血尿酸与2型糖尿病患者周围神经病变的相关性研究
曾静波,庄晓明,穆 珺
血尿酸与2型糖尿病患者周围神经病变的相关性研究
曾静波*,庄晓明,穆 珺
(首都医科大学附属复兴医院内分泌科,北京 100038)
探讨血清尿酸(SUA)水平与2型糖尿病(T2DM)患者糖尿病周围神经并发症(DPN)的关系。选取2011年3月至2013年3月在首都医科大学附属复兴医院内分泌科住院及门诊就诊的T2DM患者920例。采集血清进行生化指标[空腹血糖(FBG),糖化血红蛋白(HbA1c),总胆固醇(TC),甘油三酯(TG),低密度脂蛋白胆固醇(LDL-C),高密度脂蛋白胆固醇(HDL-C),SUA]检测,同时对入组患者是否患有DPN进行诊断并记录。SUA水平四分位法分为4个水平,logistic回归分析不同尿酸水平与DPN发病率的关系。logistic回归分析结果显示SUA水平>3.5mg/dl即第2个四分位后,SUA即为DPN的危险因素,且随着SUA水平的升高,其影响程度增加,OR值分别为2.95(2.02~8.76),3.06(1.75~6.45),4.15(0.84~6.74),均<0.05。SUA是DPN的一个危险因素,在DPN的临床治疗中除了降糖、降脂和降压之外,有效地降低SUA水平应该成为治疗中的一个重要环节。
尿酸;糖尿病,2型;糖尿病并发症;糖尿病神经病变
糖尿病患者随着病程的发展渐出现各种慢性糖尿病并发症,其中微血管并发症所导致的糖尿病周围神经并发症(diabetic peripheral neuropathy,DPN)可诱发糖尿病足及糖尿病坏疽,严重危害患者的生存质量及寿命。多种因素可能会加重周围神经病变的症状,诸如持续高血糖、高脂血症、高血压等,其发病机制也涉及多元醇旁路、氨基己糖、蛋白激酶C(protein kinase C,PKC)、氧化应激、炎症反应以及非酶糖基化,除了这些可能的发病机制外,其他的机制如神经生长因子、聚腺苷二磷酸-核糖聚合酶(poly-ADP-ribose polymerase,PARP)等也影响着DPN的发生及发展[1,2]。2型糖尿病(type 2 diabetes mellitus,T2DM)患者是高尿酸血症的高发人群,近来,一些研究表明高尿酸血症与冠心病、脑梗死等糖尿病大血管并发症的发生相关[3,4],另有研究显示血清尿酸(serum uric acid,SUA)水平与糖尿病性肾病有相关性[5,6],是否高尿酸血症也会影响糖尿病微血管并发症周围神经病变的发生及发展?目前关于这方面的研究尚不多,本研究拟通过对SUA水平与T2DM患者DPN的相关性分析来探讨SUA水平对糖尿病微血管并发症周围神经病变的影响,为DPN的临床治疗提供进一步的理论依据。
1 对象与方法
1.1 研究对象
选取2011年3月至2013年3月在首都医科大学附属复兴医院住院及门诊就诊的T2DM患者共920例,年龄45~75岁。入选患者符合WHO1999糖尿病诊断标准,初次诊断均在30岁后,初次诊断后有过至少1年的口服降糖药治疗史,目前未进行周围神经病变药物治疗。除外合并糖尿病急性并发症(如糖尿病酮症及糖尿病高渗昏迷)、服用降尿酸药物、肾透析、肾移植、发热、心力衰竭、恶性肿瘤及妊娠患者。
1.2 方法
1.2.1 一般临床资料收集 采用横断面研究,收集入选920例患者年龄、性别、身高、体质量、腰围、臀围,血压、糖尿病病程、吸烟史。其中有2例患者因收集资料缺失,未能参与统计分析。
1.2.2 标本检测 于采集临床资料当日采集空腹肘静脉血,将采集标本3000r/min离心10min后分离血清及血浆,冻存于-80℃冰箱中。待入组患者结束后统一测定生化指标,包括空腹血糖(fasting blood glucose,FBG),空腹胰岛素,甘油三酯(triglycerides,TG),总胆固醇(total cholesterol,TC),高密度脂蛋白胆固醇(high density lipoprotein cholesterol,HDL-C),低密度脂蛋白胆固醇(low density lipoprotein cholesterol,LDL-C),糖化血红蛋白(glycosylated hemoglobin A1c,HbA1c),血清肌酐(serum creatinine,SCr),SUA。
1.2.3 DPN的诊断 按照神经病变症状评分,(Neuropathy Symptom Score,NSS)及神经病变失能评分(Neuropathy Disability Score,NDS)进行诊断[7,8]。NSS问卷包括:感觉异常的类型,症状的位置,症状持续时间,夜间静息痛,缓解因素。NDS包括:10g压力的尼龙丝检查,128Hz音叉检查振动觉,用针检查两点辨别感觉、用棉花絮检查轻触觉、膝腱反射及跟腱反射。每个参数分值为0~2分,以NSS及NDS的评分总值进行诊断,诊断为DPN的条件为:NSS问卷6分,可不考虑NDS评分,或NDS 3~5分同时NSS至少5分。同时根据病史除外颈腰椎病变、脑梗死及格林巴利综合征。
1.3 统计学处理

2 结 果
2.1 入选患者一般临床资料
主要临床资料见表1。共入组920例患者,男406例(44.2%),女512例(55.8%),年龄(57.3±10.1)岁,糖尿病病程为(6.70±5.68)年,HbA1c为(7.5±1.3)%,体质量指数(body mass index,BMI)为(24.2±2.9)kg/m2,SUA水平为(5.11±2.21)mg/dl。

表1 2型糖尿病患者的临床特征
T2DM: type 2 diabetes mellitus; BMI: body mass index; SBP: systolic blood pressure; DBP: diastolic blood pressure; HbA1c: glycosylated hemoglobin A1c; FBG: fasting blood glucose; BUN: blood urea nitrogen; SUN: serum urea nitrogen; SCr: serum creatinine; TC: total cholesterol; TG: triglycerides; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; SUA: serum uric acid. 1mmHg=0.133kPa
2.2 血清尿酸四分位分组各组DPN的发生比例
根据SUA水平按四分位法将所有观察患者分为4组:≤3.5,3.6~4.8,4.9~5.6,≥5.7mg/dl。观察不同组别DPN的发生比例,结果显示4组发生比例分别为9.1%,19.9%,33.5%,35.2%,随着SUA水平的升高,其发生比例有升高的趋势。
2.3 血清尿酸与DPN的logistic回归分析
logistic向后回归分析法分析不同变量年龄、糖尿病病程、BMI、收缩压(systolic blood pressure,SBP)、舒张压(diastolic blood pressure,DBP)、HbA1c、FBG、SCr、TC、TG、LDL-C、HDL-C、尿蛋白/肌酐(urinary albumin:creatinine ratio,A/C)及SUA对DPN的影响,结果显示糖尿病病程、DBP、HbA1c、SCr、TG、尿A/C及SUA为DPN的危险因素(表2)。经对危险因素进行调整后,logistic回归分析结果显示不同SUA水平对DPN发生率的影响是不一样的,SUA≤3.5mg/dl时对DPN无影响,在SUA水平>3.5mg/dl后,SUA即为DPN的危险因素,且随着SUA水平的升高,其影响程度增加,第2~4分位OR值分别为2.95(2.02~8.76),3.06(1.75~6.45),4.15(0.84~6.74),均<0.05(表3)。
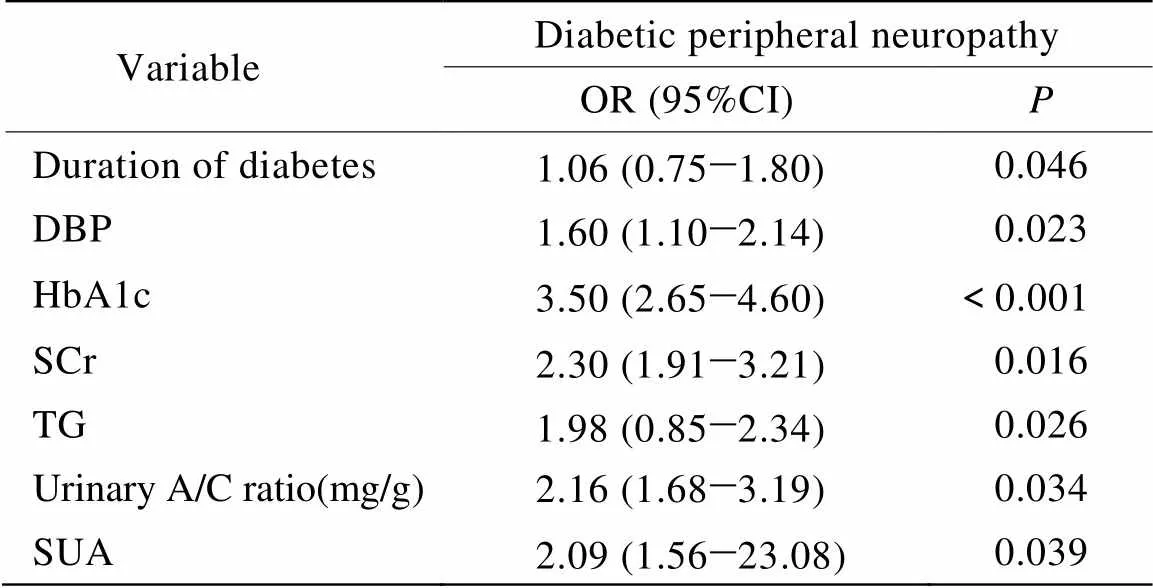
表2 logistic回归向后逐步退法进入方程的自变量及参数估计
DBP: diastolic blood pressure; HbA1c: glycosylated hemoglobin A1c; SCr: serum creatinine; TG: triglycerides; A/C: albumin∶creatinine; SUA: serum uric acid
3 讨 论
本项对920例T2DM患者的横断面研究结果显示,SUA可能是DPN的一个危险因素。
目前已有的研究结果显示,高尿酸血症是代谢综合征以及T2DM的一个重要发病原因,降低高脂喂养大鼠的SUA水平可以改善大鼠的代谢综合征,高尿酸血症同高胰岛素血症明显相关,较高的SUA水平可以预测T2DM以及代谢综合征的发生[9,10]。进一步的研究发现,SUA水平在糖尿病慢性并发症的发生发展中也起了一定的作用,用别嘌醇降低SUA水平后可以明显改善T2DM模型小鼠已受损的肾脏组织,人群研究也发现对1型糖尿病患者进行随访,发现基线SUA水平可以预测糖尿病进程中的持续大量白蛋白尿[11],另有研究显示SUA水平与T2DM肾病的发生发展明显相关[12]。
DPN是T2DM的一个重要并发症,目前其发病机制仍在研究及探讨中,可能有多种因素如持续高血糖、高脂血症及高血压等引发DPN,作为糖尿病及其慢性并发症发生发展中的一个重要危险因素,SUA水平可能在DPN的发生发展中也发挥了作用,目前关于SUA与DPN的研究尚少,一项病例对照研究观察比较了DPN的T2DM患者与无DPN的患者的SUA水平,他们的结果显示DPN组SUA水平明显高于无DPN组[13]。而另一项对608名泰国T2DM患者的研究发现,SUA水平与DPN显著相关[5]。我们对920名T2DM患者的研究结果显示,SUA可能是DPN的一项危险因素,随着SUA水平的升高,DPN的发生比例有增长趋势,并且在SUA水平>3.5mg/dl后,DPN的发生比例即显著增加,与其他研究结果较一致。目前认为DPN发生机制包括多元醇旁路、氨基己糖、蛋白激酶C、氧化应激、炎症反应、非酶糖基化、神经生长因子、聚腺苷二磷酸-核糖聚合酶等,SUA可能是通过影响氧化应激、炎症反应以及一氧化氮(nitric oxide,NO)来引起DPN的发生发展。有研究提示,SUA升高可以诱发氧化应激,可以激活炎症反应,增加炎症因子C反应蛋白的表达而诱发DPN[14,15]。另外SUA可以使内皮细胞NO合成酶功能下降[16],NO水平下降而导致血管功能障碍,使得组织缺血,周围神经功能也因此而受损。

表3 血清尿酸与糖尿病周围神经病变的logistic回归分析
Adjusted for duration of diabetes, diastolic blood pressure, HbA1c, serum creatinine, triglycerides, urinary albumin: creatinine ratio(mg/g)
综上所述,我们的研究结果提示在T2DM的临床治疗中除了降糖、降脂和降压之外,有效地降低SUA水平可能应该成为T2DM治疗中的一个重要环节。但由于本研究未采用肌电图以及病理活检来诊断DPN,因此仍需进一步的研究来证实,另外尚需更多的研究来探讨SUA在DPN的发生发展中的具体作用机制。
[1] Brederson JD, Joshi SK, Browman KE,. PARP inhibitors attenuate chemotherapy-induced painful neuropathy[J]. J Peripher Nerv Syst, 2012, 17(3): 324−330.
[2] Pan P, Dobrowsky RT. Differential expression of neuregulin-1 isoforms and downregulation of erbin are associated with Erb B2 receptor activation in diabetic peripheral neuropathy[J]. Acta Neuropathol Commun, 2013, 1(1): 39.
[3] Shankar A, Klein BE, Nieto FJ,. Association between serum uric acid level and peripheral arterial disease[J]. Atherosclerosis, 2008, 196(2): 749−755.
[4] Resl M, Clodi M, Neuhold S,. Serum uric acid is related to cardiovascular events and correlates with N-terminal pro-B-type natriuretic peptide and albuminuria in patients with diabetes mellitus[J]. Diabet Med, 2012, 29(6): 721−725.
[5] Chuengsamarn S, Rattanamongkolgul S, Jirawatnotai S. Association between serum uric acid level and microalbuminuria to chronic vascular complications in Thai patients with type 2 diabetes[J]. J Diabetes Complications, 2014, 28(2): 124−129.
[6] Behradmanesh S, Horestani MK, Baradaran A,. Association of serum uric acid with proteinuria in type 2 diabetic patients[J]. J Res Med Sci, 2013, 18(1): 44−46.
[7] Dyck PJ, Litchy WJ, Lehman KA,. Variables influencing neuropathic endpoints: the Rochester Diabetic Neuropathy Study of healthy subjects[J]. Neurology, 1995, 45(6): 1115−1121.
[8] Young MJ, Boulton AJ, MacLeod AF,. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population[J]. Diabetologia, 1993, 36(2): 150−154.
[9] Viazzi F, Leoncini G, Vercelli M,. Serum uric acid levels predict new-onset type 2 diabetes in hospitalized patients with primary hypertension: the MAGIC study[J]. Diabetes Care, 2011, 34(1): 126−128.
[10] Bhole V, Choi JW, Kim SW,. Serum uric acid levels and the risk of type 2 diabetes: a prospective study[J]. Am J Med, 2010, 123(10): 957−961.
[11] Jalal DI, Rivard CJ, Johnson RJ,. Serum uric acid levels predict the development of albuminuria over 6 years in patients with type 1 diabetes: findings from the Coronary Artery Calcification in Type 1 Diabetes study[J]. Nephrol Dial Transplant, 2010, 25(6): 1865−1869.
[12] Hovind P, Rossing P, Tarnow L,. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes: an inception cohort study[J]. Diabetes, 2009, 58(7): 1668−1671.
[13] Kiani J, Habibi Z, Tajziehchi A,. Association between serum uric acid level and diabetic peripheral neuropathy (a case control study)[J]. Caspian J Intern Med, 2014, 5(1): 17−21.
[14] Kanbay M, Segal M, Afsar B,. The role of uric acid in the pathogenesis of human cardiovascular disease[J]. Heart, 2013, 9(11): 759−766.
[15] Matheus AS, Tibiriçá E, da Silva PB,. Uric acid levels are associated with microvascular endothelial dysfunction in patients with type 1 diabetes[J]. Diabet Med, 2011, 28(10): 1188−1193.
[16] Choi YJ, Yoon Y, Lee KY,. Uric acid induces endothelial dysfunction by vascular insulin resistance associated with the impairment of nitric oxide synthesis[J]. FASEB J, 2014, 28(7): 3197−3204.
(编辑: 李菁竹)
Correlation of serum uric acid level with diabetic peripheral neuropathy in type 2 diabetic patients
ZENG Jing-Bo*, ZHUANG Xiao-Ming, MU Jun
(Department of Endocrinology, Fuxing Hospital Affiliated to Capital Medical University, Beijing 100038, China)
To determine the correlation of serum uric acid (SUA) level with diabetic peripheral neuropathy (DPN) in the patients with type 2 diabetes mellitus (T2DM).A total of 920 T2DM in- and out-patients in our department from March 2011 to March 2013 were enrolled in this cross-sectional study. Biochemical parameters, such as fasting blood glucose (FBG), glycosylated hemoglobin Alc (HbAlc), total cholesterol (TC), triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and SUA were measured. DPN was diagnosed by neuropathy symptom score and neuropathy disability score. The subjects were stratified according to quartiles of SUA levels. Logistic regression analysis was used to analyze odds ratios between SUA and the prevalence of DPN.The adjusted logistic regression indicated that the level of SUA was a risk factor of DPN after 2nd quartile (>3.5mg/dl); and with the increase in the level, its effect on prevalence of DPN from 2nd to 4th quartiles became more significant [OR=2.95 (2.02 to 8.76), 3.06 (1.75 to 6.45), and 4.15 (0.84 to 6.74),<0.05].Level of SUA is a risk factor for the prevalence of DNP in T2DM patients. In the clinical management of DPN, monitoring SUA should be one of important treatment in addition to controlling glucose and lowering blood pressure.
uric acid; diabetes mellitus, type 2; diabetes complications; diabetic neuropathies
(2011SRC0761).
R587.1; R587.25
A
10.3724/SP.J.1264.2014.000132
2014−04−21;
2014−06−23
北京市优秀人才培养专项经费资助项目(2011SRC0761)
曾静波, E-mail: abosong@126.com

