium influx during hypoxia/reoxygenation in primary cultured rat hepatocytes. Pharmacology 1999;58:160-170.
49 Gasbarrini A, Borle AB, Farghali H, Bender C, Francavilla A, Van Thiel D. Effect of anoxia on intracellular ATP, Na+i, Ca2+i, Mg2+i, and cytotoxicity in rat hepatocytes. J Biol Chem 1992;267:6654-6663.
Received June 28, 2013
Accepted after revision August 18, 2013
Author Affiliations: Department of Gastroenterology, Laboratory of Medical Investigations LIM37 Discipline of Liver and Gastrointestinal Transplantation (Figueira ERR, Cecconello I and D'Albuquerque LAC); Discipline of Anesthesiology (Rocha-Filho JA); School of Medicine, Medical Student and Scientific Research in Medicine FAPESP (Nakatani M, Buto MFS, Tatebe ER and Andre VO), Hospital das Clinicas, University of São Paulo, Brazil
Estela RR Figueira, MD, Department of Gastroenterology, University of São Paulo School of Medicine, Rua dos Ingleses 586, apt 194, 01329-000, São Paulo, SP, Brazil (Email: estelafigueira@me.com) This study was presented as oral abstract at the Annual Meeting of Americas Hepato-Pancreato-Biliary Association (AHPBA), February 20-24, 2013, Miami Beach, FL, USA.
© 2014, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(14)60005-9
 Hepatobiliary & Pancreatic Diseases International2014年1期
Hepatobiliary & Pancreatic Diseases International2014年1期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Samaritan donor interchange in living donor liver transplantation
- ZBTB20 is involved in liver regeneration after partial hepatectomy in mouse
- Graft cholangiopathy: etiology, diagnosis, and therapeutic strategies
- Hepatobiliary & Pancreatic Diseases International (HBPD INT)
- Graft-to-recipient weight ratio lower to 0.7% is safe without portal pressure modulation in right-lobe living donor liver transplantation with favorable conditions
- Platelet count reduction and outcomes in living liver donors
